Objective: To determine the effect of botulinum toxin A on spasticity and somatosensory evoked potentials of hand muscles in patients who have undergone cerebrovascular accident.DESIGN: Preliminary, prospective, before-after study design.
Patients: Six subjects prospectively followed after application of botulinum toxin A in the rehabilitation department of a university hospital.
METHODS: All patients underwent botulinum toxin A injection to the upper extremity muscles in varying combinations and carried out a home-based exercise programme. Primary outcome measure was median somatosensory evoked potential of hand muscles (N20). Secondary outcome measures were: spasticity assessed clinically by Modified Ashworth Scales (MAS); functional ability analysis assessed by Physician’s Rating Scale (PRS); and functional difficulties reported by patients or their care-givers by patient disability and care-giver burden rating scale (PD & CBRS).
RESULTS: MAS, PRS and PD & CBRS improved with botulinum toxin A treatment. In the affected limb, N20 potentials were impaired compared with those in the unaffected side. With botulinum toxin A treatment, although improvement in overall N20-P25 amplitudes was significant, as a result of limited sample size, post hoc pair-wise comparisons with Bonferroni correction failed to yield any significant pairs.
CONCLUSION: The improvement in the median somatosensory evoked potentials following botulinum toxin A treatment suggests that central somatosensory patterns in hemiplegia can be modified by peripheral inputs.
Key words: stroke; spasticity; upper extremity; somatosensory evoked potentials; botulinum toxin.
J Rehabil Med 2012; 44: 541–546
Correspondence address: Aynur Basaran, Konya Beyhekim State Hospital, Physical Medicine and Rehabilitation Clinic, Yazir, Selcuklu TR-42250 Konya, Turkey. E-mail: aynurbasaran@hotmail.com
Submitted July 14, 2011; accepted January 31, 2012
Introduction
Loss of upper extremity function is a common and devastating outcome of stroke. Approximately 75% of patients initially show a motor deficit in the upper limb, and recovery is generally poor (1–2). Approximately 50% of all stroke survivors are left with a non-functional arm (3).
Muscle weakness, loss of dexterity and spasticity are the major features of upper motor neurone syndrome that lead directly to disability (4). Upper extremity spasticity interferes with activities of daily living (ADL); it may contribute to overall functional disability and may slow rehabilitation, especially in patients with residual muscle power (4).
The degree of initial motor deficit was found to be the most important determinant of motor and functional recovery (5). However, many patients who regain adequate motor function in the upper extremity are still unable to use the limb because of gross sensory deficits, including astereognosis, loss of 2-point discrimination, and proprioception (6).
Somatosensory evoked potentials (SEPs) are a useful, objective, quantitative, and direct method of assessing the integrity of somatosensory and motor pathways of the central nervous system (CNS) (1, 5, 7). SEPs assess the neural activity of the dorsal horn (by recording the spinal N13 potential) and dorsal column-lemniscus medialis (by recording brainstem P14 and cortical N20, P27 and N30 potentials) systems of the lemniscal pathways (8).
Several studies have examined the value of these potentials in the prediction of overall functional recovery (1, 5) or upper limb function (1, 5, 9). Studies show that an early clinical measurement of the motor deficit was superior to neurophysiological measurements. However, adding SEPs to clinical information improved the precision of prediction (5). The SEPs were retained as a significant predictor for long-term outcome and improved the predictive accuracy by approximately 8% when measured at baseline (1). In other studies, clinical measurements of sensory loss were also retained as significant predictors (1, 10). These findings stress the prognostic value of sensory impairment in outcome.
Among all the SEP parameters, it has been shown that the N20-P25 amplitude has positive correlation with both clinical outcome parameters and can serve as an independent predictor of outcome (5). N20 latency measurements have moderate correlation with outcome Medical Research Council scale score. Other authors have come to the same conclusion (5, 11).
N20 generates from a largely cortical-subcortical area (12, 13), and changes have, as expected, an important effect on prognosis, as they correlate with the degree of proprioceptive loss that determines poor recovery (9). Furthermore, SEP results reflect the integrity of a large cerebral zone where the key sensory and motor structures are situated. Besides being of more complex origin than motor output, SEP contributes to the prediction of the more complex functional recovery, as compared with pure motor restoration (9). Evoked potential studies performed at the beginning of rehabilitation can contribute to the prediction of functional recovery after stroke (7).
The aim of this study was to assess median SEP (N20 potentials) in both the affected and non-affected side in stroke patients. In addition, follow-up studies were performed after successful treatment with botulinum toxin A (BoNT-A) to evaluate its influence on the SEP pattern.
Patients and Methods
Participants
The trial included 6 subjects who were recruited on their admission to Zonguldak Karaelmas University, Department of Physical Medicine and Rehabilitation, Zonguldak, Turkey. Inclusion criteria were: having a history of a single stroke; having complaints for 1–3 years previously at the time of participation in the study; spasticity > 2 in at least one of the forearm pronators, wrist flexors, or finger flexors according to the Modified Ashworth Scale (MAS), which is articulated in 6 levels. Exclusion criteria were: having cognitive impairment; behavioural disturbances, or severe chronic disease likely to interfere with the ability to give informed consent, or to cooperate in the study; presenting with fixed contractures; having received previous BoNT-A therapy or alcohol/phenol injections; and the presence of any contraindication for BoNT-A or local anaesthesia. Subjects taking oral antispastic drugs were included in the study only if the dosage had not been changed during the month before and throughout the study.
All the patients who participated in the study provided informed consent before the outset of the study procedures. The protocol was approved by Zonguldak Karaelmas University Ethics Committee (No: 2009/08).
Intervention
Selection of target muscles was performed with the clinical examination. Biceps brachii, brachioradialis, pronator teres, flexor carpi ulnaris, flexor carpi radialis, flexor digitorum superficialis, flexor digitorum profundus, flexor pollicis longus, adductor pollicis and lumbricales were treated with varying combinations. BoNT-A (Botox®, Allergan, Irvine, CA, USA) was reconstituted in 2 ml normal saline and applied using a monopolar, Teflon-coated 27-gauge needle electrode under local anaesthesia. Electroneuromyography (ENMG) (Barrett Engineering, Fortuna, CA, USA) with passive and active guidance was used for identification of muscles (by monitoring involuntary and voluntary muscle activity). Maximum doses were 300 U per session and 50 U per injection site.
All patients received a home-based exercise programme including motor training and stretching to decrease spasticity and improve muscle strength, length, and functional ability of the upper limb. A volar hand splint was prescribed for all patients, and they were asked to use it a night.
Outcome measurements
Our primary outcome measure was median SEP (N20 latency and amplitude) to assess the proprioceptive status. Secondary outcome measures were the assessment of spasticity (forearm pronator, wrist flexor and finger flexor) by MAS, which is articulated in 6 levels, functional ability assessed by Physician’s Rating Scale (PRS), and functional difficulties that were reported by the patients or their care-givers using the Patient Disability, and Care-giver Burden Rating Scales (PD & CBRS).
The patients were assessed on admission and in the first and third months after application. All measurements were performed by the same investigator (NB), except SEP (UE).
Somatosensory evoked potentials. SEPs were measured through stimulation of the left and right median nerves at the wrist using a computerized 4-channel electromyography system (Medelec Synergy, Oxford, England). The first measurement was obtained from the paretic limb. The stimulus rate was set at 5 Hz and stimulation was provided until visible twitch of the thumb muscles was seen. Silver-silver chloride electrodes were placed over contralateral somatosensory areas (2 cm behind C3 and C4), and the reference electrode was placed at Fz based on the 10–20 International System. To ensure the reproducibility of the evoked response components, a minimum of two trials were performed. The evoked potentials were calculated by averaging the recordings at every 250 stimuli, and the responses were filtered through a bandpass of 30 to 3 Hz. The absolute latency for N20 and the peak-to-peak amplitude (PPA) of the N20-P25 were recorded.
Modified Ashworth Scale. In the clinical evaluation, spasticity at the forearm pronation, wrist and fingers were measured using MAS articulated at 6 levels, which measures resistance to passive movement according to the following scores: 0, no increase in muscle tone; 1, slight increase in muscle tone, giving a catch and release or by minimal resistance at the end range of motion (ROM) when the joint is moved in flexion or extension; 2, slight increase in muscle tone, giving a catch followed by minimal resistance throughout the remainder (less than half) of the ROM; 3, more marked increase in muscle tone through most of the ROM; 4, considerable increase in muscle tone; and 5, limb rigid in flexion or extension (14).
Physician’s Rating Scale (PRS). This scale evaluates the upper extremity by measuring the ability to move selected muscle groups: active elbow extension, active supination in extension and flexion, active wrist dorsiflexion, wrist dorsiflexion (angle of movement), finger opening, thumb in function, associated increase in muscle tone and two-handed function. Each item was assigned a point and the total maximum score was 47 (4).
Patients’ Disability and Care-giver Burden Rating Scale (PD & CBRS). In the first part of this scale (patient disability), there are 8 items: cleaning the palm of the hand, cutting fingernails, putting the paretic arm through a sleeve, cleaning under the armpit, cleaning around the elbow, standing balance, walking balance, and the ability to perform home arm physiotherapy. In the second part (care-giver burden), there are 4 items: cleaning the palm, cutting fingernails, dressing, and cleaning under the armpit. Each category is graded as follows: 0 = no disability/care-giver burden; 1 = mild disability/ care-giver burden; 2 = moderate disability/care-giver burden; 3 = severe disability/care-giver burden; 4 = maximum disability/care-giver burden. The patient completed the rating of disability and the care-giver completed the care-giver burden scale (15).
Data analysis
Repeated measurements analysis of variance (ANOVA) was used to compare values for pretreatment, and for the first and third months of treatment. Bonferroni corrected p-values were used for post hoc pair-wise comparisons. Paired sample t-test was used to compare the affected limb with the unaffected limb. Kolmogorov-Smirnov test was used to test normal distribution of continuous data.
PASW Statistics version 18 (SPSS Inc., Chicago, IL, USA) was used in recording and analysis of the data. Statistical significance level was set to 0.05 for all the analyses. Results were presented as mean (standard deviation (SD)) [minimum–maximum].
Results
Six patients were included in the study, age range 42–69 years (mean age 55.0 years (SD 11.73 years)), with a mean post-stroke interval of 22.67 months (SD 6.89 months) (range 15–33 months). In all the cases, the lesion was ischaemic. One patient was diagnosed with right-side hemiparesis and the remaining 5 patients with left-side hemiparesis. Motor recovery evaluated by Brunnstrom staging of the affected upper extremity was 2.83 (SD 0.75) [2–4] and of the hand was 2.67 (SD 0.82) [2–4].
Four patients were receiving oral antispastic therapy at the time of inclusion in the study. One of them was on baclofen (40 mg/day); one on tizanidine (18 mg/day); one on gabapentin (1800 mg/day); and one on both baclofen (40 mg/day) and gabapentin (1800 mg/day). None of the patients changed drug dosage during the follow-up.
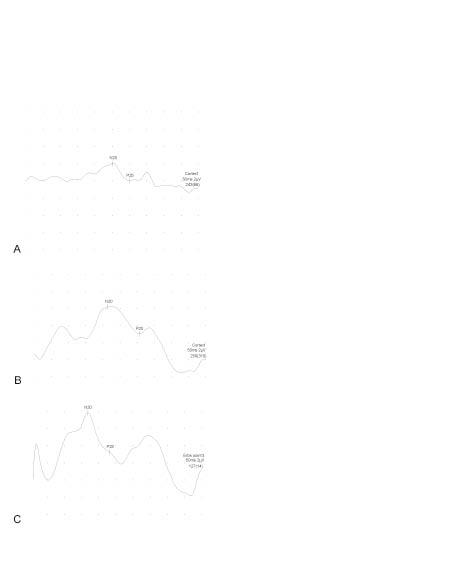
According to the clinical evaluation, target muscles are treated in varying combinations. Total dosage of BoNT-A was 300 U for all the patients. Muscle tone analysis according to MAS, functional ability analysis (PRS) and functional difficulties reported by the patients or their care-givers (PD & CBRS) improved with BoNT-A treatment (Table I). On admission, N20 latency in the affected limb was longer and N20-P25 amplitude was lower than that of the unaffected side (Table II). With BoNT-A treatment, although the improvement in overall N20-P25 amplitudes was significant, as a result of limited sample size, post hoc pair-wise comparisons with Bonferroni correction failed to yield any significant pairs. N20 latencies of the groups were not statistically significantly different (Table III, Fig. 1).
| Table I. Changes in muscle tone analysis according to Modified Ashworth Scale (MAS), functional ability analysis (PRS) and functional difficulties reported by patients or their care-givers (PD & CBRS) with botulinum toxin treatment |
| Clinical parameters | Pre-treatment Mean (SD) [Min–Max] | First month Mean (SD) [Min–Max] | Third month Mean (SD) [Min–Max] |
| MAS | | | |
| Forearm pronator | 3.33 (0.52) [3–4]a | 1.83 (0.41) [1–2]a | 2.17 (0.75) [1–3] |
| Wrist flexor | 3.17 (0.75) [2–4]ab | 1.67 (0.52) [1–2]a | 2.16 (0.75) [1–3]b |
| Finger flexor | 3.50 (0.84) [2–4]a | 1.83 (0.75) [1–3]a | 2.50 (0.84) [2–4] |
| PRS | 3.67 (4.72) [0–13]ab | 6.83 (3.19) [4–13]a | 5.67 (4.27) [2–14]b |
| PD & CBRS | | | |
| PD | 21.67 (5.28) [12–26]ab | 14.50 (4.59) [6–19]a | 14.83 (4.22) [7–19]b |
| CBRS | 10.17 (1.47) [8–12]ab | 6.00 (1.41) [4–8]a | 5.83 (1.33) [4.–8]b |
| a,bSame superscripts in a row denote statistically significant difference in post hoc pairwise comparisons (p < 0.05 with Bonferroni correction).PRS: Physician’s Rating Scale; PD & CBRS: Patient Disability Score and Caregiver Burden Rating Scale; SD: standard deviation. |
| Table II. Results of somatosensory evoked potential parameters on admission |
| | Affected limb Mean (SD) [Min–Max] | Unaffected limb Mean (SD) [Min–Max] | p |
| N20 latency, ms | 22.85 (1.81) [20.25–25.00] | 20.49 (1.10) [18.90–21.80] | 0.035* |
| N20-P25 amplitude, µV | 1.51 (0.73) [0.71–2.40] | 2.63 (0.91) [1.80–4.30] | 0.003* |
| *Statistically significant difference (p < 0.05). SD: standard deviation. |
| Table III. Changes in N20 latencies and N20-P25 amplitudes in stroke patients, with botulinum toxin treatment |
| | Pre-treatment Mean (SD) [Min–Max] | First month Mean (SD) [Min–Max] | Third month Mean (SD) [Min–Max] | p* |
| N20 latency, ms | 22.85 (1.81) [20.25–25.00] | 20.74 (0.73) [19.95–21.75] | 20.58 (2.34) [16.75–24.10] | 0.15 |
| N20-P25 amplitude, µV | 1.51 (0.73) [0.71–2.40] | 2.67 (1.66) [0.47–4.70] | 2.74 (1.88) [0.69–4.90] | 0.03** |
| *Overall p-values. **No statistically significance differences were observed in post hoc pair-wise comparisons with Bonferroni correction. SD: standard deviation. |
Fig. 1. The cortical somatosensory evoked potentials that resulted from electric stimulation of median nerve of the spastic limb (A) before, (B) after first month and (C) third months of botulinum toxin injection.
Discussion
In recent years, selective application of BoNT-A into spastic muscles has been shown to be an effective option in spasticity management. Many studies have demonstrated its efficacy in the treatment of spasticity due to stroke and shown that the functional ability of the upper limb has improved, while disability has reduced (4, 15–18).
Earlier studies have focused especially on the reduction in spasticity and motor gains with BoNT-A treatment. To the best of our knowledge, there are no published studies that focus on sensory functions, except for two studies documented in subjects other than hemiplegia. One study, by Park et al. (19), documented improvement in cortical SEPs with associated reduction in spasticity that occurred after BoNT-A injection in children with cerebral palsy. The other study, by Naumann & Reiners (20), investigated the long-latency reflexes of hand muscles in idiopathic focal dystonia and their modification by BoNT-A. This study documented a significant reduction in long-latency reflex 2 (LLR 2, occurring at ~50 ms) amplitudes on the clinically affected side with BoNT-A treatment. In our study, the N20 potential amplitude, which is related to especially proprioception, increased, and N20 latency decreased compared with its pretreatment levels, in accordance with the study of Park et al. However, the difference was not statistically significant, which may have been due to small sample size.
The perception of limb position and movement, termed “kinaesthesia”, requires 3 sources of input that are tactile, visual and proprioceptive (21). Goodwin et al. (22) demonstrated that the receptors for kinaesthesia are located in the muscles rather than in the joints, and muscle spindles, especially, are involved.
The muscle spindle has two different types of ending; primary and secondary. Primary endings respond to the size of a muscle length change and its speed (23), that probably contributes both to the sense of limb position and movement. Secondary endings respond only to the length change itself, so contribute only to the sense of position (21).
Signals of conscious proprioception coming from the proprioceptors are transmitted by the posterior column-medial lemniscus pathway to the cerebrum (24). The SEP is largely mediated via larger-diameter Ia sensory fibres in the peripheral nerve and dorsal column-medial lemniscal system in the CNS (25). N20 potentials show the neural activity of dorsal column-lemniscus medialis systems of the lemniscal pathways (8). Median nerve N20 potential alteration in stroke patients’ affected arms has been documented previously, and is consistent with the results of our study (5, 9, 12). Moreover, it has been demonstrated in our study that treatment with BoNT-A seems to improve N20 potentials.
Similarly, Park et al. (19) have documented improvement in cortical SEPs after BoNT-A injection in children with spastic palsy, with more frequent improvement observed in the younger age group. In another study, Pfeiffer et al. (26) showed that, after taking diazepam, the SEP waveforms improved as the spasticity decreased. These findings suggest that the neurophysiological characteristics underlying spasticity may partially contribute to the abnormal SEP responses in patients with spasticity (19).
As for the question of “how does the improvement occur?” our hypothesis is that in case of spasticity, holding the muscle in contracted/shortened length for a prolonged period of time, the muscle spindle will be less stretched, which reduces its signalling. As a result, proprioception deteriorates with prolonged spasticity. This means that treating spasticity not only reduces spasticity but also improves the proprioception that is under the burden of spasticity. This hypothesis can explain the improvement in N20 potentials with BoNT-A treatment.
This hypothesis is supported by a well-known phenomenon that SEPs are depressed during muscle contraction (27, 28) and abolished completely during strong muscle co-contraction (29). SEP depression during muscle contraction appears to be mediated through several central and peripheral gating mechanisms (29–31).
The term “gating” is the modulation of somatosensory information during its path from the periphery to the primary somatosensory cortex resulting in SEP attenuation during and before voluntary movement (29, 31, 32). Centrifugal gating is the suppression of SEPs carried out by inhibitory interaction between the given sensory signals and the efferent signals induced by the motor command from the motor-related areas. On the other hand, centripetal gating is the modulation exerted by interaction between the given sensory afferents and the afferent signals evoked by kinaesthesia (31).
Intramuscular BoNT-A affects not only the extrafusal motor endplates, but also causes paralysis of intrafusal muscle fibres (33), and hence reduces the group I spindle afferent discharge (34) and possibly also group II muscle afferent discharge (35). After toxin injection, the reduction in spindle afferent input to the spinal cord would lead to decreased tonic presynaptic gating (36). The substantial changes in afferent input could influence not only the spinal pathway (35), but also the higher central nervous system pathway (31, 37, 38).
Eventually, consistent with the results of Park et al. (19) the increase in SEP amplitudes with muscle and muscle spindle paralysis with BoNT-A is reasonable. Nevertheless, although the reduction in SEP latency after BoNT-A injection in our study is consistent with the results of Park et al. (19), the mechanism of the changes in cortical SEP latency remains a mystery. This difference may be related to the different neurophysiological background of spasticity (19), associated not only with centripetal gating, but also with centrifugal gating.
From the perspective of central neuroplasticity, after BoNT-A application the increase in sensory inputs to the central somatosensory system might lead to a new structuring in the parietal cortex. This change or remodelling in areas representative of the extremity in the cerebral cortex might explain the increase in SEP responses; N20-P25 amplitudes. Although we think that reduction in spasticity plays a major role in SEP improvement, since central and peripheral mechanisms are not single-acting, every effort under rehabilitation practice influences central activity. Lindberg et al. (39) have demonstrated that decreased cerebral activity during passive movements in the representation for the upper extremity with time after stroke can be reversed with training.
It has long been known that in the post-stroke period, important changes occur within a few weeks in the injured cerebral cortex area (40). Similarly, the changes in the early period of treatment with BoNT-A might be associated with neuroplastic changes in the cerebral cortex. All of our patients were late ischaemic stroke cases. The treatment was applied after the changes took place in the natural course of recovery. In addition, before and after application of BoNT-A, values of each patient were compared. Thus, the changes in the SEP values may be attributed to spasticity reduction, or to BoNT-A application.
Muscle spindles are the starting point in the proprioceptive functioning to the nervous system. Under the burden of spasticity, they might fail to function well, and proprioception may further deteriorate in addition to central injury, leading to clumsiness and inability to perform delicate and fine movements, and resulting in failure in stroke rehabilitation and a functionless upper extremity. The probable improvement in conscious proprioception, in particular, may lead to increased benefits of stroke rehabilitation above the pharmacological effect of BoNT-A treatment.
Although our sample size was small, such a finding may help to elucidate the mechanism of effect of BoNT-A in spasticity. However, further studies with larger patient series are needed to confirm these preliminary findings.
In conclusion, alterations in N20 potentials were determined in stroke patients. The improvement in median SEPs following BoNT-A treatment suggests that central somatosensory patterns in hemiplegia can be modified by peripheral inputs. This sheds new light on rehabilitation of hemiplegia cases treated with BoNT-A, not only with respect to motor recovery, but also for somatosensory re-education.
ACKNOWLEDGEMENTS
Financial disclosure. We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, if applicable, we certify that all financial and material support for this research (e.g. National Institutes of Health or National Health Service grants) and work are clearly identified in the title page of the manuscript.
References