Rachael Rietdijk, B.App.Sc, Leanne Togher, PhD and Emma Power, PhD
From the Disipline of Speech Pathology, Faculty of Health Sciences, The University of Sydney, Sydney, Australia
OBJECTIVE: To describe the effectiveness of using telehealth programs to provide training or support to family members of people with traumatic brain injury.
DESIGN: Systematic review.
DATA SOURCES: Intervention studies were identified by searching Medline, CINAHL, PsycINFO, Web of Science, Scopus, the Cochrane library, Embase, PsycBITE and ProQUEST.
STUDY SELECTION: Studies included in the review reported an intervention involving family members of adults or children with traumatic brain injury, delivered at a distance through use of technology (including telephone, websites or videoconferencing). Reliability of inclusion of studies in the review was high (Kappa = 0.816) based on a second reviewer evaluating a random sample of 25% of the 830 references originally identified from the database search.
DATA EXTRACTION: Data describing the participants, interventions and outcomes were extracted from each study. The quality of studies was evaluated using the PEDro-P scale.
DATA SYNTHESIS: The review identified 7 randomised controlled trials, 4 non-randomised controlled trials and 5 case series studies. The studies involved a variety of program formats and intervention targets. All but one study reported positive outcomes of the telehealth programs, however very few studies used blinded assessors.
CONCLUSIONS: Telehealth programs for family members of people with traumatic brain injury are feasible, with positive outcomes reported. Further research is needed to strengthen the evidence for the use of telehealth in comparison to face-to-face interventions, and to provide information to guide clinical decision-making.
Key words: Traumatic brain injury; family; carers; caregivers, telehealth, systematic review
J Rehabil Med 2012; 44: 00–00
Correspondence address: Mrs Rachael Rietdijk, Disipline of Speech Pathology, Faculty of Health Sciences, University of Sydney, PO Box 170 Lidcombe NSW 1825, Australia. E-mail: rman7827@uni.sydney.edu.au
Submitted February 26, 2012; accepted, July 11, 2012
INTRODUCTION
Traumatic brain injury (TBI) is the leading cause of disability in people aged under 40, with the annual incidence estimated to be 100 per 100,000 population per year (1). TBI places a high level of burden on families, who face a lifetime of supporting a family member who sustained a TBI as a young person. When a person with TBI leaves hospital, family members assume increased responsibility for providing support, but receive limited access to services (2). Recent research has indicated that 71% of family caregivers feel anxious about their role when the person with TBI is discharged from hospital (3). The ongoing demands of supporting a family member with TBI increase the risk of depression, anxiety and other psychological symptoms, with up to a third of family caregivers reporting high levels of psychological distress (4).
Brain injuries affect families as a whole, and therefore the whole family unit requires support, not only the person with the brain injury (5). Family caregivers have identified increased support and information would be helpful for reducing anxiety and feelings of burden (3, 6) and that the provision of specific information regarding how to care for the person with TBI and achieve the highest possible level of functioning would be beneficial (7). Providing services to caregivers can result in improved outcomes for people with TBI (8), as well as improving caregiver depression, health and problem-solving (9).
Although providing support and training to families is beneficial, it is challenging for brain injury services to meet this need given the large geographical areas that each service must cover. Even in metropolitan areas, families report it is often difficult to access support from brain injury specialist centres due to work or childcare commitments (5). Lack of transportation, and the cognitive and physical impairments of people with TBI can also make it difficult to organise attendance at appointments. Telehealth (the use of information and communication technology to deliver health services over a distance) has been identified as one strategy that can expand and decentralize service delivery for people with disabilities (10). The delivery of telehealth programs may use synchronous technology (e.g. telephone call, videoconferencing, instant messaging) which allows real-time connection between two parties, or asynchronous technology (e.g. self-guided web exercises, discussion boards, email) where information stored or sent by one party is retrieved at a later time (11). Some programs use a combination of these modes.
This is a potentially useful approach for meeting the needs of families of people with TBI for information and training. The benefits of this approach can include increased access for families in rural areas, reduced costs of services and reduced travel time (12). Extending follow-up into the home, potentially through use of telehealth, has been identified as a component of effective caregiver programs (13). Recent qualitative research (5) found that family caregivers identified interventions using the internet or telephone as an ideal solution for providing timely access to information and for offering the possibility of informal peer support networks. A telehealth approach meets many of the features of services valued by caregivers, such as accessibility, flexibility and efficiency (5).
Many services are therefore starting to make use of telehealth, and develop policies around its use. For example, in the United States, the Caregivers and Veterans Health Services Omnibus Act of 2010 (14) entitles caregivers of veterans with TBI to online training. Although telehealth services for caregivers are becoming more frequently offered, there are unanswered questions about many aspects of this approach, and there are no systematic reviews within this field that are specific to TBI. However, there are several related systematic reviews that suggest this approach may have potential, and that it would be worthwhile to conduct a systematic review in this area. For example, a broad systematic review of telehealth studies across a variety of conditions found an overall trend in the literature supporting the effectiveness of such interventions (15). A systematic review of telehealth interventions for carers of people with dementia found that interventions had moderate effects on improving caregiver stress and depression (16).
Aims
The aim of this systematic review was to identify and synthesise current evidence for the use of telehealth to provide training or support to family members of people with TBI. The effectiveness of interventions was evaluated through identifying statistically significant results and/or large effect sizes within studies. The questions of interest were:
1) Do telehealth programs involving family members improve the functioning of people with TBI?
2) Do telehealth programs involving family members improve the psychological wellbeing, skills, knowledge or burden of family members of people with TBI?
3) Do telehealth programs involving family members improve the overall functioning of families of people with TBI?
4) Are telehealth programs as effective as face-to-face programs in achieving improved outcomes for people with TBI and their families?
5) What features contribute to the effectiveness of such programs (as determined from the results of controlled studies comparing alternative forms of interventions, or from qualitative feedback from participants)?
6) What characteristics of family members affect the outcomes of such programs?
METHOD
There was no registered protocol for the review.
Inclusion and exclusion criteria
The inclusion and exclusion criteria for the review were defined in relation to the populations and interventions of interest. We included sources that reported any telehealth intervention involving family members of a person with TBI of any severity and of any age (adult or child). Family members could include parents, partners, siblings, extended family or close friends who had taken on a caregiving role. In studies involving a mixed sample of conditions, it was required that at least 50% of the sample be family members of a person with a TBI. Telehealth was defined as any intervention delivered at a distance using technology, including telephone, websites or videoconferencing. There were no inclusion criteria imposed on the outcomes of the intervention or on the study design.
Sources were therefore excluded from the review on the basis of the following criteria: (i) does not relate to traumatic brain injury rehabilitation; (ii) does not relate to family members of a person with TBI; (iii) less than 50% of participants were family members of a person with a TBI; (iv) not related to training / support for family members of a person with a TBI; (v) training / support not delivered via telehealth; (vi) duplicates other results reported more fully elsewhere; (vii) did not have at least an abstract available for review or (viii) no data reported on outcome measures or process measures relating to the program.
Search strategy
The initial search strategy was developed by identifying key words for the population and interventions of interest. Sources meeting the inclusion criteria were identified from the initial search. The backward citations within these sources and the forward citations (identified using Web of Science) were examined to determine any further citations meeting inclusion criteria. The search strategy was then further refined to ensure it captured as many relevant sources as possible.
The search strategy was applied to the following databases with no limit on date of publication: Medline, CINAHL (Cumulative Index to Nursing and Allied Health Literature), PsycINFO, Web of Science, Scopus, all databases within the Cochrane library, Embase, PsycBITE (Psychological Database for Brain Impairment Treatment Efficacy) and all databases within ProQUEST. The search strategy (with syntax adjusted for individual databases) was as follows: “brain injury” AND (partner or spouse or family or friend or carer or caregiver or staff) AND (train* or treat* or therapy or intervention or rehabilitation or reintegration) AND (telehealth or telemedicine or telerehabilitation or videoconferencing or “internet resources” or “telephone support” or (web-based near intervention)). The term “staff” was included as interventions involving paid carers were initially considered for this review, however, the scope was later restricted to family caregivers. No restrictions were placed on the language or dates of search results. The search strategy was finalised on 24th September 2011. Five additional sources were added to the review up to 28th December 2011 through reference lists of included articles, citation alerts originating from the search strategy or forwarded articles in press from authors who were aware of the review.
Study selection
Exclusion decisions were initially made based on the title and abstract. Where further information was required, the full text of the papers was reviewed to determine whether they met inclusion criteria. In cases where it was difficult to determine whether results from the same group of participants were reported across multiple sources, study authors were contacted to provide further information. Links between papers reporting separate outcome data from the same group of participants were noted.
An independent rater examined 25% of the sources (n = 208) obtained from the database search after duplicate references had been removed and applied the exclusion criteria as described. The inter-rater point-by-point agreement was 98.6% (205/208). Kappa was 0.816 (p <0.001), 95% confidence interval (CI) (0.483, 0.816) indicating almost perfect agreement (17).
Data extraction
The following categories of data were extracted from included sources: (i) number of participants with TBI in each intervention group; (ii) age group of participants with TBI; (iii) description of TBI participants based on inclusion criteria; (iv) number of family members in each intervention group; (v) description of family members based on inclusion criteria, (vi) participants in intervention; (vii) technology used for intervention; (viii) target of intervention; (ix) description of intervention; (x) number of hours or contacts involved in the intervention; (xi) comparison condition (if applicable); (xii) time to follow-up; (xiii) measures of outcomes, including outcomes for participants with TBI or family members as well as process measures (e.g. satisfaction, ease of use, costs) and (xiv) results on outcome measures (such as p statistics and effect sizes where available), including any analyses of caregiver subgroups.
The strength of the evidence was evaluated by classifying the level of evidence provided by the included sources using the Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence (Table I) (18). The risk of bias within randomised and non-randomised controlled studies was assessed using the PEDro-P scale (19). The criteria on which this rating is based are shown in Table III. Although the focus of this scale is usually on outcomes for the clinical population, for the purposes of this review, ratings were also completed for studies comparing intervention groups based on caregiver outcomes or client satisfaction. PEDro-P ratings were not completed on sources for which only an abstract was available, as the abstract alone provided insufficient information. Ratings were used from the PsycBITE website (20) where available. Where no rating was available, two independent raters evaluated the papers, and any disagreements between the two ratings were resolved through consensus.
|
Table I. Levels of evidence for questions of treatment benefits |
|
|
Level of evidence |
Type of study |
|
1 |
Systematic review of randomized trials or n-of-1 trials |
|
2 |
Randomized trial or observational study with dramatic effect |
|
3 |
Non-randomized controlled cohort/follow-up study |
|
4 |
Case-series, case-control studies, or historically controlled studies |
|
5 |
Mechanism-based reasoning |
|
Based on Oxford Centre for Evidence-Based Medicine (18). |
|
RESULTS
Study selection
Fig. 1 outlines the search results and the process of exclusion of sources. 1,353 sources were identified from use of the search strategy. Following removal of duplicates and application of the exclusion criteria, there were 19 sources meeting the inclusion criteria. Five additional sources were identified following the original database search. This formed a corpus of 24 sources for the review.
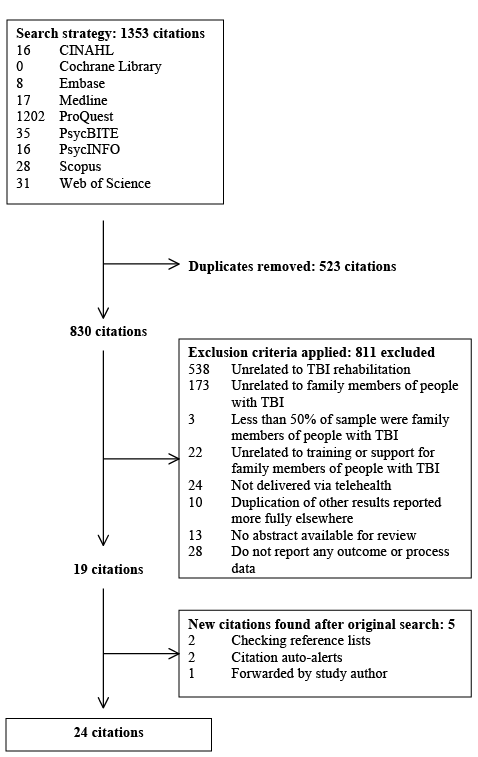
Fig. 1. Screening of studies for eligibility.
Study characteristics
Study design. Table II outlines the included studies. Outcomes and description of some studies are reported across multiple papers, which are grouped together in individual rows in Table II. The search located 7 randomized controlled trials (RCTs) (Level 2) reported across 13 papers (21–33), 4 non-RCTs (Level 3) reported across 6 papers (34–39) and 5 case-series studies (Level 4) (40–44). No systematic reviews were identified. Four of the RCTs (21–25, 33) were large studies including 120 or more caregivers of people with TBI. One RCT (23) was a replication of an earlier RCT (22) using a more diverse sample of participants over a longer time period. All studies were published within the last 15 years.
|
Table II. Description of participants in studies |
|
||||||
|
Study |
TBI participants (n)a |
TBI age |
TBI characteristics |
Caregiver participants (n)a |
Caregiver characteristics |
Intervention participants |
Target of intervention |
|
Level 2: RCTs |
|||||||
|
Bell et al., 2004, 2005 (21, 22) |
Rx: n = 79/85 Ctrl: n = 78/86 |
16+ |
Mod-severe TBI; |
Rx: n = 79/85 Ctrl: n=78/86 |
Family members or significant others |
Both TBI and caregiver (focussed on TBI) |
Management of family concerns |
|
Bell et al., 2011 (23) |
Rx: n = 169/210 Ctrl: n = 174/223 |
16+ |
Mod-severe TBI; |
Rx: n = 169/210 Ctrl: n = 174/223 |
Family members or close friends |
Both TBI and caregiver (focussed on TBI) |
Management of family concerns |
|
Salazar et al., 2000 (24), Warden et al., 2000 (25) |
Rx: n = 53/53 Ctrl: n = 67/67 |
Adult |
Mod-severe TBI; < 3 months post-injury |
Rx: n = 53/53 Ctrl: n = 67/67 |
Family members |
Both TBI and caregiver (focussed on TBI) |
Cognitive rehabilitation |
|
Wade et al., 2006 (26, 27), Carey et al., 2008 (28) |
Rx: n = 22/26 Ctrl: n = 20/20 |
5–16 |
Mod-severe TBI; 1–24 months post-injury |
Rx: n = 22/25 Ctrl: n = 20/20 |
Parents and school-aged siblings |
Both TBI and caregiver |
Family problem-solving |
|
Wade et al., 2008, 2009 (29, 30) |
Rx: n = 5/5 Ctrl: n = 4/4 |
11–18 |
Mod-severe TBI; < 2 years post-injury |
Rx: n = 5/5 Ctrl: n = 4/4 |
Parents and siblings |
Both TBI and caregiver |
Family problem-solving |
|
Wade et al., 2010, 2011 (31, 32) |
Rx: n = 16/20 Ctrl: n = 19/21 |
11–18 |
Mod-severe TBI |
Rx: n = 16/20 Ctrl: n = 19/21 |
Parents and siblings |
Both TBI and caregiver |
Family problem-solving |
|
Wade et al, 2011 (33) |
120 randomized |
12–17 |
Mod-severe TBI |
Family of 120 TBI participants |
Family members |
Both TBI and caregiver |
Family problem-solving |
|
Level 3: N-RCTs |
|||||||
|
Brown et al., 1999 (34) |
Rx: n = 48/70 (34/48 were TBI) Ctrl: n = 35/49 (12/35 were TBI) |
Adult |
Primarily mod-severe TBI but also some mild TBI and some stroke |
Rx: n = 52/74 (residing > 40 km from hospital) Ctrl: n = 39/57 (residing < 40 km from hospital) |
Family members or significant others |
Caregiver only |
Discussion topics chosen by participants |
|
Hauber et al., 2002 (35) |
Rx: n = 5/7 Ctrl: n = 4/5 |
16–52 |
Reduced state of consciousness |
Rx: n = 5/7 Ctrl: n = 4/5 |
Parents or wives |
Caregiver only |
Support to caregiver |
|
Wade et al., 2004, 2005 (36, 37, 38) |
Rx: n = 3/3 Ctrl: n = 3/3 |
5–16 |
Mod-severe TBI; 15+ months post-injury |
8/8 parents, 5/5 siblings |
Parents and siblings |
Both TBI and caregiver |
Family problem-solving |
|
Woods et al., 2011 (39) |
Rx: n = 25 Ctrl: n = 23 |
Child |
Mild-severe ABI (incl. traumatic/non-traumatic) |
48 mothers, 13 fathers |
Parents |
Caregiver only |
Behaviour |
|
Level 4: Case Series |
|||||||
|
Gilkey et al., 2009 (40) |
n = 23/31 |
5–17 |
Mod-severe TBI |
n = 23/31 |
Parents and school-aged siblings |
Both TBI and caregiver |
Family problem-solving |
|
Rotondi et al., 2005 (41) |
n = 17/23 |
Adult |
Males with mod-severe TBI |
n = 17/23 |
Female significant others |
Caregiver only |
Topics directed by participants |
|
Sander et al., 2009 (42) |
n = 15/15 |
16+ |
Mild-severe TBI; from rural community |
n = 15/15 |
Parents, spouses, other caregivers |
Caregiver only |
Management of cognitive and behavioural problems |
|
Wade et al., 2009 (43) |
n = 9/9 (all completed at least one session, but 5/9 completed the program) |
3–8 |
Mod-severe TBI; < 2 years post-injury |
n = 13/13 |
Parents |
Caregiver only (child with TBI involved in observed play sessions) |
Parenting skills |
|
Wade et al., 2011 (44) |
n = 9/10 |
3–9 |
Mod-severe TBI |
n = 12/13 |
Parents |
Caregiver only (child with TBI involved in observed play sessions) |
Parenting skills |
|
an represents the number of participants completing follow-up over the number of participants initially allocated to groups. RCT: randomized controlled trial; N-RCT: non-randomized controlled trial; TBI: traumatic brain injury; Rx: intervention group; Ctrl: control group. |
|||||||
Nine papers reporting results of RCTs and 4 papers reporting results of non-RCTs could be appraised using the PEDro-P scale. The results for each paper are shown in Table III. Sources for which only an abstract was available and case series studies were not rated using the PEDro-P scale. The mean PEDro-P rating for the RCTs was 5.6/10 (range 3/10 to 7/10) and for the non-RCTs was 2/10 (range 1/10 to 3/10).
|
Table III. Methodological quality of studies |
|||||||||||||
|
|
Level |
PEDro-P Ratinga |
|||||||||||
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
TOTAL |
||
|
Bell et al., 2005 (22) |
2 |
Y |
Y |
Y |
N |
N |
N |
Y |
Y |
Y |
Y |
Y |
7/10 |
|
Bell et al., 2011 (23) |
2 |
Y |
Y |
Y |
Y |
N |
N |
Y |
N |
Y |
Y |
N |
6/10 |
|
Salazar et al., 2000 (24) |
2 |
Y |
Y |
Y |
Y |
N |
N |
N |
Y |
Y |
Y |
Y |
7/10 |
|
Wade et al., 2006 (26) |
2 |
Y |
Y |
N |
Y |
N |
N |
N |
Y |
Y |
Y |
Y |
6/10 |
|
Wade et al., 2006 (27) |
2 |
Y |
Y |
Y |
Y |
N |
N |
N |
Y |
Y |
Y |
Y |
7/10 |
|
Carey et al., 2008 (28) |
2 |
N |
Y |
Y |
Y |
N |
N |
N |
Y |
N |
N |
N |
4/10 |
|
Wade et al., 2008 (29) |
2 |
Y |
Y |
Y |
N |
N |
N |
N |
Y |
N |
N |
N |
3/10 |
|
Wade et al., 2010 (31) |
2 |
Y |
Y |
Y |
N |
N |
N |
N |
Y |
N |
Y |
Y |
5/10 |
|
Wade et al., 2011 (32) |
2 |
Y |
Y |
Y |
N |
N |
N |
N |
Y |
N |
Y |
Y |
5/10 |
|
Brown et al., 1999 (34) |
3 |
Y |
N |
N |
N |
N |
N |
N |
N |
N |
Y |
N |
1/10 |
|
Hauber et al., 2002 (35) |
3 |
Y |
N |
N |
N |
N |
N |
N |
Y |
N |
N |
N |
1/10 |
|
Wade et al., 2004 (36) |
3 |
Y |
N |
N |
N |
N |
N |
N |
Y |
N |
Y |
Y |
3/10 |
|
Wade et al., 2005 (37) |
3 |
Y |
N |
N |
N |
N |
N |
N |
Y |
N |
Y |
Y |
3/10 |
|
aPEDro-P rating criteria are as follows: (1) Eligibility criteria and source specified (this criterion is not included in the total score); (2) Random allocation; (3) Concealed allocation; (4) Intervention groups similar; (5) Subjects blinded; (6) Therapists blinded; (7) Assessors blinded; (8) Retention of 85% participants; (9) Intention to treat analysis; (10) Between-group statistical comparisons; (11) Point and variability measures. Y: Yes; N: No. |
|||||||||||||
Participants
Fourteen studies involved only participants with TBI and their family members and excluded any other conditions, and two studies involved a mixed sample with at least 50% being participants with TBI and/or their family members (34, 39). Nine studies (including 4 studies reported across multiple papers) focussed on children (under 18) with TBI and their family members (26–33, 36–40, 43, 44). All of these studies related to interventions directed towards parents. Siblings were also included in the intervention in 5 studies (including 4 studies reported across multiple papers) (26–31, 36–38, 40, 44). Seven studies (including two studies reported across multiple papers) focussed on adults with TBI and their family members (21–25, 34, 35, 41, 42). Six of these studies targeted a heterogeneous caregiver group composed of parents, spouses or other close family members or friends, while one study specifically targeted female significant others (41).
Nine studies (including 6 studies reported across multiple papers) involved both the person with TBI and the caregiver as participants in the intervention (21–33, 36–38, 40, 43, 44) and the remaining 7 studies involved the caregivers only in the intervention, with no specific training provided to the person with TBI. This was the case for caregivers of people in reduced states of consciousness (35), for interventions which targeted parenting skills (39, 43, 44), for interventions involving a peer support group (34, 41) and for one other study which targeted family caregivers in rural areas (42).
Interventions
Table SI (available from http://www.medicaljournals.se/jrm/content/?doi=10.2340/16501977-1058) describes the interventions and outcomes investigated by each study. The studies involved a variety of technology formats and intervention targets. Ten studies used videoconferencing; including 8 studies (with 4 studies reported across multiple papers) which used videoconferencing combined with self-guided web sessions (26–33, 36–38, 40, 43, 44) and 2 studies which used videoconferencing alone (35, 42). Five studies (including 2 studies reported across multiple papers) used interventions delivered via a telephone call or group teleconferencing (21–25, 34, 39) and 1 study involved web-based resources only (41).
Six studies (including 2 studies reported across multiple papers) involved programs that were broad in their focus and provided general information and responded to concerns or topics that were raised by participants (21–25, 34, 35, 41). Ten studies (including 4 studies reported across multiple papers) involved a set structured program, with specific goals such as family problem-solving skills (26–33, 36–38, 40), parenting skills (39, 43, 44) or strategies for cognitive and behavioural problems (42). These programs were also adapted to the individual needs of participants.
Most of the interventions were completed over a time period of 3 to 6 months involving weekly to fortnightly sessions, or in one case, access to a web-based resource for a 6 month period (41). Two studies involved programs of longer duration; up to 9 months (same study reported across 2 papers) (21, 22) or up to 21 months (23), however with a lower frequency of contact compared to other programs.
Comparison Conditions
Twelve studies involved a comparison condition, including 7 RCTs (reported across 13 papers) (21–33) and 4 non-RCTs (reported across 6 papers) (34–39). In 6 studies (including 2 studies reported across multiple papers), those in the comparison condition received no intervention or a lower intensity intervention (21–23, 26–28, 31–33, 35), sometimes described as “usual care”. Some of these studies provided a lower intensity telehealth intervention: a computer with access to general web-based resources without the content that the intervention group received. In 4 studies, those in the comparison condition received an equivalent program to the intervention group but with some variation in the mode of delivery. Two of these 4 studies involved comparison to an equivalent face-to-face program (34, 39). The other 2 studies (reported across 5 papers) involved comparison to an equivalent program using slightly different technology, such as a different webcam (29, 30, 36–38). One study (reported across 2 papers) involved comparison to a higher intensity inpatient program (24, 25).
Outcomes
Eight studies (including 4 studies reported across multiple papers) measured outcomes only at completion of the intervention, without any further follow-up (26–32, 36–38, 40, 41, 43, 44). Four studies included long-term follow-up after intervention had been completed: at 6 months (34, 39), 1 year (same study reported across 2 papers) (24, 25), or 18 months (42). Three studies (including 1 study reported across 2 papers) had set follow-up points based on time post-injury or time post-discharge (21–23, 35). One study did not report on the time of follow-up (33).
Most studies included some reporting on measurement of aspects of the intervention process, such as the number of contacts or participants’ ratings of satisfaction with the intervention. Ten of the studies measured family members’ satisfaction with aspects of the telehealth intervention (27, 30, 34, 36, 39–44), including factors such as whether they would recommend the intervention to others, whether they preferred the telehealth intervention to a face-to-face equivalent, whether the intervention met their expectations, ease and comfort in using technology and the helpfulness or value of the intervention. All of these studies concluded that the overall response of family members to the telehealth interventions was positive. However, 29–38% of family members across 5 studies (27, 30, 36, 40, 44) stated they believed a face-to-face intervention would have been preferable or more helpful. No studies reported any formal cost analysis, although one study (24) that provided cost estimates noted the telehealth intervention was much less expensive than the intensive inpatient comparison condition which had similar outcomes.
Most studies included measurement of outcomes relating to the person with TBI. Four studies (22–24, 35) included measures of overall functioning. One RCT (22) found significant between-group differences when a telehealth program was compared to usual care, however, this was not replicated in a later RCT (23). Another RCT (24) found no significant difference in overall adjustment between a group that received a low intensity telehealth intervention and a group which received an intensive inpatient program, which suggested that a telehealth program may have equivalent benefits to intensive face-to-face rehabilitation. Measures of behavioural status were included in 7 studies (including 1 study reported across 2 papers) (26, 29, 31–33, 37, 39, 43). Four studies relating to the family problem-solving intervention program had significant findings for behavioural outcomes. This included two RCTs (including 1 study reported across 2 papers) (26, 31, 32) which showed significant between-group differences for this program when compared to a lower intensity intervention, and 2 further studies (29, 37) which showed significant pre-post differences on behaviour measures. Measures of psychological wellbeing were included in 5 studies (22–24, 29, 37). There were significant between-group differences when compared to usual care in 1 RCT (22) which were not replicated in a later RCT (23), significant pre-post differences in 1 study (29) and no significant between-group differences between a low intensity telehealth intervention group and an inpatient group in an RCT (24). Return to work was investigated in 2 RCTs (23, 24), with no significant differences found in comparison to either usual care or intensive inpatient rehabilitation. Quality of life measures were included in 2 studies (22, 23), with significant between-group differences when compared to usual care in one RCT (22) which again was not replicated in the later RCT (23). Measures of cognitive functioning were included in one RCT (24) with no significant difference at follow-up between the low intensity telehealth intervention and the inpatient group.
Eight studies (including 1 study reported across 2 papers) (27–29, 33, 34, 38, 39, 42, 43) included measurement of outcomes relating to the family members. Six studies (27, 29, 33, 34, 38, 39) involved measurement of aspects of the family member’s psychological well-being such as depression, anxiety, stress and mood. Three studies relating to the family problem-solving intervention had positive findings: 1 RCT (27) found significant between-group differences for parental psychological well-being when compared to a low intensity program, and 2 further studies (29, 38) found significant pre-post differences. One study (34) of a telephone group support program also found significant pre-post differences for caregiver psychological well-being. Measurement of parenting skills was included in 3 studies (27, 39, 43), either measured by blinded assessors or by report from the parents. One study (43) found significant pre-post differences in positive and problematic parenting behaviours as rated by blinded assessors. Measurement of application of knowledge was included in 1 study (42), which found at the 18 month follow-up point all caregivers reported having used information from the intervention program. Measurement of caregiver burden or needs was included in 1 study (34) but the results were not significant.
Six studies (including 1 study reported across 2 papers) (29, 32, 34, 35, 37–39) involved measurement of overall family functioning or interaction style. Family interaction, communication or relationships was measured in 4 studies (29, 32, 34, 37) with significant pre-post differences for the intervention group in 2 studies (29, 37), both of which involved the family problem-solving intervention. The family burden associated with caring for a person with TBI was measured in 2 studies (38, 39), with significant pre-post differences for the intervention group in 1 study (38). The proportion of family needs which were met or unmet was measured in 1 study (35), with only descriptive data reported due to the small sample size.
Four studies (including 2 studies reported across multiple papers) (22, 23, 26–28, 31, 32) conducted subgroup or regression analyses to investigate whether family member characteristics moderated treatment efficacy. Two studies reported across 4 papers (26, 27, 31, 32) investigated family income or socio-economic status (SES) as a moderating factor, with 2 papers (26, 32) reporting that improvements in parent-reported child behaviour outcomes were significant for lower SES families but not for higher SES families, although for teen self-reported behaviour outcomes the opposite pattern was found (32). Two studies investigated ethnicity as a moderating factor (22, 23), with one study finding that the telehealth intervention was most effective for non-Hispanic white families compared to those of other ethnicities. One paper (28) compared outcomes of a telehealth intervention for parents with prior technology experience to parents without experience, and found that outcomes for parental depression and anxiety were significantly better for participants with prior experience.
DISCUSSION
The variety of intervention programs in the studies reviewed in this paper demonstrates the potential for using telehealth to meet the needs of family members of people with TBI. The considerable diversity across the studies demonstrates the feasibility of using telehealth to expand the scope and reach of many types of interventions. The studies reviewed here illustrate telehealth could be used to increase access to services for families in rural areas, to train family members in the skills required to facilitate recovery after TBI, to provide appropriate and timely intervention for problems arising at home, or to create a forum for peer support without needing to share the same location.
This review provides preliminary evidence of the efficacy of supporting family members of people with TBI via telehealth, which is consistent with findings of previous general systematic reviews within telehealth (15, 16). The studies reviewed here reported a range of significant outcomes relating to the functioning of the person with TBI as well as to the psychological wellbeing, support skills and burden of family members. Several studies demonstrated that participants reported training to be beneficial over the long-term after program completion, or that improvements in outcomes were maintained over time. The replication of positive findings across multiple studies of the family problem solving intervention (26–33, 36–38, 40) create a strong base of evidence for this particular program, although all studies to date originate from the one research group. Overall, all but one of the studies (23) in this review reported some level of positive outcomes for the telehealth program being investigated, which suggests this is a promising approach.
A recent review of systematic reviews in telehealth (45) noted that there is generally a lack of high quality evidence for the effectiveness of such programs, and a need for more thorough economic analyses. This review found similar issues. Most of the reviewed studies did not use blinded assessors for measuring outcomes, relying on self-report measures only, which means that positive results may be inflated by social desirability biases. Use of observational measures of family members’ skills that could be completed via videoconferencing (such as in Wade et al. (43)) would assist to further strengthen the evidence for the effectiveness of telehealth programs. It will also be necessary to replicate positive findings for interventions across multiple studies to confirm preliminary positive results, as is illustrated by the differing findings of the two studies by Bell et al (22, 23). Furthermore, none of the studies reviewed here provided a detailed analysis of costs, which is an important factor in clinical decision-making.
It is also relevant to compare telehealth programs with equivalent face-to-face programs. In some situations, a telehealth intervention may be the only option available to families. However, in other circumstances, it is important to know the potential impact of replacing a traditional face-to-face service with a telehealth service. Only a few of the studies reviewed here involved a comparison group receiving an equivalent face-to-face intervention, and only one of these studies, a non-randomised controlled trial (34) reported between-groups statistics. This particular study found no significant differences in caregiver outcomes between the telehealth and the face-to-face group, although the rural caregivers in the telephone group reported higher satisfaction than the metropolitan caregivers in the face-to-face group. Another key issue is the impact on rapport between the client and the professional (11). One study in this review found that the therapeutic alliance was rated highly even with use of a telehealth approach (36). These potential differences between intervention modes need to be explored across more studies, including comparison of measures of cost and effectiveness, and exploration of how using a telehealth mode affects communication processes (46). As concluded in previous systematic reviews (12), the current literature does not provide sufficient evidence for guiding decision-making regarding modes of service delivery.
This review also sought to identify the features that contribute to the effectiveness of telehealth programs for family members of people with TBI, through identifying controlled trials comparing different intervention formats or from qualitative feedback from study participants. The results of several studies (26, 27, 31, 32) suggested an interactive skills-based program is more effective than providing caregivers with general information. There were no other studies involving comparison of program features or formats. In one study, qualitative feedback indicated that family members found coaching sessions with a therapist via videoconferencing to generally be more valuable than self-guided web sessions (44). However, few studies in this review provided information about program elements that contributed to effectiveness, and further research is required. The unexpected non-significant findings of two studies (23, 24) illustrate the importance of exploring the key features or ingredients of interventions to determine what makes them work. This is particularly complex with psychosocial interventions in which programs involve multiple components and are often individualised for the needs of participants. It may therefore be appropriate to include process evaluations in telehealth studies to assist with understanding why interventions work or do not work (47).
Another important factor for clinical decision-making is whether telehealth interventions are appropriate for all family caregivers. Overall, caregivers reported high satisfaction with telehealth interventions, which is consistent with previous research (15). However, in studies in which family members were asked about their preferences, approximately a third of the sample reported they believed a face-to-face intervention would have been preferable or more helpful. This suggests that not all family caregivers will be receptive to a telehealth approach. Several studies offered preliminary evidence that factors such as ethnicity, SES or prior experience with technology may moderate treatment efficacy, which was a similar finding to a systematic review of telehealth supports for carers of people with dementia (16). Another review of telehealth interventions suggested that gender, age and attitudes towards computers can influence outcomes (11). These factors could be explored further in future studies.
In conclusion, there are a growing number of studies involving the use of telehealth to provide support and training to carers of people with TBI. This systematic review has found that research to date has focussed on feasibility, user satisfaction and preliminary explorations of effectiveness, with mostly positive findings in each of these areas. However, the diversity in the characteristics of intervention programs, populations, caregiver groups and outcome measures used in these studies prevented any pooling of data for meta-analysis. As with other systematic reviews, the results here may be influenced by the publication bias towards studies with positive findings, which may skew the impression of the effectiveness of interventions. There is also the issue of time lag between the completion of trials and the publication of results. In a field such as telehealth, clinical practice is rapidly evolving with improvements in access to and quality of technology, and current best practice may be moving ahead of the research reported in publications. This review, although it reflects current research, may not reflect the most recent developments in practice or technology. The methodological issues already identified, such as the use of self-report measures of outcomes, also limit the conclusions that can be drawn from this review.
Acknowledgements
We would like to thank Elise Bogart for her assistance with reliability coding for this review.
This research was supported by an Australian Postgraduate Award.
References
