OBJECTIVE: To compare the accuracy of combinations of clinical examination findings for predicting a positive response to injection of local anaesthetic into the subacromial bursa.
DESIGN: Prospective, cohort, diagnostic validity design.
SUBJECTS: Consecutive patients with shoulder pain recruited from primary care physiotherapy and general medical practices.
METHODS: All subjects underwent a standardised clinical examination (index test) followed by a diagnostic injection of xylocaineTM into the subacromial bursa (reference standard test) performed under ultrasound guidance. Clinical examination variables associated with a positive anaesthetic response (≥ 80% post-injection reduction in pain intensity) were identified (p < 0.20) and diagnostic accuracy was calculated.
RESULTS: Of the 196 subjects who received a subacromial bursa injection, 66 subjects (34%) reported a positive anaesthetic response. Strain injury (adjusted odds ratio (AOR) 2.3), anterior shoulder pain (AOR 2.3) and absence of pain with external rotation at 90º abduction (AOR 3.9) were the strongest clinical predictors of positive anaesthetic response. Clinical prediction model variables demonstrated 100% specificity (3 positive tests) but low sensitivity (maximum 40%) for a positive anaesthetic response. Combinations of 9 other clinical variables also demonstrated 100% specificity (7 or more positive tests), and improved sensitivity (95 to 100%) for a PAR compared with clinical prediction model variables when less than two findings were present.
CONCLUSION: Combinations of these clinical tests may assist the clinician to differentiate subacromial pain from other shoulder conditions and guide selection of targeted pain management interventions. Additional diagnostic tests may be required when clinical criteria are not satisfied.
Key words: shoulder pain; physical examination; sensitivity and specificity; anesthetics, local; primary health care.
J Rehabil Med 2012; 44: 877–884
Guarantor’s address: Angela Cadogan, 520 Bower Avenue, Parklands, Christchurch 8083, New Zealand. E-mail: acadogan@vodafone.co.nz
Submitted September 21, 2011; accepted August 9, 2012
Introduction
Shoulder pain is a common and disabling complaint with a reported prevalence in the general population of at least 16% (1), and up to 34% in those over the age of 65 years (2). Subacromial disorders including subacromial bursa (SAB) pathology, rotator cuff disease and rotator cuff tears are the most commonly reported shoulder disorders, accounting for up to 70% of shoulder pain seen in primary care practice (3). It is generally accepted that the SAB is the main source of pain in rotator cuff disease due to its anatomic location, mechanical vulnerability and rich nociceptive innervation (4).
The SAB may be affected by a number of conditions including primary synovitis (bursitis) (5), crystal deposition, calcific loose bodies (6), rotator cuff disease or may occur secondary to repeated mechanical ‘impingement’ against the acromial arch (7). Specific pain management interventions are advocated in the management of painful bursal conditions including corticosteroid injections and surgical bursectomy (8), and barbotage procedures for calcific lesions (9). The success of any treatment intervention however, is dependent upon identification of the SAB as the pain source in the first instance. The early detection of painful bursal pathology would therefore assist the clinician in more efficient differentiation of subacromial pain from other shoulder conditions and facilitate timely application of appropriate treatment to reduce the considerable functional disability and adverse health and psychosocial consequences associated with ongoing shoulder pain (10, 11).
Subacromial disorders may be difficult to differentiate from other sources of shoulder pain due to the complex regional anatomy, and the similar clinical presentations of different shoulder disorders (12). The majority of previous studies have assessed the diagnostic ability of isolated physical examination tests, reporting poor specificity of these tests for identifying subacromial pathology (12–15), and their limited ability to differentiate between early stage “impingement” (bursal pathology) and more advanced rotator cuff disease (13). However, in clinical practice, diagnosis is rarely based upon the result of a single test. The two most popular methods for evaluating diagnostic accuracy of combinations of clinical tests are clinical prediction models (16) and establishing the best combination of tests based upon minimum numbers of positive clinical findings (17). To our knowledge these two methods of interpreting diagnostic accuracy for combinations of clinical data have not previously been compared in the same shoulder pain cohort to determine which method provides the largest improvement in post-test probability of a positive ‘case’.
The majority of previous diagnostic studies used surgery as the reference standard procedure, but while this provides visualisation of pathology, it does not allow one to determine whether the observed pathology is the primary source of symptoms. Thus, diagnostic injections of local anaesthetic into the subacromial region are considered the reference standard test for identification of subacromial pain (7), with marked reduction in post-injection pain intensity following injection of local anaesthetic into the SAB being indicative of a positive anaesthetic response (PAR) and a likely subacromial pain source. In addition to providing valuable diagnostic information, a PAR may also provide an indication of the therapeutic value of targeted pain management interventions such as corticosteroid injections.
The aim of this study was to compare the diagnostic accuracy of combinations of clinical examination findings for predicting a PAR to a guided subacromial diagnostic block into the SAB.
METHODS
This study formed part of a wider prospective, blinded diagnostic accuracy study in which clinical examination and imaging variables (index tests) were compared with results of guided diagnostic injection of local anaesthetic (reference standard) into the SAB, acromioclavicular joint (ACJ) and glenohumeral joint (GHJ) (18). Subjects were recruited from community-based medical and physiotherapy practices across Christchurch, New Zealand. The New Zealand Ministry of Health Regional Ethics Committee (Upper South A) granted ethical approval for the study. Informed consent was gained from all subjects prior to participation in the study and the rights of all subjects were protected.
Consecutive patients over the age of 18 years, presenting to their general practitioner or physiotherapist for the first time with a new episode of shoulder pain and with the ability to follow verbal instructions were eligible for inclusion in the study (Fig. 1). Exclusion criteria were known fractures or dislocations around the shoulder complex, referred pain from the cervical spine, sensory or motor deficit involving the upper limb, previous surgery to the shoulder or cervical spine, or contraindications to injection procedures.
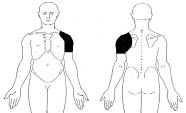
Fig. 1. Location of primary pain required for inclusion in the study.
A total of 373 patients were referred to the study between July 2009 and June 2010 resulting in 208 subjects being included. The mean time between clinical examination and the SAB diagnostic block was 4 days (standard deviation (SD), 3 days; range 1–19). All included subjects initially completed self-report questionnaires consisting of the SF-8TM health survey (19), Shoulder Pain and Disability Index (SPADI) (20) and Fear Avoidance Beliefs Questionnaire (FABQ) (21). This was followed by a standardised clinical examination (history and physical examination) conducted by an experienced clinician.
Clinical examination
Patient history consisted of standardised questionnaires including details of pain intensity, location and behaviour, mechanism of onset, past history and medical history. The physical examination consisted of the following tests: active range of motion (ROM) of the cervical spine (22), inspection for swelling or muscle atrophy, recording of symptom responses associated with passive ROM (23) and resisted muscle tests, orthopaedic tests selected according to evidence for reported diagnostic accuracy (14) and performed as originally described; Hawkins-Kennedy test (24), drop-arm test (25), empty can test (26), external rotation lag sign (27), Speed’s test (28), apprehension-relocation test (29) and pain responses to palpation of the shoulder region (30). During the physical examination, those tests provocative of typical pain were identified for use in pre- and post-injection testing. Indeterminate results of clinical examination tests were recorded and coded as missing data.
Subacromial bursa diagnostic block
For the subacromial diagnostic block (reference standard) procedure, subjects were positioned supine with the arm in external rotation. Under aseptic conditions, a 22-gauge needle was used to inject 5mL of 1% lidocaine hydrochloride (xylocaineTM) into the SAB under ultrasound guidance using an anterior approach. Needle placement within the SAB was confirmed by ultrasound. As the contents of the syringe were emptied into the bursa, infiltration of the SAB was verified by visualisation of bursal distension. The SAB injection procedure used in this study has been described in detail elsewhere (18).
Immediately prior to the injection, all subjects were examined using up to 6 tests identified during the clinical examination as being provocative of typical symptoms. Pre-injection pain intensity was recorded for each clinical test on a 100 mm visual analogue scale (VAS) where 0 mm indicated “no pain” and 100 mm represented “worst imaginable pain”. Tests were repeated between 5 and 15 min following the diagnostic block and post-injection pain intensity VAS scores recorded again. The mean change in pain intensity from all clinical tests was then calculated. A positive anaesthetic response was determined by 80% or more post-injection reduction in pain intensity (80% PAR). This is similar to the criteria for PAR used in other studies involving diagnostic blocks (31–33) and represents a high level of confidence that the target structure is a major contributor to symptoms.
The investigator performing the clinical examination and pre- and post-injection clinical tests was blinded to any diagnostic or treatment information from referring practitioners. The radiologist who performed the SAB diagnostic block was blinded to any clinical information and to the results of pre-injection provocative clinical testing.
The sample size was estimated using methods for estimates for diagnostic accuracy studies described by Flahault et al. (34) with the minimal acceptable lower confidence limit set at 0.75 and expected sensitivity/specificity both set at 0.90, with adjustment following sub-group analysis of the first 100 cases to maintain precision of confidence interval estimates.
Statistical analysis
The Fisher exact test (dichotomous variables) and univariate logistic regression analyses (continuous variables) were performed for all demographic, self-report questionnaires and clinical examination variables for a PAR to SAB diagnostic block using the Statistical Package for the Social Sciences (SPSS) version 17.0. Variables demonstrating univariate association with PAR to SAB diagnostic block at the p ≤ 0.200 level were included in multiple logistic regression analyses and stepwise backward variable elimination was performed using Akaike’s Information Criterion (AIC) (35) to derive the best prediction model. A multiple regression analysis was carried out using “R” statistical software (36). The goodness of fit for the model was assessed using the Hosmer-Lemeshow test.
Diagnostic accuracy statistics including sensitivity, specificity, predictive values, positive likelihood ratios (PLR) and negative likelihood ratios (NLR) and 95% confidence intervals (CI) were then calculated to assess the discriminatory ability of the prediction model, and for combinations of clinical variables associated with PAR to SAB diagnostic block (p ≤ 0.20) according to the minimum number of variables present. Confidence Interval Analysis software (37) was used for calculation of diagnostic accuracy statistics.
Results
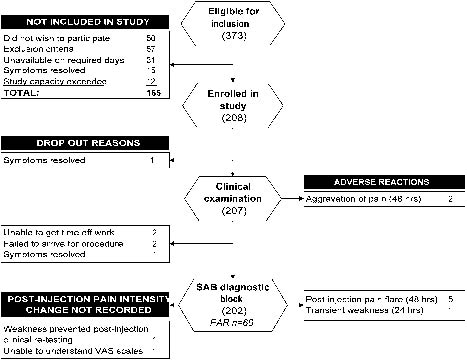
Demographic data for those included in the study are presented in Table I. Symptom duration was significantly less (Mann-Whitney, p < 0.001) in those excluded from the study (median 2 weeks; IQ range, 4 weeks). Details of progression of subjects through the study, drop-out explanations and adverse events are presented in Fig. 2.
| Table I. Demographic information of the subjects in the positive (n=69) and negative (n=133) anaesthetic response groups |
| Demographic information | All subjects | | PAR Group Mean (SD) | | NAR Group Mean (SD) |
| Mean (SD) | Range | | |
| Age, years | 42 (14) | 18–81 | | 42 (12) | | 42 (15) |
| Height, cm | 172 (10) | 147–199 | | 171 (9) | | 172 (10) |
| Weight, kg | 80.6 (18.0) | 50.3–189.0 | | 80.2 (21) | | 81 (17) |
| Symptom duration, weeksa | 7 (13)a | 0–175 | | 7 (14)a | | 7 (12)a |
| VAS, worst | 62 (23) | 3–100 | | 62 (22) | | 64 (24) |
| VAS, mean | 37 (22) | 1–100 | | 36 (18) | | 37 (23) |
| VAS, best | 9 (18) | 0–98 | | 7 (13) | | 10 (20) |
| SF8 physical component score | 44 (8) | 23–61 | | 44 (8) | | 44 (8) |
| SF8 mental component score | 52 (9) | 27–66 | | 53 (8) | | 52 (9) |
| SPADI pain score, % | 50 (22) | 0–100 | | 50 (21) | | 51 (22) |
| SPADI disability score, % | 30 (23) | 0–96 | | 28 (22) | | 31 (22) |
| SPADI total, % | 38 (21) | 0–98 | | 36 (20) | | 39 (21) |
| FABQ physical activity score, % | 64 (22) | 0–100 | | 62 (23) | | 66 (22) |
| FABQ work score, %b | 27 (23) | 0–81 | | 26 (23) | | 27 (24) |
| FABQ total score, %b | 41 (19) | 0–87 | | 40 (18) | | 41 (19) |
| Male gender, % | 51 | | | 47 | | 55 |
| Right hand dominant, % | 87 | | | 88 | | 87 |
| Dominant arm affected, % | 53 | | | 52 | | 53 |
| ACC claim, % | 93 | | | 92 | | 92 |
| Physiotherapist referrals, % | 98 | | | 99 | | 97 |
| Employment status | | | | | | |
| In paid employment, % | 80 | | | 82 | | 80 |
| On modified duties, % | 9 | | | 9 | | 9 |
| Off work, % | 3 | | | 0 | | 5 |
| Co-existent medical conditions, % | 34 | | | 32 | | 35 |
| Smoker, % | 19 | | | 19 | | 19 |
| avariable not normally distributed; median (interquartile range) are presented. bonly cases ‘in paid employment’ used in analysis. PAR: positive anaesthetic response (≥ 80% post-injection reduction in pain intensity); NAR: negative anaesthetic response (< 80% reduction in post-injection pain intensity); VAS: 100 mm visual analogue pain score in previous 48 h; SPADI: Shoulder Pain & Disability Index; FABQ: Fear Avoidance Beliefs Questionnaire; ACC: Accident Compensation Corporation. |
Fig. 2. Diagram showing progression of subjects through the study, drop-out explanations and adverse events. SAB: subacromial bursa; PAR: positive anaesthetic response; VAS: visual analogue scale.
Two hundred and seven subjects completed the clinical examination. Variables where missing data exceeded 5% included ‘family history of shoulder pain’ (15% ‘unsure’), atrophy in the supraspinous or infraspinous fossa (9% indeterminate), painful arc abduction (1% ‘unsure’ if typical symptoms were reproduced; 11% had insufficient active ROM abduction) and passive ROM cross-body adduction in external rotation (7% unable to achieve full external rotation). Frequency distributions of clinical examination findings for the PAR and NAR groups are presented in Table II.
| Table II. Distribution of main clinical examination findings of the subjects in the positive (n = 66) and negative (n = 130) anaesthetic response groups |
| Clinical examination variables | Total number of positive tests (n) |
| All subjects | PAR group | NAR group |
| History | | | |
| Past history of shoulder pain | 64 | 22 | 42 |
| Family history of shoulder pain | 37 | 13 | 24 |
| Mechanism of onset | | | |
| Traumatic | 74 | 17* | 57 |
| Strain | 81 | 36** | 45 |
| Repetitive | 22 | 9 | 13 |
| Unknown | 18 | 3 | 15 |
| Pain location | | | |
| Anterior | 63 | 28* | 35 |
| Superior | 31 | 10 | 21 |
| Lateral shoulder/arm | 57 | 17 | 40 |
| Posterior | 10 | 4 | 6 |
| Pain aggravated by overhead activity | 187 | 63 | 124 |
| Referred pain extending below the elbow | 28 | 9 | 19 |
| Nature of pain constant/intermittent | 61 | 21 | 40 |
| Night pain disturbs sleep | 100 | 37 | 63 |
| Unable to sleep on the affected side | 105 | 39 | 66 |
| Physical examination | | | |
| Cervical spine pain on testing | 100 | 36 | 64 |
| AROM elevationa – symptoms reproduced | 163 | 52 | 111 |
| AROM HBB – symptoms produced | 136 | 40 | 96 |
| Painful arc abduction | 101 | 35 | 66 |
| Resisted tests – symptoms reproduced | 172 | 60 | 112 |
| Any resisted testb | 172 | 60 | 112 |
| Resisted abduction or external rotation | 154 | 50 | 104 |
| Resisted internal rotation | 93 | 33 | 60 |
| PROM – symptoms reproduced with testing | | | |
| Glenohumeral abduction | 153 | 45* | 108 |
| External rotation (0º) abduction | 136 | 45 | 91 |
| External rotation (90º) abduction | 147 | 39*** | 108 |
| Internal rotation (90º) abduction | 107 | 31 | 76 |
| Horizontal adduction (IR) | 130 | 38 * | 92 |
| Horizontal adduction (ER) | 115 | 32 ** | 83 |
| Orthopedic tests | | | |
| Hawkins-Kennedy test | 125 | 38 | 87 |
| Drop-arm test | 20 | 8 | 12 |
| Empty can test (pain or weakness) | 163 | 57 | 106 |
| External rotation lag sign | 7 | 3 | 4 |
| Speeds test | 125 | 36 | 89 |
| Apprehension/relocation (pain) | 73 | 22 | 51 |
| Palpation – typical symptoms reproduced | | | |
| Greater tuberosity | 105 | 33 | 72 |
| Lesser tuberosity | 81 | 22 | 59 |
| Long head of biceps tendon | 103 | 34 | 69 |
| *p < 0.050. **p < 0.010. ***p < 0.001. aelevation through flexion. bsymptoms reproduced with any of: resisted abduction, external rotation or internal rotation. PAR: positive anaesthetic response (≥ 80% post-injection reduction in pain intensity); NAR: negative anaesthetic response (< 80% post-injection reduction in pain intensity); AROM: active range of motion; PROM: passive range of motion; IR: internal rotation; ER: external rotation; SAB: subacromial bursa; max: maximum; CAL: coracoacromial ligament. |
Two hundred and two subjects received the SAB diagnostic block with needle placement within the SAB and visualisation of bursal distension confirmed in all 202 cases. Post-injection change in pain intensity was obtained from 200 subjects. Due to the known limitations of VAS scales for measuring change in pain intensity when pre-injection pain levels are low (< 20 mm) (38), only cases where pre-injection pain intensity exceeded 20 mm were included in the analysis. Four cases were subsequently excluded from the analysis in which pre-injection pain intensity was less than 20 mm on the VAS scale, resulting in 196 cases being included in the analysis. An 80% PAR was reported by 66 of the 196 (34%) cases following the SAB injection. Eleven subjects (6%) reported a post-injection increase in pain intensity.
No demographic or self-report variables were associated with a PAR to SAB diagnostic block (p ≤ 0.20). Table III presents univariate odds ratios (OR), contingency cell counts and diagnostic statistics for potential clinical examination predictors associated with PAR to SAB diagnostic block (p ≤ 0.20). The most efficient clinical examination predictors of a PAR to SAB diagnostic block were anterior shoulder pain (adjusted odds ratio (AOR) 2.3), strain mechanism of injury (AOR 2.3) and the absence of symptom provocation during passive ROM external rotation (at 90º abduction) (AOR 3.9) (Table III). Hosmer-Lemeshow statistics indicated the goodness of fit of the model was adequate (χ26 = 3.24, p = 0.778). The diagnostic accuracy of combinations of prediction model variables is presented in Table IV. The highest sensitivity (0.40; 95% CI, 0.29, 0.52) was observed when 1 of the 3 variables were present, and highest specificity (1.00; 95% CI, 0.97, 1.00) was observed when all 3 clinical variables were present.
| Table III. Diagnostic accuracy of individual clinical examination variables |
| Dichotomous variables | Contingency cell counts | Diagnostic accuracy | Association |
| TP | FN | FP | TN | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | PLR (95% CI) | NLR (95% CI) | OR (95% CI) | AOR (95% CI) |
| Strain injury | 36 | 30 | 45 | 85 | 0.55 (0.43–0.66) | 0.65 (0.57–0.73) | 0.44 (0.34–0.55) | 0.74 (0.65–0.81) | 1.58 (1.13–2.17) | 0.70 (0.51–0.91) | 2.3** (1.2–4.2) | 2.3* (1.2–4.4) |
| Anterior shoulder pain | 28 | 38 | 35 | 95 | 0.42 (0.31–0.54) | 0.73 (0.65–0.80) | 0.44 (0.33–0.57) | 0.71 (0.63–0.78) | 1.58 (1.05–2.33) | 0.79 (0.61–0.98) | 2.0* (1.0–3.7) | 2.3* (1.2–4.5) |
| Unable to sleep on affected side | 39 | 22 | 66 | 62 | 0.64 (0.51–0.75) | 0.48 (0.40–0.57) | 0.37 (0.29–0.47) | 0.74 (0.64–0.82) | 1.24 (0.95–1.58) | 0.75 (0.50–1.06) | 1.7 (0.9–3.1) | |
| HBB – asymptomatic | 25 | 40 | 28 | 96 | 0.39 (0.28–0.51) | 0.77 (0.69–0.84) | 0.47 (0.34–0.60) | 0.71 (0.62–0.78) | 1.70 (1.08–2.65) | 0.80 (0.63–0.97) | 2.1* (1.1–4.1) | |
| PROM GHJ abd asymptomatic | 20 | 45 | 19 | 108 | 0.31 (0.21–0.43) | 0.85 (0.78–0.90) | 0.51 (0.36–0.66) | 0.71 (0.63–0.77) | 2.06 (1.19–3.54) | 0.81 (0.67–0.96) | 2.5* (1.2–5.2) | |
| PROM ER90º asymptomatic | 26 | 39 | 20 | 108 | 0.40 (0.29–0.52) | 0.84 (0.77–0.90) | 0.57 (0.42–0.70) | 0.74 (0.66–0.80) | 2.56 (1.56–4.21) | 0.71 (0.56–0.86) | 3.6*** (1.8–7.2) | 3.9*** (1.9–8.0) |
| PROM IR90º asymptomatic | 32 | 31 | 47 | 76 | 0.51 (0.39–0.63) | 0.62 (0.53–0.70) | 0.41 (0.30–0.52) | 0.71 (0.62–0.79) | 1.33 (0.95–1.84) | 0.80 (0.59–1.04) | 1.7 (0.9–3.1) | |
| PROM CB adduction (IR) asymptomatic | 27 | 38 | 33 | 92 | 0.42 (0.30–0.54) | 0.74 (0.65–0.81) | 0.45 (0.33–0.58) | 0.71 (0.62–0.78) | 1.57 (1.04–2.36) | 0.79 (0.62–0.98) | 2.0* (1.1–3.7) | |
| Negative Hawkins-Kennedy test | 27 | 38 | 36 | 87 | 0.42 (0.30–0.54) | 0.71 (0.62, 0.78) | 0.43 (0.31, 0.55) | 0.70 (0.61–0.77) | 1.42 (0.95–2.10) | 0.83 (0.64–1.03) | 1.7 (0.9–3.2) | |
| Variables were selected based upon association with an 80% PAR (p ≤ 0.200). Total cell counts are less than 196 for some variables due to missing data. *p < 0.050; **p < 0.010; ***p < 0.001. TP: true positive; FN: false negative; FP: false positive; TN: true negative; CI: confidence interval; PPV: positive predictive value; NPV: negative predictive value; PLR: positive likelihood ratio; NLR: negative likelihood ratio; OR: univariate odds ratio; AOR: multivariate adjusted odds ratio; HBB: hand-behind-back; PROM: passive range of motion; GHJ abd: glenohumeral joint abduction; ER90º: external rotation (at 90º abduction); IR90º: internal rotation (at 90º abduction); CB: cross body; IR: internal rotation. |
Diagnostic accuracy of combinations of all clinical examination variables identified as being associated with a PAR to SAB diagnostic block (p < 0.20) are presented in Table V. Sensitivity was highest (1.00; 95% CI, 0.95, 1.00) and negative likelihood ratio lowest (0.00, 95% CI, 0.00, 1.79) when none of the clinical finding were present. Specificity (1.00; 95% CI, 0.97, 1.00) and positive likelihood ratio (infinity; 95% CI estimates 1.71, 509.00) were highest when at least seven clinical findings were present. The area under the receiver operating curves (0.686; 95% CI, 0.598, 0.774) indicated that the optimal diagnostic point was represented by four positive clinical findings (sensitivity 0.55, specificity 0.70).
| Table IV. Diagnostic accuracy of prediction model variables |
| Number of positive clinical findings | Contingency cell counts | Diagnostic accuracy | |
| TP | FN | FP | TN | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | PLR (95% CI) | NLR (95% CI) | OR (95% CI) |
| 1 of 3 | 26 | 39 | 62 | 66 | 0.40 (0.29–0.52) | 0.52 (0.43–0.60) | 0.30 (0.21–0.40) | 0.63 (0.53–0.72) | 0.83 (0.57–1.15) | 1.16 (0.89–1.50) | 0.7 (0.4–1.2) |
| 2 of 3 | 22 | 43 | 18 | 110 | 0.34 (0.24–0.46) | 0.86 (0.79–0.91) | 0.55 (0.40–0.69) | 0.72 (0.64–0.78) | 2.41 (1.40–4.13) | 0.77 (0.62–0.91) | 3.3 (1.6–6.7) |
| 3 of 3 | 6 | 59 | 0 | 128 | 0.09 (0.04–0.19) | 1.00 (0.97–1.00) | 1.00 (0.61–1.00) | 0.68 (0.62–0.75) | ~ (1.45–444.00)a | 0.91 (0.84–0.98)a | 3.2 (2.6–3.9) |
| a0.5 added to cells to estimate confidence intervals. Clinical examination tests: strain mechanism of injury, anterior shoulder pain and passive range of motion external rotation (at 90º abduction) does not reproduce typical symptoms. Total cell counts are less than 196 for some variables due to missing data. For abbreviations see Table III. |
| Table V. Diagnostic accuracy of combinations of clinical examination variables |
| | Contingency cell counts n | Diagnostic accuracy | |
| Number of positive clinical examination findings | TP | FN | FP | TN | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | PLR (95% CI) | NLR (95% CI) | OR (95% CI) |
| ≥ 1 | 66 | 0 | 123 | 4 | 1.00 (0.95–1.00) | 0.03 (0.01–0.08) | 0.35 (0.29–0.42) | 1.00 (0.51–1.00) | 1.03 (1.02–1.09) | 0.00 (0.00–1.79) | 0.7 (0.6–0.7) |
| ≥ 2 | 61 | 3 | 92 | 26 | 0.95 (0.87–0.98) | 0.22 (0.16–0.30) | 0.40 (0.33–0.48) | 0.90 (0.74–0.96) | 1.22 (1.09–1.38) | 0.21 (0.07–0.62) | 5.8 (1.7–19.8) |
| ≥ 3 | 50 | 15 | 62 | 61 | 0.77 (0.66–0.86) | 0.50 (0.41–0.58) | 0.45 (0.36–0.54) | 0.80 (0.70–0.88) | 1.53 (1.22–1.91) | 0.47 (0.28–0.73) | 3.3 (1.7–6.6) |
| ≥ 4 | 36 | 27 | 34 | 92 | 0.57 (0.45–0.69) | 0.73 (0.65–0.80) | 0.51 (0.40–0.63) | 0.77 (0.69–0.84) | 2.12 (1.48–3.03) | 0.59 (0.42–0.78) | 3.6 (1.9–6.8) |
| ≥ 5 | 23 | 40 | 14 | 112 | 0.37 (0.26–0.49) | 0.89 (0.82–0.93) | 0.62 (0.46–0.76) | 0.74 (0.66–0.80) | 3.29 (1.83–5.90) | 0.71 (0.57–0.85) | 4.6 (2.2–9.8) |
| ≥ 6 | 13 | 51 | 4 | 125 | 0.20 (0.12–0.32) | 0.97 (0.92–0.99) | 0.77 (0.53–0.90) | 0.71 (0.64–0.77) | 6.55 (2.34–18.48) | 0.82 (0.70–0.91) | 8.0 (2.5–25.6) |
| ≥ 7 | 7 | 58 | 0 | 129 | 0.11 (0.05–0.21) | 1.00 (0.97–1.00) | 1.00 (0.65–1.00) | 0.69 (0.62–0.75) | ~ (1.71–509)a | 0.89 (0.82–0.97)a | 3.2 (2.6–4.0) |
| ≥ 8 | 3 | 62 | 0 | 130 | 0.05 (0.02–0.13) | 1.00 (0.97–1.00) | 1.00 (0.44–1.00) | 0.68 (0.61–0.74) | ~ (0.73–265)a | 0.95 (0.90–1.01)a | 3.1 (2.5–3.8) |
| Nine | 1 | 65 | 0 | 130 | 0.02 (0.00–0.08) | 1.00 (0.97–1.00) | 1.00 (0.21–1.00) | 0.67 (0.60–0.73) | ~ (0.24–142)a | 0.98 (0.94–1.02)a | 3.0 (2.5–3.7) |
| a0.5 added to cells to estimate confidence intervals. Clinical examination results: strain mechanism of injury; anterior shoulder pain; unable to sleep on affected side; HBB, PROM GHJ abduction, PROM external rotation (at 90o abduction), PROM internal rotation (at 90º abduction), cross-body adduction (in internal rotation) do not provoke typical symptoms; negative Hawkins-Kennedy test. Variables were selected based upon association with an 80% PAR (p ≤ 0.200). Total cell counts are less than 196 for some variables due to missing data. For abbreviations see Table III. |
Discussion
The ability to accurately identify those patients likely to report a PAR to subacromial diagnostic block can inform diagnostic decision making regarding the source of pain, guide referral for further investigation of specific subacromial pathology or specialist consultation and ultimately guide the selection of targeted pain management interventions such as corticosteroid injection (39). Accurate identification of subacromial pain may also guide treatment selection within conservative management programmes targeted at commonly reported causes of subacromial pain including scapula dyskinesis (40) and humeral head stability (41). The consequences of delayed diagnosis of subacromial pain may include prolonged diagnostic processes with extended periods of pain and declining functional ability and a delay in implementation of appropriate management with resulting adverse effects on treatment outcome.
The clinical prediction model identified three variables that were able to rule-in an 80% PAR to SAB diagnostic block with 100% specificity (95% CI, 0.97, 1.00) when all 3 variables were positive (strain mechanism of injury, pain primarily located in the anterior shoulder region and when typical shoulder symptoms were not provoked during passive ROM external rotation performed at 90º abduction). This provides a basis for clinical decision making regarding application of specific treatment interventions for subacromial pain and, when present, these 3 findings provide justification for the use of more invasive or expensive investigation or treatment interventions, and may have more diagnostic value in specialist settings where the prevalence of painful bursal pathology is likely to be higher.
The 3 variables identified in the clinical prediction model however, could not rule-out a PAR to a SAB diagnostic block, with the highest sensitivity being only 40% (1 of 3 findings present). Possible explanations for the low sensitivity include the heterogeneity of subacromial pain and pathology in primary care populations, and the relatively low prevalence of 80% PAR (34%). Structures that occupy the subacromial region including the SAB and components of the rotator cuff, cross the anatomical boundaries we arbitrarily set for anterior shoulder pain (42). Thus lesions of the SAB or the rotator cuff, in the absence of anterior shoulder pain may still report relief from subacromial injections of local anaesthetic. Similarly, subacromial pain is known to result from mechanisms other than ‘strain’ including trauma, repetitive activity or insidious onset, and also as a result of inflammatory disease (43).
In contrast, using combinations of the 9 clinical variables was more effective (Table III), with the ability to rule-out an 80% PAR (sensitivity) improved to 100% when none of the variables were present, and could also be ruled-out with a high level of confidence when less than two variables were present (sensitivity 0.95; 95% CI, 0.87, 0.98 and NLR 0.21; 95% CI, 0.07, 0.62). Specificity of a PAR also increased with increasing numbers of positive tests, however, there was a trade-off with decreasing numbers of subjects satisfying the criteria that included higher numbers of positive tests. When 6 findings were positive, subjects were almost seven times more likely to report a PAR to SAB diagnostic block (specificity 0.97), and when 7, 8 or 9 clinical tests were positive, specificity increased to 100%. In cases where clinical findings present diagnostic uncertainty (3, 4 of 5 positive clinical findings), a clinically-administered diagnostic injection of local anaesthetic into the subacromial region may be required to confirm the diagnosis. This is a simple and inexpensive diagnostic procedure when performed ‘blind’ in primary care with low associated risks, and in competent hands, injection accuracy approaches that of guided procedures (44).
A limitation of note is that we cannot determine precisely which structures were anaesthetised. Recognised procedures were followed in our protocol to ensure accurate needle placement into the SAB using ultrasound guidance, and infiltration of the SAB was confirmed in all cases by visual observation of bursal distension on ultrasound. However, as no contrast agent was used, it was not possible to track movement of injectate to surrounding tissues following the SAB injection. It is therefore possible that structures in close anatomic proximity to the SAB were also exposed to the anaesthetic, including structurally compromised portions of the rotator cuff and the acromioclavicular joint. For this reason the results reflect predictors of anaesthetic response to subacromial injection, without the assumption of isolated bursal involvement.
In conclusion, both clinical prediction model variables, and combinations of 9 history and physical examination variables were able to identify the presence of painful subacromial conditions in primary care patients. This provides confidence in distinguishing subacromial pain from other sources of shoulder pain and aids more judicious selection of patients for more expensive or invasive investigations for specific subacromial conditions, or targeted pain relief interventions, such as corticosteroid injections. However, compared with the clinical prediction model variables, the absence of at least 2 of the 9 clinical examination findings was more accurate in ruling-out painful subacromial conditions. Additional diagnostic procedures including the use of diagnostic injections of local anaesthetic may be required to confirm the anaesthetic response when these clinical criteria are not satisfied.
ACKNOWLEDGEMENTS
We thank Dr Tony Young of Christchurch Radiology Group for support in-kind, Dr Mark Coates for radiological expertise and Mr Khalid Mohammed for his advice during the study. Thanks to Industrial Research Ltd for their technical support for the hand-held dynamometer. Funding support provided by the Health Research Council of New Zealand (Clinical Research Training Fellowship) (AC), New Zealand Manipulative Physiotherapists Association, Physiotherapy New Zealand and AUT University Faculty of Health and Environmental Science. No funding agency influenced interpretation of data or conclusions drawn. The authors declare they have no conflicts of interest.
REFERENCES