Ingvild Kjeken, PhD1, Ingvild Bø, PT2, Aud Rønningen, OT2, Cristina Spada, MD2, Petter Mowinckel, MSc1, Kåre Birger Hagen, PhD1 and Hanne Dagfinrud, PhD1
From the 1National Resource Center for Rehabilitation in Rheumatology, Department of Rheumatology, Diakonhjemmet Hospital, Oslo and 2Lillehammer Hospital for Rheumatic Diseases, Lillehammer, Norway
OBJECTIVE: To evaluate the mean overall effects over a 1-year period of a multidisciplinary in-patient rehabilitation programme for patients with ankylosing spondylitis.
DESIGN: Observer-blinded, randomized controlled trial, with assessments made after 4 and 12 months.
Patients: Forty-six patients received a 3-week in-patient rehabilitation programme and 49 patients received treatment as usual.
METHODS: Primary outcomes were disease activity measured with the Bath Ankylosing Spondylitis Disease Activity Scale (BASDAI), and function measured with the Bath Ankylosing Spondylitis Functional Index (BASFI). Secondary outcomes included well-being, spinal and hip mobility, and health-related quality of life measured with the Medical Outcome Study Short Form-36. Overall treatment effects were estimated with Mixed models repeated measures analyses.
RESULTS: Significant overall treatment effects in favour of the rehabilitation group were found in the BASDAI score (mean difference over the 1-year period –10.0, 95% confidence interval: –3.7 to –16.3), in well-being (–7.3, 95% confidence interval: –1.0 to –14.7), and in the Medical Outcome Study Short Form-36 variables social functioning, role physical, role mental and bodily pain (mean differences ranging from 5.8 (pain) to 10.7 (role physical)).
CONCLUSION: A 3-week in-patient rehabilitation programme had positive overall effects on disease activity, pain, function and well-being, and should be considered an important complement to medical disease management in persons with ankylosing spondylitis.
Key words: rehabilitation; ankylosing spondylitis; physical therapy; occupational therapy; randomized controlled trial.
J Rehabil Med 2013; 00: 00–00
Guarantor’s address: Ingvild Kjeken, National Resource Center for Rehabilitation in Rheumatology, Diakonhjemmet hospital, PO Box 23, Vinderen, NO-0319 Oslo, Norway. E-mail: ingvild.kjeken@diakonsyk.no
Accepted August 16, 2012; Epub ahead of print Nov 9, 2012
INTRODUCTION
Ankylosing spondylitis (AS) is a chronic, progressive rheumatic disease characterized by inflammation and ankylosis of the axial skeleton, especially sacroiliitis, which is regarded as the hallmark of the disease. The main clinical features are inflammatory back pain, joint stiffness and fatigue, resulting in varying degrees of structural and functional impairments and reduced general health (1–3). In addition, peripheral joint involvement is reported in approximately one-third of patients, and AS may also be associated with extra-spinal manifestations, i.e. enthesitis, anterior uveitis, and bowel and heart disease. The disease presents at around 20–30 years of age, the overall prevalence is reported to be between 0.1% and 1.4%, and the male to female ratio is approximately 2 to 1.
Over the last years a revolution in the treatment of AS has taken place, in terms of improved understanding of basic disease mechanisms, new imaging techniques and criteria for classification and early diagnosis, use of biological drugs (tumour necrosis factor alpha (TNF-α)-blockers), and increased insight into the risk of cardiovascular disease (CVD) (1, 4–7). With the huge advances in pharmacological treatment, it is debatable whether rehabilitation programmes are still needed for people with AS. However, recent studies have shown that a combination of biological treatment and physical therapy (PT) (8), occupational therapy (OT) (9), or multi-disciplinary rehabilitation programmes (10–12), gave synergetic effects and produced positive benefits on pain, function and health-related quality of life, indicating that non-pharmacological interventions will also be important for AS patients in the future. Rehabilitation is therefore still considered one of the main treatment strategies (13), and the Assessment of SpondyloArthritis International Society (ASAS) and European League Against Rheumatism (EULAR) working group state that optimal management of AS comprises a combination of pharmacological and non-pharmacological treatment, the latter including education, exercise and physiotherapy (14).
However, even though several studies show beneficial effects of physiotherapy and rehabilitation programmes (10, 11, 15–17), more information is needed to optimize the delivery of these interventions. Furthermore, the results concerning the duration of the effect of rehabilitation are conflicting, and doubts remain about sustained improvement over long periods (13, 16, 17). Thus, the aim of this study was to evaluate the mean overall effects over a 1-year period of a multidisciplinary in-patient rehabilitation programme for patients with AS compared with treatment as usual.
PATIENTS AND METHODS
Study design
In this randomized controlled trial, participants were assessed at baseline (before group allocation), and after 4 and 12 months. After baseline assessments, patients were randomly assigned to the intervention group (a 3-week in-patient rehabilitation programme at Lillehammer Hospital for Rheumatic Diseases) or control group (treatment as usual). Participants in the rehabilitation group completed the programme within 3 months of baseline. Since many of the participants lived a long distance from the hospital, 4-month assessments were patient-reported outcomes (PROs) collected by posted questionnaires. Twelve-month assessments were a combination of PROs and a clinical examination. The study was approved by the Regional Committee for Medical Research Ethics and the Data Inspectorate, and all patients consented to participate. The trial is registered in the ISRCTN-register (ISRCTN5685576).
Study participants
From February 2006 to April 2010, a total of 100 participants with AS previously diagnosed by a rheumatologist based on the modified New York criteria (18), were consecutively included at two Norwegian hospitals; the out-patient clinic at Lillehammer Hospital for Rheumatic Diseases at Lillehammer, and the Department of Rheumatology at Diakonhjemmet Hospital in Oslo.
Other inclusion criteria were: age between 18 and 65 years; a score on the Bath Ankylosing Spondylitis Disease Activity Scale (BASDAI) of ≥ 40 mm; and ability to communicate in Norwegian. Exclusion criteria were: coronary heart disease; pregnancy; impaired function due to other significant medical problems; surgery or rehabilitation within the last 6 months; or cognitive or mental impairment. In addition, participants in the control group were excluded at the 4-month control if they reported participation in multidisciplinary rehabilitation after baseline assessment. Also, participants in both groups were excluded at the 12-month control if they had started biological therapy during the trial period or reported multidisciplinary rehabilitation after the 4-month assessment.
Randomization and procedures
Patients were randomly assigned to the intervention or control group. A statistician not involved in the study made a computer-generated randomization list. Concealed, opaque envelopes prepared by a secretary were used to allocate the patients to either the intervention or the control group. The envelopes were stored in a locked closet and were opened by the assessor after baseline assessments and inclusion was completed. In this trial, the patients and therapists delivering the intervention were aware of the treatment assigned. However, to achieve observer blinding, a second blinded assessor performed the 12-month assessment, and in the posted appointment for the assessment, patients were asked not to inform the assessors about their group allocation.
Intervention
Rehabilitation group. The rehabilitation programme was aimed at reducing symptoms, improving physical function and enhancing self-management. At admission, a physician, nurse, physiotherapist and occupational therapist examined the patient. Based on the assessments and interviews, an individualized plan for the rehabilitation stay was developed, including patient-specific long- and short-term goals.
Current recommendations for management of AS encompass exercises as a cornerstone of treatment (19). The physiotherapist designed a weekly exercise programme, which was a combination of exercises in the gym, in a hot water pool, and outdoor physical activities (Table I). In line with best practice in physiotherapy, doses, intensity, and frequency of the different elements in the package was individually adopted, to ensure an optimal starting level and progression for each patient. As recent research has revealed that AS is associated with an increased risk of CVD (5, 20), at least one of the daily exercise bouts had sufficient intensity for developing cardio-respiratory fitness (controlled by use of a heart rate monitor). In addition, participants received individual physiotherapy when needed, including manual techniques.
The Canadian Occupational Performance Measure (COPM) was used as a part of the baseline assessments (21), and the activity limitations and participation restrictions described in the COPM interview gave directions for the occupational therapy intervention. Depending on the problems described by the patient, the intervention could include teaching of energy conservation and alternative working methods, use of assistive technology, and discussing home and workplace accommodations. As sleep disturbances and daytime fatigue have been described as major problems by people with AS, information concerning fatigue management and sleep hygiene, including trying out high-quality mattresses and ergonomic pillows during the rehabilitation stay, was emphasized (22). After discharge, participants received community-based physiotherapy when appropriate.
|
Table I. Example of a weekly exercise programme |
|||||
|
Goal |
Type of exercise |
Duration (min) |
Intensity |
Frequency (sessions per week) |
|
|
Pool (group) |
Warm-up |
10 |
3–5 |
||
|
Mobility |
Individually adopted exercises |
15 |
8–12 reps × 3 |
||
|
Cardio-respiratory fitness |
Interval training |
4 × 4 |
High (90%) to moderate (70%) intensity |
||
|
Gym |
Muscle strength, stability and mobility |
5–10 exercises (use of different types of fitness equipment) |
30–45 |
Mobility: 8–12 reps × 3 Strength/stability: to exhaustion |
2–3 |
|
Outdoors |
Cardio-respiratory fitness |
Nordic walking |
45–60 |
55–90%a (heart rate monitor) |
3 |
|
aPercentage of age-predicted maximal heart rate. |
|||||
Control group. Participants received treatment as usual, which could include consultations with a rheumatologist or physician, community-based physiotherapy and/or self-management in terms of physical activity and exercises. Both groups received relevant medication. The control group was offered a rehabilitation stay after completion of the study.
Assessments
Socio-demographic characteristics (age, sex, employment status and marital status) and disease variables (duration of symptoms, time since diagnosis, co-morbidity and sedimentation rate (SR)) were recorded at baseline.
The following outcomes were measured at 4- and 12-month follow-ups:
Primary outcomes in the trial were disease activity and physical function, assessed with two PROs; the BASDAI (23) and the Bath Ankylosing Spondylitis Functional Index (BASFI) (24). Both measures are AS-specific and have shown good evidence for validity, reliability and responsiveness across a wide variety of settings (25).
BASDAI comprise 6 100-mm visual analogue scales (VAS), (0 = low disease activity), related to major symptoms relevant to AS; fatigue, spinal pain, joint pain, localized tenderness, and degree and length of morning stiffness. A sum-score was calculated as mean of the 2 morning stiffness items and the 4 remaining items (23).
BASFI is the mean score of 10 questions addressing physical function (daily activities and paid work), assessed on VAS scales (0 = good function) (24).
Secondary outcomes were spinal and hip mobility measured by the Bath Ankylosing Spondylitis Metrology Index (BASMI) (26), well-being measured by the Bath Ankylosing Spondylitis Global Score (BAS-G) (27), and health-related quality of life measured by the Medical Outcome Study Short Form-36 (SF-36) (28).
The BASMI is the sum of 5 clinical examinations of spinal column and hip joints, with ratings classified in categories from 0 to 2 (0 = normal mobility) (26).
The BAS-G score is the mean of patients’ VAS scores of their well-being over the last week and last 6 months, respectively (0 = high well-being) (27).
SF-36 is a generic health measure with 8 subscales (physical functioning, role limitations due to physical problems, bodily pain, general health perceptions, vitality, social functioning, mental health, and role limitation due to emotional problems) (28). Each scale is expressed with values from 0–100, where low scores indicate poor health.
Other assessments. The COPM is an individualized instrument designed to describe and measure patients’ perception of activity performance and satisfaction with performance over time, and was used to capture activity limitations and participation restrictions at baseline (21, 29). The COPM assessment started with an interview addressing patient-specific AS-related activity limitations and participation restrictions within 9 areas: personal care, functional mobility, community management, (termed self-care), paid/unpaid work, household management, play/school, (termed productivity) and quiet recreation, active recreation and socialization (termed leisure). The final COPM score was the mean of patients’ ratings of 5 prioritized activities for performance (performance score) and satisfaction with performance (satisfaction score) on 1–10-point scales (where 10 = good performance/high satisfaction).
At baseline and 12-month control, patients also recorded performed physical activities and exercises (hours per week) in the previous 4 weeks in a diary with 8 suggested individual and group modalities, respectively. In addition, the diary had open labels where participants could describe and record modalities other than the 8 listed.
All participants also recorded weekly hours with physiotherapist, occupational therapist, nurse and physician in the 4 weeks before baseline and before 12-month follow-up, and in the period between baseline and 4-month control.
Sample size
Sample size was calculated based on the results of a study of a large AS cohort at Diakonhjemmet Hospital (2). In this study population (n = 312), the mean BASDAI score among participants with a VAS score ≥ 40 mm (n = 203) was 62 mm (standard deviation (SD) 16). The ASAS Working group’s criterion for improvement defines a clinical relevant improvement as ≥ 20% of the mean of the outcome (30). We therefore calculated that a sample size of 50 patients for each group was required to detect a difference of 12.4 in the BASDAI scores, with an expected 25% loss to follow-up after 12 months.
Statistical analyses
All participants were analysed according to initial group allocation, but follow-up data was not collected for participants who were excluded during the trial period.
Differences at baseline between participants in the two treatment groups were examined by independent samples t-test for means, Mann-Whitney tests for medians and χ2 for proportions. Within-group differences were examined by paired-samples t-tests. Treatment effects (mean differences between the groups after 4 and 12 months, and for the overall effect for the total trial period, respectively) were estimated with Mixed models repeated measures analysis (31). This analysis give estimates for the differences between the two groups at 4 and 12 months, respectively, as well as an estimate of the mean difference between the two groups over the one-year trial period, which was the main outcome in this study. The model includes the interaction of treatment and time (i.e. 4 and 12 months). For each variable we adjusted for gender and individual baseline values. A parametric bootstrap procedure was applied to ascertain the robustness of the findings. The adequacy of the model was assessed using Cook’s d and the CovRatio for fixed effects and covariance parameters. The analysis was performed using Statistical Analysis System version 9.2 (SAS Institute, Cary, NC, USA). p-values ≤ 0.05 were considered statistically significant.
RESULTS
Study participants
A total of 522 consecutive patients were assessed for eligibility. Of the 215 patients who met the inclusion criteria and were invited to participate in the study, 115 refused, most frequently due to work obligations (Fig. 1).

Fig. 1. Flow of participants through the trial.
The remaining 100 were randomized into receiving rehabilitation (rehabilitation-group, n = 51), or treatment as usual (control-group, n = 49). Five participants in the rehabilitation group were excluded from the study during their rehabilitation stay, because their AS diagnosis was not confirmed (no sacroiliitis on radiographs). Thus, all results are calculated based on 46 participants in the rehabilitation group and 49 in the control group. The 5 excluded participants had higher SR and reported poorer function on PROs compared with the other participants at baseline.
Four participants in the rehabilitation group and 6 in the control group were excluded prior to follow-up assessments, most frequently due to participation in rehabilitation programmes at other centres in the trial period (n = 6). A total of 80% and 63% completed 4-and 12-month assessments in the rehabilitation group, vs 71% and 61% in the control group.
The baseline characteristics of participants were well matched between the two groups, except for a significantly lower proportion of women in the rehabilitation group (p = 0.02) (Table II).
Use of medication at baseline and in the trial period was similar and stable in both groups, except for use of biological therapy. At baseline, the ratio between rehabilitation and control group was 1 vs 6 (p = 0.18). After 4 and 12 months it was 1 vs 4 (p = 0.39) and 1 vs 3 (p = 0.82), respectively, as two of the participants using biological therapy in the control group were excluded or dropped out before the 4-month control and 1 before the 12-month control.
|
Table II. Baseline characteristics of participants allocated to rehabilitation (rehabilitation group) or treatment as usual (control group) |
||||
|
All participants (n = 95) |
Rehabilitation group (n = 46) |
Control group (n = 49) |
p-valuesa |
|
|
Demographic variables |
||||
|
Age, years, mean (SD) |
49.0 (9.8) |
49.4 (10.3) |
48.6 (9.4) |
0.69 |
|
Female, n (%) |
33 (34.7) |
10 (21.7) |
23 (46.9) |
0.02 |
|
Education > 12 years, n (%) |
37 (41.6) |
20 (45.5) |
17 (37.8) |
0.60 |
|
Living alone, n (%) |
30 (31.9) |
16 (34.8) |
14 (29.2) |
0.72 |
|
Still working, n (%) |
58 (69.9) |
28 (71.8) |
30 (68.2) |
0.91 |
|
Disease variables |
||||
|
Disease duration, years, mean (SD) |
15.5 (10.9) |
14.9 (9.6) |
16.1 (12.0) |
0.62 |
|
Symptoms duration, years, mean (SD) |
23.6 (11.2) |
23.8 (11.3) |
23.5 (11.1) |
0.89 |
|
Sedimentation rate, mean (SD) |
10.8 (10.2) |
9.3 (8.3) |
12.1 (11.5) |
0.18 |
|
Co-morbidity, yes, n (%) |
60 (63.2) |
29 (63.0) |
31 (63.3) |
1.0 |
|
Medication, n (%) |
||||
|
Analgesics |
29 (30.5) |
13 (28.3) |
16 (32.7) |
1.0 |
|
NSAIDs |
72 (75.8) |
34 (73.9) |
38 (77.6) |
0.78 |
|
DMARDs |
4 (4.2) |
3 (6.5) |
1 (2.0) |
0.49 |
|
Biological therapy |
7 (7.4) |
1 (2.2) |
6 (12.2) |
0.18 |
|
Physiotherapy, h/week, median (range) |
0 (0, 3) |
0 (0, 2) |
0 (0, 3) |
0.44 |
|
Exercises and physical activity, h/week, median (range) |
2.8 (0, 16.5) |
3.0 (0, 16.5) |
2.4 (0, 8.3) |
0.10 |
|
aDifference between groups (independent samples t-test for means, Mann-Whitney tests for medians and χ2 for proportions). NSAIDs: non-steroidal anti-inflammatory drugs; DMARDs: disease-modifying anti-rheumatic drugs. |
||||
There were no significant differences in baseline levels of physiotherapy, exercising and physical activity, or any other treatment modality.
Median hours of physiotherapy per week in the first 4 months after baseline in the control group was 0.2 h of physiotherapy (range 0–2), and the median hours per week for the other modalities was 0 (physician range 0–0.3, nurse range 0–0.25, and occupational therapist range 0–0.06). As the rehabilitation group received 3 weeks of multidisciplinary rehabilitation between baseline and 4-month follow-up, the total amount of treatment received was significantly larger for all treatment modalities in this group, compared with the control group.
When comparing self-reported weekly hours of physiotherapy, exercising and physical activity in the last 4 weeks before baseline and 12-month follow-up, the number of hours of physiotherapy decreased, while the level of exercise and physical activity increased in both groups. However, there were no significant differences within or between the two groups in terms of treatment, exercise or physical activity in the 4 weeks prior to baseline and 12-month follow-up, respectively.
At baseline COPM interviews, participants prioritized a total of 375 activity limitations and participation restrictions. The distribution among the 9 activity and participation areas, and specific activity limitations/participation restrictions prioritized by ≥ 20 of participants are visualized in Fig. 2.
into significantly improved physical function, as measured by the BASFI. Physical impairment in AS has been shown to be independently caused by reversible components (such as patient-reported disease activity) and irreversible components (e.g. structural damage of the spine) (36). The results demonstrate that there is no linear relationship between symptoms and function, and suggest that a relatively large reduction in symptoms is needed to improve physical function. Also, as demonstrated in other studies, there are several other important components influencing function, such as environmental and personal factors (32, 33, 37, 38).
There were, however, significant positive overall treatment effects in the SF-36 variables social functioning, role physical, role mental and bodily pain. This indicates that generic and disease-specific instruments capture different aspects of health and functioning, and support combining different measures in rehabilitation studies.
We therefore also used the patient-specific instrument COPM to capture participants’ descriptions of activities and participation-areas that were important to address in the rehabilitation programme. Problems with regular exercising, poor sleep and lack of energy for social activities were frequently described in the COPM interviews. The overall positive treatment effects obtained in the current study may therefore partly be attributed to the individually designed exercise programmes, and to the focus on sleep, fatigue management and activity limitations and participation restrictions in the PT- and OT-interventions.
After we designed our study, new diagnostic criteria and measures of disease activity have been developed. In these criteria, sacroiliitis on imaging is not required for diagnosing AS, as long as the patient is HLA-B27-positive and has ≥ 3 other spondyloarthritis features (7). The exclusion of participants without X-ray-verified sacroiliitis may thus have been too conservative. In addition, use of the new ASDAS score might have increased the possibility of detecting clinically relevant treatment effects.
We used a rather strict inclusion criterion of BASDAI scores
Primary outcomes
There was a significant treatment effect in favour of the rehabilitation group in the BASDAI score after 4 months (mean difference between groups –14.2, 95% confidence interval (CI): –22.8, –5.7), but not in the BASFI score (Fig. 3 and Table III).
After 12 months, there were no significant differences between the two groups in any of the primary outcomes.
There was, however, a significant positive overall mean treatment effect in the 1-year trial period in the BASDAI score (mean difference over the 1-year period –10.0, 95% CI: –3.7 to –16.3).
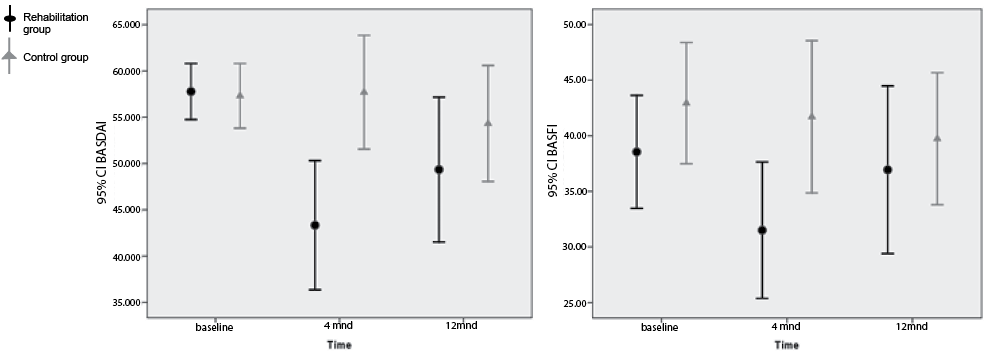
Fig. 3. Scores of the primary outcomes Bath Ankylosing Spondylitis Disease Activity Scale (BASDAI) and Bath Ankylosing Spondylitis Functional Index (BASFI) at baseline, and at 4-and 12-month follow-ups, respectively. The plot shows error bars with mean values and 95% confidence intervals (CIs). Lower values indicate fewer symptoms or better function.
Secondary outcomes
There were significant treatment effects in favour of the rehabilitation group in the SF-36 variables role physical, role mental, vitality and bodily pain after 4 months, but no significant differences in any secondary outcome after 12 months.
There were, however, significant positive overall treatment effects in well-being (–7.3, 95% CI: –14.7 to –1.0), and in the SF-36 variables social functioning, role physical, role mental and bodily pain (mean differences ranging from 5.8 (pain) to 10.7 (role physical)) (Table III).
|
Table III. Mean (95% confidence interval (CI)) scores for treatment effects with p-values, estimated with mixed models linear repeated measures analysisa |
|||||||
|
Rehabilitation group Mean (95% CI) |
Control group Mean (95% CI) |
Treatment effect Mean (95% CI) |
p-value |
Overall treatment effect Mean (95% CI) |
p-value |
||
|
Primary outcomes |
|||||||
|
BASDAIb (0–100, 0 is low disease activity) |
–10.0 (–16.3, –3.7) |
0.002 |
|||||
|
Baseline |
57.8 (54.7, 60.8) |
56.9 (53.4, 60.4) |
|||||
|
4 months |
43.2 (37.3, 49.2) |
57.5 (51.3, 63.6) |
–14.2 (–22.8, –5.7) |
0.001 |
|||
|
12 months |
49.6 (43.0, 56.2) |
54.5 (48.1, 60.9) |
–4.9 (–14.2, 4.3) |
0.30 |
|||
|
BASFIb (0–100, 0 is good function) |
–3.6 (–8.8, 1.6) |
0.17 |
|||||
|
Baseline |
38.6 (33.5, 43.6) |
42.4 (36.8, 48.0) |
|||||
|
4 months |
33.6 (28.7, 38.6) |
39.6 (34.6, 44.7) |
–6.0 (–13.1, 1.1) |
0.10 |
|||
|
12 months |
38.0 (32.5, 43.5) |
38.6 (33.6, 43.8) |
–0.70 (–8.2, 6.8) |
0.86 |
|||
|
Secondary outcomes |
|||||||
|
BAS-Gb (0–100, 0 is high well-being) |
–7.3 (–14.7, –1.0) |
0.02 |
|||||
|
Baseline |
56.2 (51.0, 61.3) |
57.5 (52.2, 62.7) |
|||||
|
4 months |
46.1 (40.1, 52.1) |
52.5 (46.3, 58.7) |
–6.4 (–15.1, 2.3) |
0.15 |
|||
|
12 months |
41.7 (34.9, 48.5) |
50.5 (44.1, 56.8) |
–8.8 (–18.1, 0.5) |
0.06 |
|||
|
BASMIb (0–10, 0 is good mobility) |
0.01 (–0.6,0.6) |
0.97 |
|||||
|
Baseline |
3.0 (2.3, 3.6) |
2.6 (2.1, 3.1) |
|||||
|
4 months |
– |
– |
– |
||||
|
12 months |
2.8 (2.4, 3.3) |
2.8 (2.4, 3.2) |
0.01 (–0.6, 0.6) |
0.97 |
|||
|
SF-36 physical functionc (0–100, 0 is poor health) |
2.2 (–2.8, 7.1) |
0.39 |
|||||
|
Baseline |
71.1 (66.4, 75.8) |
65.4 (60.4, 70.5) |
|||||
|
4 months |
71.1 (66.3, 75.8) |
68.9 (64.0, 73.8) |
2.2 (–4.6, 9.0) |
||||
|
12 months |
70.0 (64.6, 75.3) |
68.0 (63.0, 72.9) |
2.0 (–5.3, 9.3) |
||||
|
SF-36 social functioningc (0–100, 0 is poor health) |
7.0 (0.03, 13.9) |
0.05 |
|||||
|
Baseline |
70.4 (63.8, 76.9) |
69.7 (62.9, 76.5) |
|||||
|
4 months |
73.4 (66.7, 80.1) |
65.6 (58.7, 72.5) |
7.8 (–1.8, 17.4) |
0.11 |
|||
|
12 months |
72.1 (64.6, 79.7) |
66.1 (59.2, 73.1) |
6.0 (–4.3, 16.3) |
0.25 |
|||
|
SF-36 role physicalc (0–100, 0 is poor health) |
7.7 (0.3, 15.0) |
0.04 |
|||||
|
Baseline |
55.7 (49.2, 62.2) |
47.5 (39.5, 55.4) |
|||||
|
4 months |
59.5 (52.5, 66.5) |
47.7 (40.5, 54.8) |
11.9 (1.8. 21.9) |
0.02 |
|||
|
12 months |
57.2 (49.3, 65.1) |
54.2 (46.9, 61.6) |
3.0 (–7.8, 13.8) |
0.59 |
|||
|
SF-36 role mentalc (0–100, 0 is poor health) |
10.7 (3.2, 18.1) |
0.006 |
|||||
|
Baseline |
79.5 (72.7, 86.3) |
81.4 (75.6, 87.2) |
|||||
|
4 months |
79.5 (72.3, 86.6) |
65.8 (58.6, 73.1) |
13.6 (3.5, 23.8) |
0.01 |
|||
|
12 months |
80.7 (72.8, 88.7) |
73.2 (65.7, 80.8) |
7.5 (–3.5, 18.5) |
0.18 |
|||
|
SF-36 mental healthc (0–100, 0 is poor health) |
3.8 (–0.6, 8.2) |
0.09 |
|||||
|
Baseline |
77.7 (73.5, 81.9) |
72.5 (67.5, 77.5) |
|||||
|
4 months |
78.4 (74.2, 82.5) |
72.6 (68.3, 76.8) |
5.8 (–018, 11.8) |
0.06 |
|||
|
12 months |
74.4 (69.8, 79.1) |
73.2 (68.8, 77.5) |
1.3 (–5.1, 7.7) |
0.69 |
|||
|
SF-36 vitalityc (0–100, 0 is poor health) |
5.2 (–0.4, 10.8) |
0.07 |
|||||
|
Baseline |
37.2 (31.6, 42.9) |
32.8 (27.9, 37.8) |
|||||
|
4 months |
43.1 (37.8, 48.4) |
34.7 (29.2, 40.2) |
8.4 (0.8, 16.1) |
0.03 |
|||
|
12 months |
36.8 (30.8, 42.8) |
35.6 (30.1, 41.2) |
1.2 (–7.0, 9.4) |
0.78 |
|||
|
SF-36 bodily painc (0–100, 0 is poor health) |
5.8 (0.5, 11.0) |
0.03 |
|||||
|
Baseline |
36.4 (33.0, 39.8) |
32.4 (28.2, 36.6) |
|||||
|
4 months |
45.1 (40.1, 50.0) |
36.9 (31.8, 42.0) |
8.2 (1.1, 15.3) |
0.02 |
|||
|
12 months |
43.2 (37.6, 48.8) |
40.2 (34.9, 45.4) |
3.0 (–4.7, 10.7) |
0.44 |
|||
|
SF-36 general healthc (0–100, 0 is poor health) |
2.4 (–3.0, 7.7) |
0.39 |
|||||
|
Baseline |
49.3 (43.6, 54.9) |
49.0 (43.8, 54.3) |
|||||
|
4 months |
52.9 (47.8, 58.0) |
46.7 (41.4, 52.0) |
6.3 (–1.1, 13.6) |
0.09 |
|||
|
12 months |
48.8 (43.1, 54.6) |
50.9 (45.6, 56.3) |
–2.1 (–10.0, 5.8) |
0.60 |
|||
|
aAdjustment for the baseline mean value of the variable and for gender. bNegative values favour the intervention group. cPositive values favour the intervention group. BASDAI: Bath Ankylosing Spondylitis Disease Activity Scale; BASFI: Bath Ankylosing Spondylitis Functional Index; BAS-G: Bath Ankylosing Spondylitis Global Score; BASMI: Bath Ankylosing Spondylitis Metrology Index; SF-36: Medical Outcome Study Short Form-36. |
|||||||
DISCUSSION
The main aim of this study was to evaluate the overall effects of a multidisciplinary in-patient rehabilitation programme for patients with AS. The results demonstrate that the rehabilitation programme resulted in sustained improvement over a 1-year period, in terms of significant reductions in disease activity and pain, and improved function and well-being.
The improvement in patient-reported disease activity (BASDAI) is noteworthy, as this captures the patients’ experienced reduction in the main AS symptoms pain, stiffness and fatigue, which are important determinants for daily functioning and health-related quality of life (3, 32, 33).
Inflammation is recognized as the main driver of the disease process, and increases the risk of co-morbidity such as CVD (20). Even if the BASDAI does not include a biomarker of inflammation, studies have shown that this measure is highly correlated with the new AS disease activity score (ASDAS), which includes inflammatory markers (34, 35). The finding that a multi-disciplinary rehabilitation programme significantly reduced disease activity over a 1-year period therefore indicates that rehabilitation still has an important role in AS management.
The decrease in disease activity did not, however, translate into significantly improved physical function, as measured by the BASFI. Physical impairment in AS has been shown to be independently caused by reversible components (such as patient-reported disease activity) and irreversible components (e.g. structural damage of the spine) (36). The results demonstrate that there is no linear relationship between symptoms and function, and suggest that a relatively large reduction in symptoms is needed to improve physical function. Also, as demonstrated in other studies, there are several other important components influencing function, such as environmental and personal factors (32, 33, 37, 38).
There were, however, significant positive overall treatment effects in the SF-36 variables social functioning, role physical, role mental and bodily pain. This indicates that generic and disease-specific instruments capture different aspects of health and functioning, and support combining different measures in rehabilitation studies.
We therefore also used the patient-specific instrument COPM to capture participants’ descriptions of activities and participation-areas that were important to address in the rehabilitation programme. Problems with regular exercising, poor sleep and lack of energy for social activities were frequently described in the COPM interviews. The overall positive treatment effects obtained in the current study may therefore partly be attributed to the individually designed exercise programmes, and to the focus on sleep, fatigue management and activity limitations and participation restrictions in the PT- and OT-interventions.
After we designed our study, new diagnostic criteria and measures of disease activity have been developed. In these criteria, sacroiliitis on imaging is not required for diagnosing AS, as long as the patient is HLA-B27-positive and has ≥ 3 other spondyloarthritis features (7). The exclusion of participants without X-ray-verified sacroiliitis may thus have been too conservative. In addition, use of the new ASDAS score might have increased the possibility of detecting clinically relevant treatment effects.
We used a rather strict inclusion criterion of BASDAI scores ≥ 40mm in our study, because we wanted to include the patients who were most likely to benefit from the programme. This also reduced the variation in the study population, and thereby limited the number of participants needed to gain sufficient statistical power. More than 40% of the patients (n = 218) assessed for eligibility had BASDAI scores < 40 mm. Thus, a less restrictive inclusion criterion would probably have shortened the inclusion period and ensured that the participants were more representative for those who are usually referred to multidisciplinary rehabilitation.
Furthermore, the majority of participants had a long-standing disease. In the last decade, improved imaging techniques and criteria for early diagnosis have facilitated earlier and more effective medical treatment. Future studies should explore whether additional early and targeted rehabilitation also can prevent CVD and functional limitations such as work disability.
The study was limited by withdrawals and drop-outs during the trial period. This is a common challenge when conducting RCTs with long-term follow-up. However, 6 participants were excluded due to participation in other rehabilitation programmes, indicating that this patient group consider rehabilitation to be important and beneficial. Furthermore, the Mixed model analysis is robust to missing values, because data at all time-points are used, even if patients are missing at one of the follow-ups (31).
Another limitation is that both participants and therapists knew which therapy they received or delivered. However, we tried to maintain masked conditions for assessment by asking patients not to reveal group allocation to the assessor at the 12-month follow-up. In addition, all but one outcome was self-reported using validated measures, thereby reducing the chance of results being greatly affected by observer bias.
One limitation is that ordinal data have been used inappropriately in part of the analysis and this may influence the results.
In conclusion, this randomized controlled trial demonstrates that a multidisciplinary rehabilitation programme had positive overall effects on disease activity, pain, function and well-being, and should be considered an important complement to medical disease management in persons with AS.
Acknowledgement
This work was supported by Health South-East, Norway, grant number 2006077.
REFERENCES
