OBJECTIVE: To assess the effectiveness of segmental neuromyotherapy combined with standard hospital therapy relative to standard therapy alone in patients with hemiplegic shoulder pain.
DESIGN: Randomized controlled trial.
Patients: A total of 24 patients with positive Neer’s and hand-behind-neck tests received standard therapy for shoulder pain. Half of them received additional segmental neuromyotherapy.
METHODS: Pain severity (visual analogue scale), upper-limb function (Fugl-Meyer arm score), and spasticity (Ashworth scale) were evaluated at 2 days (T1) and 1 day (T2) pre-treatment, in the middle (T3) and at the end (T4) of 4 weeks treatment, and 2 months post-treatment (T5).
RESULTS: The treatment group showed significant advantage compared with the Control group in Fugl-Meyer scores at T4 (p = 0.014) and T5 (p = 0.0078) compared with initial values. Significant advantage was also shown in the Neer’s test at T4 (p = 0.014), with borderline significance at T5 (p = 0.072). A larger decrease in pain scores reported by the treatment group at T5 (p = 0.068) may have been biased by higher rates of spatial neglect in this group.
CONCLUSION: Segmental neuromyotherapy added to standard therapy provides an advantage in pain relief and overall arm function in patients with hemiplegic shoulder pain.
Key words: stroke; hemiplegia; shoulder pain; spinal sensitization.
J Rehabil Med 2012; 00: 00–00
Correspondence address: Motti Ratmansky, Pain Rehabilitation Clinic, Loewenstein Rehabilitation Hospital, 278 Ahuza Street, Raanana 43100, Israel. E-mail: mottir@clalit.org.il
Submitted May 11, 2011; accepted April 18, 2012
INTRODUCTION
Hemiplegic shoulder pain (HSP) is a general term used to describe shoulder pain following a stroke, without pointing to a precise aetiology, as the underlying pathophysiology is usually uncertain (1, 2). It is a common sequel of stroke that greatly affects patient wellbeing, often leading to a delay in achieving rehabilitation goals and to prolongation of hospitalization (3). A large population study has suggested a prevalence of 55% among stroke patients undergoing rehabilitation (4), which is similar to the mean prevalence of 54% calculated by others (5), with no age group or gender dominance (6, 7). The variant incidence rate in HSP studies is explained by differences in the timing of pain evaluation (different time after stroke onset; evaluation of pain at rest vs during movement) and patient populations (hospitalized vs non-hospitalized) (8, 9). It also reflects the fact that HSP is a label attached to a variety of pathological conditions, including soft tissue damage due to capsulitis, tendon tear, impingement and tendonitis; gleno-humeral subluxation; central and peripheral sensitization; and spasticity-related pain (10, 11).
Numerous treatment methods have been suggested over the years, but none have been shown to be significantly superior to others. A recent review of stroke rehabilitation with an evidence-based orientation published by Teasell et al. (12), evaluated several treatment methods for HSP and found conflicting evidence that electrical stimulation or botulinum toxin injections could help reduce pain, moderate evidence that supports an active therapy-oriented approach and insufficient evidence that positioning of the shoulder or shoulder strapping prevented subluxation, decreased pain or increased functionality.
One diagnostic and treatment approach that has not yet been studied in HSP is segmental neuromyotherapy (SNMT), developed by the late Professor Andrew A. Fischer for the treatment of neuro-musculo-skeletal problems. According to SNMT theorizing, sensitization in corresponding spinal segments plays a major role in the formation of continuous pain in a given part of the body. The term coined by Fischer for this phenomenon is “spinal segmental sensitization” (SSS) (13). The SSS component of chronic pain was assessed directly in patients with pain from total hip replacement and from planter fasciitis (14, 15), supporting the notion that chronic pain is contributed by sensitization of spinal nociceptive neurones, regardless of the original provoking events. In the case of HSP, tissue pressure and injury in the paralysed shoulder is followed by local release of pro-inflammatory substances evoking peripheral sensitization. This, in turn, induces central sensitization in the relevant segments of the spinal cord, which may become chronic. The SSS may lead to muscle spasm in the corresponding myotomes through involvement of anterior horn motor neurones of the same spinal segments. Local taut muscular bands and trigger points are likely to form in such conditions. Local para-spinal muscle contraction with resultant narrowing of the intervertebral space and compression of nerve roots is another likely outcome. The SNMT approach aims at diagnosing the precise spinal segments involved in the sensitization process, and the musculoskeletal outcome of this sensitization. This is done using basic physical diagnostic modalities, such as assessment of limitations in articular range of motion, palpation to identify areas of hypersensitivity, evaluation of pain threshold by algometry, etc. The SNMT approach to treatment consists of injection of local anaesthetic agents in the involved dermatome to block the posterior branch of the dorsal spinal nerve along the involved para-spinal muscles. In addition, local anaesthetic injection is applied peripherally near the foci of irritation in local soft tissue, directly into taut bands and trigger points, using a needling and infiltration technique. Limbering exercises, local heat application and additional transcutaneous electrical nerve stimulation (TENS) treatment complete the muscular relaxation after the injections (13, 16, 17). The idea driving the SNMT approach is that effective pain relief depends on application of treatments that deal with all the components of the above chain of events.
To the best of our knowledge SNMT has not been tested scientifically in HSP. The aim of the current pilot study was to evaluate the effectiveness and practical applicability of the SNMT approach in stroke patients undergoing rehabilitation shortly after the onset of their disease. A small-scale RCT design was used to assess the impact of SNMT on both shoulder pain and arm function.
METHODS
Subjects
Twenty-four patients (9 females, 15 males; mean age: 62 years (standard deviation (SD) 9), admitted to the Loewenstein Hospital for rehabilitation after a first-event ischaemic or haemorrhagic stroke, were recruited for the study. Inclusion criteria were: (i) age between 40 and 85 years; (ii) no history of shoulder pain on the hemiplegic side before the stroke; (iii) cognitive and language functioning enabling coherent communication between the examiner and the patients; (iv) positive Neer’s test and hand-behind-neck (HBN) test (both tests have shown high predictability rates for developing HSP) (18, 19). Exclusion criteria were: (i) history of cardiac arrhythmia, unstable haemodynamic state, presence of a cardiac pacemaker; (ii) seizure in the 6 months preceding enrolment; (iii) use of pain medications in the week before enrolment; (iv) needle phobia; (v) known history of sensitivity to lidocaine.
After receiving a thorough explanation of the study goals and protocols, all subjects signed an informed consent form (in accordance with ethical standards on human experimentation and with the Declaration of Helsinki 1975, revised in 1983). The study was approved by the human rights committee of Loewenstein Hospital.
Of 84 consecutive admissions of neurological patients to the Department of Neurological Rehabilitation at Loewenstein Hospital, Raanana, Israel (during a period of 3 months), 72 were stroke patients. Of these, 24 with first-event ischaemic or haemorrhagic stroke, answered the set inclusion criteria and were found to have positive Neer’s and HBN tests for HSP. These 24 patients (9 females, 15 males; mean age: 62 years (SD 9)) were recruited for the study and randomly allocated to 1 of 2 groups using a simple randomization method (same chance of allocation to each group): control (n = 12) and SNMT (n = 12). The former group received the hospital’s standard treatment regimen for HSP, while the latter received the SNMT in addition to the hospital’s standard treatment, in the same time-period.
One patient withdrew from the SNMT group after completing 11 (of 12) treatments because of unsatisfactory results, but agreed to participate in the T4 and T5 evaluations. Another patient in the SNMT group died during the follow-up period, before the T5 evaluation, and his scores at T4 were also used in the group analysis at T5. Table I describes the characteristics of the groups. There were no significant differences between the groups in basic demographic and clinical characteristics (age, gender, time from stroke onset to enrolment in the study, type of stroke, score on the NIHSS, and shoulder subluxation score), but patients in the SNMT group had greater prevalence of right hemisphere damage and unilateral spatial neglect (Table I).
|
Table I. Group demographic and clinical characteristics
|
|
|
SNMT
(n = 12)
|
Control
(n = 12)
|
|
Females/males, n
|
8/4
|
7/5
|
|
Age, years, mean (SD)
|
64.7 (8.3)
|
59.8 (9.9)
|
|
Ischaemic/haemorrhagic stroke, n
|
10/2
|
10/2
|
|
Left/right hemispheric damage, n
|
4/8
|
8/4
|
|
TAO, days, mean (SD)
|
61.6 (40.4)
|
78.2 (50.3)
|
|
Neglect, n*
|
7
|
1
|
|
Degree of subluxation, median
|
2
|
2
|
|
NIHSS, mean (SD)
|
12.8 (5.0)
|
10.7 (4.4)
|
|
*The only factor showing significant (p = 0.004) difference between groups.
SNMT: segmental neuromyotherapy; TAO: time after onset of stroke at the recruitment for the study; NIHSS: National Institute of Health Stroke Scale; SD: standard deviation.
|
Experimental protocol
Following the assignment to groups and immediately before the beginning of treatment, two consecutive evaluations were conducted one day apart (T1 and T2). The evaluations included collection of demographic and clinical data and assessment of outcome measures, as detailed in the following section. The purpose of the repeated baseline evaluations was to calculate the standard error of measurement (SEM) for the outcome measures. The next evaluation took place at the middle of the treatment period (T3; 1 day after the 6th treatment session, before the 7th treatment session, approximately 2 weeks after T1). The 4th evaluation was conducted at the end of the treatment period (T4; 1 day after the 12th (last) treatment session, approximately 4 weeks after T1). Finally, a follow-up evaluation was performed 2 months after the end of the treatment period (T5; approximately 3 months after the baseline evaluation). Measurements performed at T3 and T4 were conducted in order to assess the short-term effect of the ongoing treatment, and measurements at T5 assessed long-term effects, after the end of treatment.
All of the above assessments were conducted by an external evaluator, blind to the patients’ group allocation. The physiotherapists and occupational therapists who treated the patients as part of the standard hospital therapy were blind to the patients’ group allocation and to the findings of the ongoing external evaluations. The physical therapist who administered the additional physical therapy, which was part of the SNMT protocol knew that the treatment was given within comparative research.
Assessment
Demographic, medical history and stroke-related clinical data were obtained from patient medical files. Brain computerized tomography (CT) and an anterior-posterior plane hemiplegic shoulder X-ray film taken with the hand hanging alongside the body, without support, added supplementary information. To assess subluxation severity, shoulder X-ray was taken at T1 and was evaluated by a radiologist using a standard 3-point scale (20, 21): 1= no subluxation, 2 = moderate subluxation, 3 = severe subluxation.
The outcome measurements were: (i) Neer’s test, performed by placing the arm in forced flexion with the arm pronated and the scapula stabilized (22); (ii) the HBN test, performed by placing the patients’ affected arm in external rotation and abduction, and asking patients to report the presence of pain evoked by this position by indicating “yes” or “no” (23) (the test is considered positive if the manoeuvre cannot be performed because of pain); both Neer’s and the HBN tests were used clinically to diagnose the presence of HSP (19); (iii) the intensity of spontaneous pain (without provocation by passive or active shoulder movement) was measured using the visual analogue scale (VAS) with values between 0 and 10, with the end-points set as “no pain sensation” and “the most intense pain sensation imaginable”; (iv) sensitivity of deep tissues to pain was measured with a pressure (kg/cm²) algometer (Pain Diagnostics and Treatment, Inc., NY, USA), with pressure pain threshold measured in the ipsi- and contralateral deltoid and supraspinatus muscles, evaluating the minimal pressure that induces pain (24, 25); (v) the Fugl-Meyer (FM) test was used to evaluate overall upper-limb function. This widely used test (26) examines the ability to move the arm and its segments in a series of qualitatively rated items (arm motor function containing 24 items and scores ranging from 0 to 66); (vi) the modified Ashworth scale, used to evaluate spasticity of the shoulder, with scores ranging from 0 (normal muscle tone) to 4 (affected upper limb is rigid in flexion or extension).
Treatment
The standard hospital treatment consists of daily occupational therapy (OT) and physiotherapy (PT) sessions, between 1–2 h altogether, 5 days a week; use of a shoulder sling; an arm support table attached to the wheelchair; and oral pain medication (500 mg paracetamol + 40 mg propoxyphene) up to 4 times a day, as needed by the patient. No other pain medication was used during the study.
Each patient in the SNMT group received 12 additional treatments (45 min each, 3 times a week, for 4 consecutive weeks). Each treatment included the following: (i) diagnosis of the relevant spinal segment by palpation for trigger points, pinching and rolling of the skin between the first and the index fingers (by author MR); (ii) intramuscular and subcutaneous injections of 5 ml 1% lidocaine solution (a) near the involved spinal segment blocking the posterior branch of the dorsal spinal nerve (para-spinal block), (b) peripheral injections near the irritative foci (pre-injection block), and (c) directly into the taut band and trigger points, using a needling and infiltration method (by author MR); (iii) 20-min combination of local heat application and TENS, using 4 surface patch electrodes over the deltoid and supraspinatus muscles (at a pulse frequency of 40 Hz, pulse width of 200 microseconds, current increased slowly to 11 mA or until a muscular twitch response was observed) (by a physical therapist); (iv) following the treatments, additional 10 min of passive stretching of the scapulae and the shoulder (by a physical therapist).
Statistical analysis
The Statistical Package for Social Sciences (SPSS ver. 17, Chicago, USA) was used for data analysis. The values of continuous variables are described by means and standard deviations (SD). Categorical variables are described by frequencies or medians. Differences in outcome measures between T1 and T4 and between T1 and T5 were calculated to evaluate the short- and long-term effects of the treatment, respectively. Multivariate analysis of variance (MANOVA) was used to assess differences between the groups (SNMT, Control), separately for the short- and long-term gains in outcome measures (i.e. the gain obtained during the treatment period, as reflected in the T1–T4 difference, and the gain obtained up to the follow-up evaluation, as reflected in the T1–T5 difference). Two separate MANOVAs were performed for each of the above time intervals (T1–T4; T1–T5): 1 for the outcome measures that evaluated shoulder pain and arm function (algometry, representing soft tissue sensitivity to pressure-induced pain over the deltoid and supraspinatus muscles; VAS, representing spontaneous pain at rest, and the FM arm score, representing arm function) and 1 for outcome measures related to muscle tone (Ashworth scores for external rotation, abduction and flexion in the shoulder). The MANOVAs were performed after assessing for normal distribution with the Kolmogorov-Smirnov test. In addition, between-groups comparisons were conducted separately for each of the outcome measures, using t-tests for continuous variables and χ2 tests for categorical variables. The error of measurement for each outcome measure was expressed by the standard error of measurement (SEM) (27), defined as: SD * √(1− R), where SD is the standard deviation of the pooled first and second baseline tests (T1 and T2) scores, performed on consecutive days immediately before the beginning of the treatment, and r is the correlation between these tests. The differences in the outcome measures between T1 and T4 and between T1 and T5 were compared with the SEM values to assess whether the change obtained reflects a real difference, beyond the SEM. p-values < 0.05 were considered significant.
RESULTS
Based on the consecutive measurements conducted at T1 and T2 in both groups, the SEM was calculated for the different outcome measures (Table II). High correlation coefficients attest for measurements’ test-retest reliability. The SEM values were used in subsequent analyses to determine whether the changes observed in the outcome measures were beyond the natural fluctuations in the measurements, thus likely to reflect treatment effects of clinical significance.
Since the data collected at T3 (in the middle of the treatment period) in most cases reflected values in between those of T1 (commencement of treatment) and T4 (end of treatment) we decided, for the sake of simplicity, to analyse and discuss the T4 values as representatives of the short-term effects of the ongoing treatment. Table III presents the values of the different outcome measures at T1 and T4, and the gains obtained in each group during the treatment period (4 weeks, from T1 to T4).
MANOVA revealed a significant effect of the group on the gain obtained during that period in outcome measures related to shoulder pain and arm function (algometry, VAS, FM arm score), indicating an advantage for the SNMT group over the Control group (degrees of freedom (df) = 5, F = 3.28, p = 0.033). As shown in Table III, the SNMT group demonstrated a significant advantage relative to the Control group in overall arm function, as reflected in the gain obtained in that period in the FM arm test (median increase of 2.5 vs median decrease of 0.5 relative to baseline, p = 0.014). The mean value of the difference between T1 and T4 in the FM score in the SNMT group (4.6), but not in the Control group) was beyond the SEM value of the test (1.83; Table II).
|
Table II. Standard error of measurement (SEM)
|
|
|
r
|
SD
|
SEM
|
|
VAS
|
0.66
|
1.51
|
0.88
|
|
FM arm score
|
0.99
|
16.7
|
1.83
|
|
Algometry deltoid
|
0.96
|
2.13
|
0.44
|
|
Algometry SSP
|
0.89
|
2.49
|
0.82
|
|
r: correlation coefficient; SD: standard deviation of test results pooled from the entire sample (segmental neuromyotherapy subjects and control subjects); VAS: visual analogue scale; FM: Fugl-Meyer score; ROM: range of motion; SSP: supraspinatus muscle.
|
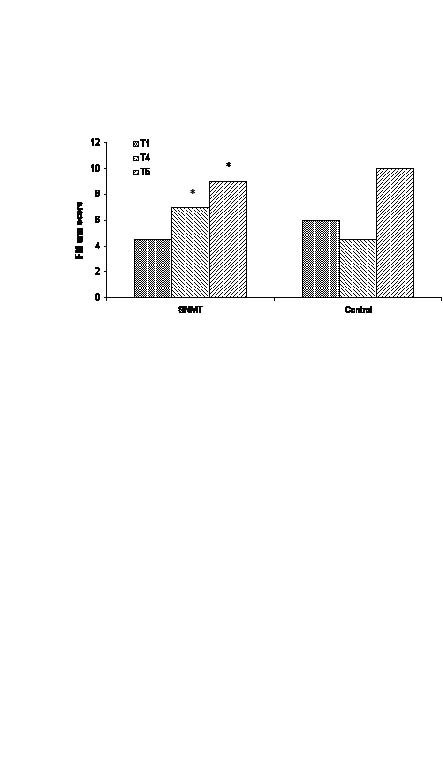
Fig. 1 shows the median values of the FM arm score of the two groups in T1, T4 and T5.
Fig. 1. Fugl-Meyer (FM) arm score values of the 2 groups in T1, T4 and T5. Median values of FM arm scores increased significantly in the segmental neuromyotherapy (SNMT) group from T1 to T4 and from T1 to T5 (*p < 0.05 and p < 0.01, respectively). The Control group did not exhibit significant changes in the FM arm score between test periods. Between-groups comparisons are shown in the text and tables.
The SNMT group also demonstrated a significant advantage over the Control group in pain relief, as reflected in the decrement shown in Neer’s test scores during the treatment period (–0.3 vs 0.0, p = 0.014). The assessment of pain threshold by algometry revealed, in both groups, a tendency towards lowering the threshold during that period, a fact that is probably related to the parallel increment in muscle tone (discussed below). This tendency was more salient in the Control group, where it surpassed the SEM values (0.82 and 0.44 for supraspinatus and deltoid, respectively; Table II), but the difference between the groups did not reach significance (p = 0.088 for the supraspinatus and higher for the deltoid). The other pain measures (HBN, VAS) did not show a significant difference between the two groups. The pain relief, as reflected in the VAS, surpassed the SEM value of the test in both groups.
A second MANOVA aimed to assess the effect of the group on muscle tone (Ashworth scores for external rotation, abduction and flexion in the shoulder) revealed an insignificant effect (df = 3, F = 0.82, p = 0.49). Given the fact that the treatment period was in the sub-acute phase of the disease, it is not surprising that patients in both groups showed an increment in muscle tone in the shoulder girdle during that period, as reflected in the Ashworth scale. As stated, this increment coincided with a slight decrease in pressure pain threshold assessed by algometry (Table III).
|
Table III. Gain of segmental neuromyotherapy (SNMT) group vs Control group at the end of the treatment period
|
|
Tests
|
SNMT
(n = 12)
|
|
Control
(n = 12)
|
|
T1
|
T4
|
Delta
|
|
T1
|
T4
|
Delta
|
|
HBN, median
|
1
|
1
|
–0.2
|
|
1
|
1
|
–0.2
|
|
Neer’s, median
|
1
|
1
|
–0.3*1
|
|
1
|
1
|
0.0
|
|
VAS, mean (SD)
|
8.1 (1.5)
|
4.0 (3.0)
|
–4.1 (3.1)
|
|
7.5 (1.6)
|
4.6 (2.1)
|
–2.9 (2.7)
|
|
FM arm score, median
|
4.5
|
7
|
2.5*2
|
|
6
|
4
|
–0.5
|
|
Ashworth ER, median
|
1
|
2.5
|
1.2
|
|
0
|
3
|
0.3
|
|
Ashworth abduction, median
|
1
|
2
|
0.7
|
|
0.5
|
2
|
1.2
|
|
Ashworth flexion, median
|
1
|
2.5
|
0.9
|
|
0
|
2
|
1.2
|
|
Algometry deltoid, mean (SD)
|
5.4 (1.8)
|
5.2 (2.2)
|
–0.2 (2.5)
|
|
5.1 (2.6)
|
4.2 (1.7)
|
–0.9 (2.1)
|
|
Algometry ssp, mean (SD)
|
7.0 (2.9)
|
6.6 (2.0)
|
–0.4 (3.0)^1
|
|
7.4 (2.4)
|
5.6 (2.2)
|
–1.8 (1.7)
|
|
*1p = 0.014; *2p = 0.02; ^1p = 0.088. p-values for between-groups comparisons reaching (*) or approximating (^) significance.
HBN: hand behind neck test; VAS: visual analogue scale; FM: Fugl-Meyer test; ER: external rotation; ssp: supraspinatus muscle; SD: standard deviation.
|
Table IV presents the scores of the different outcome measures at T1 and T5, with comparison of the gain obtained by the two groups from the commencement of treatment to the end of the follow-up period 3 months later (i.e. 2 months after the end of the treatment period). MANOVA revealed an insignificant effect of the group on the gain obtained during that period in outcome measures related to shoulder pain and arm function (df = 5, F = 1.7, p = 0.17). Nevertheless, as shown in Table IV, the SNMT group demonstrated a larger gain compared with the Control group in overall arm function, as reflected in the gain obtained in that period in the FM arm test (median increase of 1.5 vs median of 0 relative to baseline, p = 0.0078). The mean value of the difference between T1 and T5 in the FM score in the SNMT group (7.6), but not in the Control group was beyond the SEM value of the test (1.83; Table II). Two pain measures, the Neer’s test and the VAS, showed greater gain in the SNMT compared with the Control group with borderline significance (Neer’s test: –0.3 and –0.1, gains of SNMT and Control groups, respectively, p = 0.072; VAS: –3.2 (SD 3.1) and –1.4 (SD 2.5), gains of SNMT and Control groups, respectively, p = 0.068). The improvement in the VAS during that period, shown by the two groups, surpassed the SEM value of the test (0.88; Table II). The change in pressure pain threshold as assessed by algometry did not show a significant difference when comparing the two groups.
A second MANOVA aimed to assess the effect of the group on muscle tone revealed an insignificant effect (df = 3, F = 0.72, p = 0.56). The increment in muscle tone was slightly greater in the Control group, but the difference between the groups did not reach significance (Table IV).
|
Table IV. Gain of segmental neuromyotherapy (SNMT) group vs Control group at the end of the follow-up period
|
|
Tests
|
SNMT
(n = 12a)
|
|
Control
(n = 12)
|
|
T1
|
T5
|
Delta
|
|
T1
|
T5
|
Delta
|
|
HBN, median
|
1
|
1
|
–0.2
|
|
1
|
1
|
–0.2
|
|
Neer’s, median
|
1
|
1
|
–0.3^1
|
|
1
|
1
|
–0.1
|
|
VAS, mean (SD)
|
8.1 (1.5)
|
4.9 (3.7)
|
–3.2 (3.1)^2
|
|
7.5 (1.6)
|
6.2 (1.8)
|
–1.4 (2.5)
|
|
FM arm score, median
|
4.5
|
9
|
1.5*1
|
|
6
|
10
|
0
|
|
Ashworth external rotation, median
|
1
|
2
|
0.7
|
|
0
|
3
|
0.7
|
|
Ashworth abduction, median
|
1
|
2
|
0.7^3
|
|
0.5
|
2
|
1.5
|
|
Ashworth flexion, median
|
1
|
2
|
0.7^4
|
|
0
|
2
|
1.5
|
|
Algometry deltoid, mean (SD)
|
5.4 (1.8)
|
6.2 (2.9)
|
0.7 (2.2)
|
|
5.1 (2.6)
|
5.4 (2.8)
|
0.3 (2.3)
|
|
Algometry ssp, mean (SD)
|
70.0 (2.9)
|
6.6 (2.6)
|
–1.0 (4.0)
|
|
7.4 (2.4)
|
5.8 (1.9)
|
–1.7 (2.3)
|
|
p-values for between-groups comparisons reaching (*) or approximating (^) significance: *1p = 0.0078; ^1p = 0.072; ^2p = 0.068; ^3p = 0.081; ^4p = 0.091. aOne patient in this group died during the follow-up period, before the T5 evaluation. For the purposes of group analysis his scores at T4 were used also at T5.
HBN: hand behind neck test; VAS: visual analogue scale; FM: Fugl-Meyer test; ssp: supraspinatus muscle; SD: standard deviation.
|
DISCUSSION
HSP is a common sequel of stroke, with grave consequences not only for patient wellbeing, but also for the functional outcome of the affected upper limb. A recent evidence-based review of various therapeutic approaches suggested for this problem, found most treatments to have a limited effect. None of the evaluated treatments showed a significant advantage over others (12). This fact motivated us to evaluate the effectiveness in HSP of another therapeutic approach, SNMT, which was tested and found to be beneficial in other pain conditions. To the best of our knowledge, this small-scale RCT is the first attempt to assess the place for SNMT in the treatment of HSP.
In the current study, 12 stroke patients with HSP, who received SNMT (3 sessions per week during 4 weeks) in addition to the standard therapy provided for HSP in the Loewenstein Rehabilitation Hospital (daily PT and OT, shoulder sling and arm support table, oral paracetamol/propoxyphene analgesia), were compared with 12 stroke patients with HSP who received standard hospital therapy alone during this period. This was done after a process of recruitment and random allocation, as explained in the Methods section. Patients in both groups experienced a significant (more than the SEM value) relief in shoulder pain, reflected in the change in VAS scores during the treatment period. Comparison of the gains of the two groups by the end of the treatment period, revealed a significant advantage for the combined treatment (SNMT group) in pain relief, as assessed by the Neer’s test, and in upper limb function, as assessed by the FM test (Table III). The advantage of standard therapy combined with SNMT over standard therapy alone extended beyond the treatment period (4 weeks). Comparison of the magnitude of pain relief in the two groups, from the baseline assessment to the end of the follow-up period 3 months later, revealed an advantage for the SNMT group, which approximated statistical significance (p = 0.068 and 0.072 for the Neer’s test and the VAS, respectively). Comparative assessment of the magnitude of improvement in arm function (by the FM test) in the two groups, revealed that the advantage shown for the SNMT group by the end of the treatment period is maintained also at the end of the follow-up period (p = 0.044). As these figures are based on assessments conducted by an external evaluator, blind to the patients’ group allocation, and given the fact that patient allocation to groups was random, these figures provide sound evidence for the advantage of the above standard treatment protocol combined with SNMT, compared with the standard treatment alone.
The essential element in SNMT theorizing is the notion that chronic pain of various origins involves sensitization of nociceptive neurones in the segments of the spinal cord receiving their afferent sensory input from the affected part of the body (this component of chronic pain syndrome is termed “spinal segmental sensitization”). Thus, the SNMT approach to the treatment of chronic pain consists of a combined application of several therapeutic measures, physical and pharmacological (described in detail in the Introduction section), some of them shared by other treatments proposed for HSP. For example, lidocaine 1% injections were found to induce a significant reduction in shoulder pain along with an increment in passive range of motion (ROM) in 14 of 28 patients with HSP (1). This finding supports the notion, shared by the SNMT approach that injection of lidocaine to soft tissues in the shoulder is likely to exert a positive effect in patients with HSP. Non-effectiveness in other HSP patients may reflect the complexity of the underlying pathophysiology of HSP, hence the belief that a combined application of different modalities of treatment, as in the current study (patients in the SNMT group received lidocaine 1% injections in 3 target locations, local heat, TENS, and passive stretching), may better address different factors potentially applicable in a given HSP patient than a unimodal approach. In a different study of HSP, TENS (another component of the combined treatment given to the patients in the SNMT group) was found to yield a greater increment in passive ROM when applied at a frequency of 100 Hz (28). Other research groups (29–31) applied FES to the shoulder muscles, with or without PT, using lower (35–50 Hz) frequencies, and reported on the benefits to the patients. In the current study we decided to apply electrical stimulation via surface electrodes placed over the deltoid and supraspinatus muscles (as in earlier HSP studies), using a frequency of 40 Hz. The stimulation was applied immediately following the lidocaine injections, in order to achieve a muscular response, to facilitate the spread of the injectate within the muscle fibres. It should be noted that, during the planning the current study, we had no ready protocol with prescribed “dosage” for the different components of SNMT, as this was the first time that SNMT was assessed scientifically in HSP. Thus, the decision on the exact mode of application was based on literature reports on the effectiveness of unimodal treatments that form part of the SNMT approach, and on experience gained from years of clinical practice in the field of stroke rehabilitation. Earlier studies of the effectiveness of the SNMT approach in chronic pain were conducted in other clinical conditions, total hip replacement (14) and plantar fasciitis (15), with good results in both conditions, but with only limited information that could help the construction of the SNMT protocol as applied in the current study.
The addition of 3 sessions of SNMT per week to a treatment protocol composed of daily PT and OT, arm support by sling and wheelchair table, and oral paracetamol/propoxyphene, was found to yield a greater gain compared with the same treatment protocol given without additional SNMT. The meta-analysis conducted by Teasell et al. (12) found limited evidence for the effectiveness of using a shoulder sling. However, the results of the current study revealed significant pain relief (VAS gain surpassing the SEM value of the test), even in the Control group, i.e. with a combination of the above standard treatments without additional SNMT sessions. It is assumed that the combined application is likely to increase the effectiveness compared with application of each of the components alone, by targeting different elements in the multi-factorial pathophysiology of HSP.
The current study has several important limitations. First, despite the RCT design, the small cohort on the one hand and the multifactorial pathophysiology of HSP on the other hand, make any generalizations to the entire stroke population questionable. Secondly, as the groups are small, the random group allocation created an uneven distribution with respect to the affected hemisphere. Thus, it happened that 8 of 12 patients in the SNMT group have right-hemisphere damage, whereas 8 of 12 patients in the Control group have left-hemisphere damage. This means that none of the two groups represents the general stroke population in terms of hemispheric damage. Thirdly, a salient consequence of this uneven distribution is the high prevalence of unilateral spatial neglect in the SNMT but not in the Control group. The occurrence of neglect is likely to attenuate and distort pain perception (as well as the perception of other modalities of somatic sensation), originating from nociceptive stimulation in the side of the body contralateral to the lesioned hemisphere. This fact raises questions about the reliability of measures based upon subjective report, especially the VAS, and about the ability to assess correctly changes in pain sensation in the affected shoulder. These concerns apply also in a comparative design as adopted in the current study, where the VAS was used as an outcome measure (it should be noted, however, that the FM measure of arm function used here is of a more objective nature, and that the test-retest correlation coefficient calculated to compute the SEM for the VAS was quite high, albeit not as high as for the FM (0.66 vs 0.99, respectively)). Future HSP research on larger populations should consider analysing unilateral neglect as a co-variant, which is likely to affect the outcome measures independently. Fourthly, the comparative design of the current study has an inherent weakness, stemming from the fact that control patients were not given any treatment in addition to the hospital’s standard therapy, whereas the SNMT patients received 3 additional therapeutic sessions per week. For ethical reasons we designed our study this way, despite this salient weakness. Given the multiple components of the SNMT approach, adding a fictitious therapy to the Control group in order to check for a possible placebo effect, would entail one month of repeated saline injections, sham electrical stimulation, etc., which we judged to be inappropriate. Finally, in the current study, patient recruitment was based essentially on the presence of shoulder pain in the hemiplegic part of the body, irrespective of pathophysiological variance. Thus, the aim was not to assess SNMT effectiveness in combating any specific causative factor of HSP (e.g. prominent subluxation, hypertonia, peripheral nerve entrapment, central post-stroke pain, etc.). As the various aetiologies often overlap, a design aimed to assess treatment effectiveness for specific conditions would necessitate a much larger cohort and additional diagnostic measures (e.g. an ultrasound examination). The heterogeneity of mechanisms underlying HSP calls for a more extensive study aimed at evaluating the effectiveness of treatments in more homogenous subgroups of HSP.
An assessment of the applicability of SNMT in a given rehabilitation setting should take into account the substantial cost involved, especially in terms of qualified personnel time. The multiple components of each treatment session (described in the Methods section) make SNMT application a time-consuming procedure. Also, learning how to use it properly necessitates considerable practice time. Patient risk is another issue that has to be considered. As the treatment includes lidocaine injections to multiple target locations, there is a low risk in its application (however, none of the participants in the current study reported any serious inconvenience). Despite the substantial time consumption and the low risk, the positive results of the current study indicate that the SNMT approach has a place, among other therapeutic measures, in the armamentarium of clinical settings where stroke patients receive their rehabilitation treatment.
In conclusion, this small-scale RCT shows that the addition of SNMT sessions (3 times a week, during 4 weeks) to standard daily measures (PT and OT, arm support by sling and wheelchair table, and oral paracetamol/propoxyphene) significantly increases the gain of subacute stroke patients with HSP, undergoing rehabilitation. The added value is reflected both in pain relief and in the quality of arm function. Further research on a larger stroke population is required in order to corroborate and generalize the current findings to subtypes of HSP, identified on the basis of the dominant pathophysiological factors.
Acknowledgements
The authors wish to thank and acknowledge the late Professor A. A. Fisher for the development of the SNMT method and for his advice on this study. The authors also wish to thank the physical therapist, Mrs Anat Komissar, and the external evaluator, Mrs Yael Nave, for their help in conducting this study.
REFERENCES