OBJECTIVE: To investigate whether the direct application of vibratory stimuli inhibits spasticity in the hemiplegic upper limbs of post-stroke patients.
DESIGN: A randomized controlled study.
SUBJECTS: Thirty-six post-stroke patients.
METHODS: Patients were randomly allocated to the “Rest group”, “Stretch group”, or “Direct application of vibratory stimuli group”. After relaxing in a supine posture for 30 min, subjects received the interventions for 5 min. The Modified Ashworth Scale scores and F-wave parameters were recorded before, immediately after and 30 min after each intervention.
RESULTS: The Rest group showed no significant changes in F-wave parameters and Modified Ashworth Scale scores. The Stretch group showed a tendency to decrease in F-wave amplitude and F/M ratio immediately after the intervention, but not 30 min later. The Direct application of vibratory stimuli group showed significant improvements in F-wave parameters and Modified Ashworth Scale scores immediately after the intervention, which remained 30 min later. The changes in F-wave parameters and Modified Ashworth Scale scores observed in the Direct application of vibratory stimuli group significantly differed from those in the Rest group and the Stretch group.
CONCLUSION: The direct application of vibratory stimuli has anti-spastic effects in the hemiplegic upper limbs of post-stroke patients.
Key words: F-wave; Modified Ashworth Scale; muscle vibration; spasticity; stroke; vibration; vibratory stimulus.
J Rehabil Med 2011; 00: 00–00
Guarantor’s address: Shuji Matsumoto, Kirishima Rehabilitation Center, 3930-7 Takachiho, Makizono-cho, Kirishima City, Kagoshima 899-6603, Japan. E-mail: shushu@m.kufm.kagoshima-u.ac.jp
Submitted January 30, 2011; accepted November 14, 2011
Introduction
Spasticity is defined as a motor disorder characterized by a velocity-dependent increase in the tonic stretch reflex with exaggerated tendon reflexes (1). It is a hallmark of upper motor neurone lesions that is easy to identify, but difficult to quantify and to treat. In post-stroke patients, the prevalence of spasticity has been reported to be 19% after 3 months and 20% after 18 months (2, 3). The pathological increase in muscle tonus can lead to muscle shortening, abnormal posture, pain and activity limitations (4), which are major obstacles to the rehabilitation of hemiplegic patients who have had a stroke.
Several previous studies have investigated spasticity in hemiplegic patients with stroke, but found it difficult to quantify. Spasticity has generally been assessed clinically through physical examinations using measures such as the Modified Ashworth Scale (MAS). However, approaches based on electromyographic examination and biomechanical analyses of limb resistance to passive movement have recently been shown to have reliability and validity.
Although there is evidence of the short-term effects of conventional therapies, such as muscle stretching and thermotherapy, their long-term effects are unclear (5–7). Moreover, more recent approaches with proven long-term effects, such as the intrathecal application of baclofen (8) or injection of botulinum toxin (9), require practitioners to have specialized technical skills. Hence, there remains a need for a simple and efficacious therapeutic method to reduce spasticity during stroke rehabilitation.
Vibratory stimulation is useful for the treatment of motor disorders. Vibration stimulates the primary muscle spindle endings, causing Ia afferent impulses to be conducted to alpha motor neurones and Ia inhibitory interneurons in the spinal cord (10). This afferent pathway produces involuntary contraction in the vibrated muscle (that is, a tonic vibration reflex: TVR) (11), and inhibits the antagonist muscle. Vibratory stimulation is therefore traditionally applied to the antagonist of the spastic muscle, in order to decrease the spasticity of a hemiplegic limb (12, 13).
We found that vibratory stimulation applied directly to the spastic muscle of post-stroke patients with hemiplegia produced an initial intense contraction, followed by a suppression of spasticity after continuous stimulation for several minutes. We have reported previously on the efficacy of the direct application of vibratory stimulation (DAVS) in the treatment of spasticity (14). Our pilot data suggested that DAVS improved the pathological increases in muscle tonus and motor function in hemiplegic upper limbs. However, it was unclear from our before-and-after trial whether the anti-spastic activity of DAVS could be partly attributed to the effects of stretching.
The aims of the present study were to elucidate the anti-spastic effects of vibratory stimulation in DAVS for spastic hyper-reflexic patients using F-wave parameters and MAS scores.
Methods
Subjects
Thirty-six post-stroke patients with upper-limb spasticity were recruited from inpatients admitted to the Kirishima Rehabilitation Centre of Kagoshima University, Japan, between 28 April 2009 and 13 April 2010.
The inclusion criteria were as follows: 20–85 years of age; increased muscle tonus of the affected upper limb (MAS score ≥ 1); hemiplegia of the upper limb (Brunnstrom stage (15) 1–5); receiving no stimulant or relaxant medications (including anti-spasticity and anti-convulsion medications, and pharmacological injections); and normal latencies of F-waves and M-responses, indicating no peripheral nerve injury.
The exclusion criteria were as follows: onset of stroke < 4 weeks previously; abnormal upper-limb movements prior to onset of stroke; any medical condition preventing vibratory stimulation (such as severe cardiopulmonary disease or severe sensory disturbance (light touch test and position test for upper limb of Stroke Impairment Assessment Set (16) = 0)); severe aphasia that made it impossible to follow verbal instructions; bilateral hemisphere lesions; and dementia that interfered with the outcome assessments.
Stroke diagnosis was based on computed tomography (CT) or magnetic resonance imaging (MRI), as well as neurological functions. The study was conducted without altering the existing medication regimes of the patients. The procedures complied with the 1975 Declaration of Helsinki, as revised in 1983. Informed consent was obtained from each subject according to the ethical guidelines of the hospital, once they fully understood the purpose and methodology. The study was approved by the ethics committee of Kagoshima University.
Experimental procedure
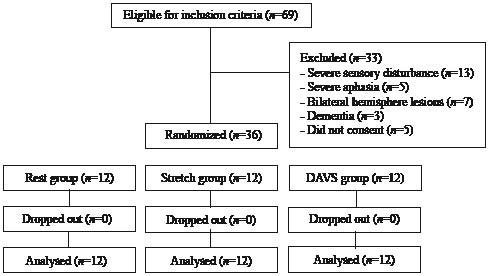
Subjects who satisfied the inclusion criteria were randomly allocated to the Rest group, the Stretch group or the DAVS group (Fig. 1). After relaxing for 30 min in the supine posture, subjects received the interventions for 5 min. The electromyographic responses (F-wave) and the MAS scores were recorded before (pre), immediately after (post 1) and 30 min after (post 2) each intervention.
Fig. 1. Trial flow-chart. DAVS: direct application of vibratory stimuli.
Interventions
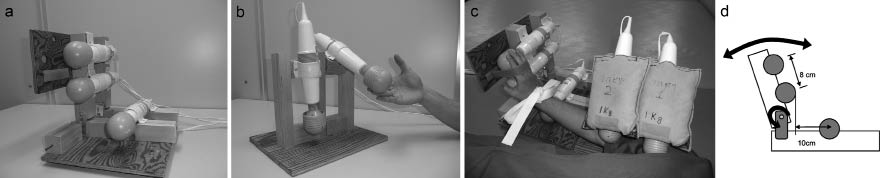
The subjects in the Rest group lay in a relaxed, supine posture for 5 min. The subjects in the Stretch group lay in a supine position with maximal extension of the elbow, wrist and finger joints, using a hand-and-forearm stimulation device without vibration, for 5 min (Fig. 2a). The subjects in the DAVS group used both a hand-and-forearm stimulation device (Fig. 2a), and an upper-arm stimulation device (Fig. 2b), developed by the authors. These consisted of 3 or 2 vibrators, respectively, a wooden frame and a cloth strap for fixation to the upper limb. Each vibrator had a spherical, rubber, vinyl-covered head (diameter = 5 cm), a frequency of 91 Hz and an amplitude of 1.0 mm (Thrive MD-01; Thrive Co., Ltd, Osaka, Japan). For the hand-and-forearm stimulation device (Fig. 2a), the wrist and metacarpophalangeal joint were fixed to the frame in the maximal extension position (Fig. 2c). The device has movable joints to adjust to the limitations of the range of motion in the wrist (Fig. 2d). Vibrations were delivered to the abdominal side of all fingers, the palm and the flexor tendon of the wrist (Fig. 2c).
For the upper-arm stimulation device, 2 vibrators were set 12-cm apart in a movable frame (Fig. 2b). A 1-kg sandbag was placed on each vibrator, to ensure that the belly of the biceps brachii muscle was stimulated with a constant force (equal pressure). Each subject lay in a supine position and all of the flexor muscles of the upper limb were stimulated simultaneously by the devices (Fig. 2c).
Fig. 2. Vibratory stimulation device. (a) Hand-and-forearm stimulation device. (b) Upper-arm stimulation device. (c) Combined vibratory stimulation devices. (d) Mobility of hand-and-forearm stimulation device.
Electromyographic examination: F-wave study
The F-wave measured by electromyographic examinations could be an indicator of alpha motor-neurone excitability (6, 17), and alterations of F-wave parameters in spasticity have been confirmed in experimental animals (18). Several authors have reported that the F-wave is larger in patients with spasticity than in healthy subjects (18–20, 21), and is more sensitive to changes in lower motor-neurone excitability than both the T-reflex and the H-reflex (19, 20, 22).
Each subject lay in a supine position with both arms supported in a relaxed position. A one-channel recording from the abductor pollicis brevis (APB) allowed instantaneous comparison of simultaneously evoked F-waves in the spastic muscle, to evaluate the effect of vibratory stimulation (18).
We used a Nihon-Kohden Neuropack with a band-pass filter of 10 Hz to 10 kHz, and a sensitivity of 5 mV and 200 mV/division, to record compound muscle action potentials (CMAPs) and F-waves, respectively. Paired silver (Ag)–silver chloride (AgCl) surface electrodes were taped to the belly and tendon of the APB after lowering the skin resistance to < 5 kΩ. The median nerve was stimulated at 1 Hz with a rigid bar electrode strapped securely to the wrist, and the cathode was positioned 3 cm proximally from the most distal wrist crease. Stimuli were 0.1 ms in duration, and ranged from 10 to 50 mA when set at an intensity 20% higher than that which elicited the largest CMAP (19, 20). One hundred F-waves were recorded following supramaximal current pulses. A stainless-steel surface electrode placed on the dorsum of each subject’s hand served as a ground electrode.
Peak-to-peak measurements were made of the M-response amplitude, and that of the 100 averaged F-responses (the F-wave amplitude), for each upper limb before and after the interventions. In addition to recording the F-wave and M-response amplitudes, we calculated the ratio of the F-wave amplitude to the M-response amplitude (the F/M ratio) and the F-wave persistence.
Measurement of muscle tonus
The extent of spasticity was measured using the MAS (23) for the biceps brachii, wrist flexor muscles and finger flexor muscles. The MAS is an established and reliable instrument, which uses a 6-point scale to score the average resistance to passive movement for each joint. To facilitate data analysis, the MAS scores (0, 1, 1+, 2, 3 and 4) were assigned numerical values designated as “computed MAS scores” (0, 1, 2, 3, 4 and 5, respectively) (24). Changes in MAS scores were calculated by the subtraction method. The evaluator was a trained physiotherapist who was blinded for the subject.
Statistical analysis
Non-parametric statistics were used for the analyses, since not all data met the criterion of normality. The effects over time of the interventions on the F-wave parameters and the MAS scores were evaluated using the Friedman test with the post-hoc Bonferroni-corrected Wilcoxon test (number of comparisons = 3). Between-group differences in the F-wave parameters and MAS scores were analysed using the Kruskal-Wallis test and the Bonferroni-corrected Mann-Whitney U test (number of comparisons = 3).
All statistical analyses were performed using IBM SPSS Statistics 18.0 (SPSS Inc., Chicago, IL, USA). A conservative level of significance with a value of 0.05/n was chosen by the Bonferroni correction, where n was the number of comparisons that were made in all post-hoc tests. Probability (p) values of the other statistical tests below 0.05 were considered statistically significant.
The strength, or magnitude, of our findings was determined by calculating the effect size r (25). According to Cohen (25), r = 0.10 is a small treatment effect, r ≥ 0.30 represents a moderate effect, and r ≥ 0.50 is a large effect.
Results
None of the subjects experienced discomfort before, during or after the 3 interventions. The electromyographic and physical examinations were completed safely in all subjects.
Patient baseline characteristics
The demographic and clinical characteristics were comparable for the 12 participants in the Rest group (median age 61 (range 27–83) years, 8 males and 4 females), the 12 participants in the Stretch group (median age 61.5 (range 41–83) years, 9 males and 3 females) and the 12 participants in the DAVS group (median age 57.5 (range 38–83) years, 8 males and 4 females) (Table I). The baseline values for the F-wave amplitude, F/M ratio, F-wave persistence, M-response, MAS score for elbow flexor muscles and MAS score for wrist flexor muscles were comparable (Table II).
| Table I. Baseline characteristics of patients |
| | Rest group (n = 12) | Stretch group (n = 12) | DAVS group (n = 12) |
| Age, years, median (range) | 61 (27–83) | 61.5 (41–83) | 57.5 (38–83) |
| Sex, male/female, n | 8/4 | 9/3 | 8/4 |
| Hemiplegic side, right/left, n | 4/8 | 5/7 | 7/5 |
| Time since onset of stroke, weeks, median (range) | 16 (7–139) | 16.5 (8–291) | 21.5 (8–156) |
| Brunnstrom stage of upper extremity, median (range) | 3 (2–5) | 3 (2–5) | 4 (2–5) |
| Brunnstrom stage of hand, median (range) | 4 (2–5) | 3 (2–5) | 3.5 (2–5) |
| DAVS group: direct application of vibratory stimuli group. |
| Table II. Baseline values of F-wave parameters and MAS scores |
| | Rest group (n = 12) | Stretch group (n = 12) | DAVS group (n = 12) |
| F-wave amplitude, µV, median (range) | 482.3 (195.0–1,951.0) | 563.0 (205.0–1097.0) | 582.0 (232.5–827.0) |
| F/M ratio, %, median (range) | 4.3 (2.9–18.6) | 5.2 (2.9–13.2) | 5.5 (1.6–5.5) |
| F-wave persistence, %, median (range) | 92.0 (76.0–100.0) | 95.0 (76.0–100.0) | 92.0 (80.0–96.0) |
| M-response, mV, median (range) | 11.0 (2.3–18.7) | 11.0 (5.7–18.0) | 11.0 (5.9–19.1) |
| MAS at elbow flexor, median (range) | 1.0 (0.0–3.0) | 1.0 (1.0–3.0) | 1.0 (0.0–3.0) |
| MAS at wrist flexor, median (range) | 2.0 (1.0–3.0) | 2.0 (0.0–4.0) | 2.0 (0.0–3.0) |
| DAVS group: direct application of vibratory stimuli group; MAS: Modified Ashworth Scale. |
F-waves
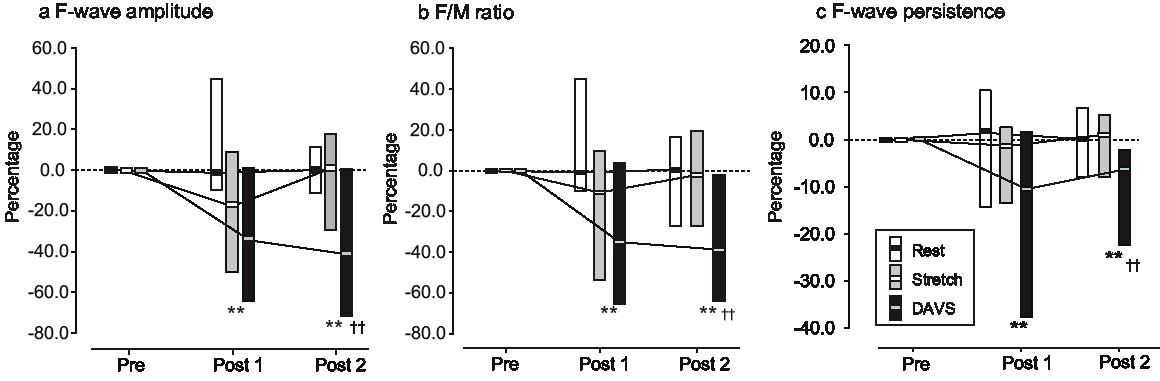
Fig. 3 shows the changes in F-wave parameters after each intervention. The Friedman test showed significant differences over time in F-wave amplitude (the Stretch group, p = 0.018; and the DAVS group, p = 0.002), F/M ratio (the Stretch group, p = 0.046; and the DAVS group, p = 0.001) and F-wave persistence (the DAVS group, p < 0.001), whereas no significant differences were observed in the Rest group.
Fig. 3. Percentage changes in F-wave parameters. The percentage changes in: (a) F-wave amplitude, (b) F/M ratio and (c) F-wave persistence are shown (post 1 and post 2). Values are medians and ranges. Significant differences between the DAVS group and the Rest group are indicated at **p < 0.01. Significant differences between the DAVS group and the Stretch group are indicated at ††p < 0.01.
The Kruskal-Wallis test showed significant differences over time among the 3 groups in F-wave amplitude (post 1, p = 0.001; and post 2, p < 0.001), F/M ratio (post 1, p = 0.001; and post 2, p < 0.001) and F-wave persistence (post 1, p = 0.001; and post 2, p < 0.001). However, no significant differences were found for the M-responses.
The post-hoc test revealed a significant decrease in the DAVS group in F-wave amplitudes (pre vs post 1, p = 0.003, r = –0.86; and pre vs post 2, p = 0.003, r = –0.86), F/M ratios (pre vs post 1, p = 0.003, r = –0.86; and pre vs post 2, p = 0.002, r = –0.88) and F-wave persistence (pre vs post 1, p = 0.003, r = –0.86; and pre vs post 2, p = 0.002, r = –0.89). In the Stretch group, the post-hoc test indicated a tendency to decrease at post 1 compared with pre (F-wave amplitudes, p = 0.026, r = –0.64; F/M ratios, p = 0.041, r = –0.59), but it indicated no significant differences at post 2 compared with pre (F-wave amplitudes, p = 0.666, r = –0.13; F/M ratios, p = 1.0, r = 0.0). In the Rest group, no significant differences were observed in any F-wave parameters during any sessions.
The DAVS group (post 1, p < 0.001, r = –0.76; and post 2, p < 0.001 r = –0.76) demonstrated significant decreases in F-wave amplitudes compared with the Rest group, whereas the DAVS group (post 2, p < 0.001, r = –0.74) showed a significant decrease in F-wave amplitude compared with the Stretch group. The DAVS group (post 1, p < 0.001, r = –0.76; and post 2, p < 0.001 r = –0.76) demonstrated significant decreases in F/M ratio compared with the Rest group, whereas the DAVS group (post 2, p = 0.001, r = –0.70) showed a significant decrease in F/M ratio compared with the Stretch group. The DAVS group (post 1, p = 0.001, r = –0.66; and post 2, p = 0.001, r = –0.69) demonstrated significant decreases in F-wave persistence compared with the Rest group, whereas the DAVS group (post 2, p = 0.001, r = –0.71) showed significant decreases in F-wave persistence compared with the Stretch group.
MAS scores
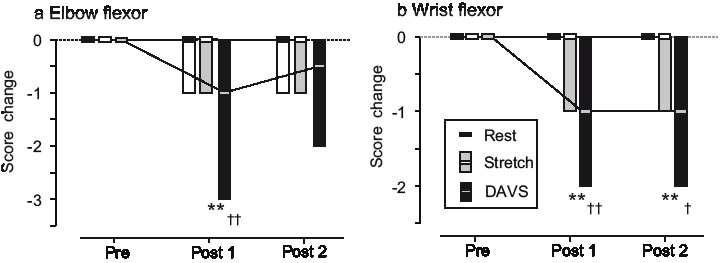
Fig. 4 shows the changes in MAS scores for elbow flexor muscles and wrist flexor muscles after each intervention. The Friedman test showed significant differences over time in the MAS scores for elbow flexor muscles (p = 0.03) and wrist flexor muscles (p = 0.009) in the DAVS group, whereas no significant differences were observed in the Rest group and the Stretch group. The Kruskal-Wallis test showed significant differences over time among the 3 groups in the MAS scores for elbow flexor muscles (post 1, p < 0.001; and post 2, p < 0.001) and wrist flexor muscles (post 1, p < 0.001; and post 2, p < 0.001).
Fig. 4. Modified Ashworth Scale (MAS) score changes. The MAS score changes for (a) the elbow flexor and (b) the wrist flexor following the 3 interventions are shown. Values are medians and ranges. Significant differences between the DAVS group and the Rest group are indicated at **p < 0.01. Significant differences between the DAVS group and the Stretch group are indicated at †p < 0.05 and ††p < 0.01.
The post-hoc test revealed decreases in the MAS scores for elbow flexor muscles (pre vs post 1, p = 0.008, r = 0.80) and wrist flexor muscles (pre vs post 1, p = 0.005, r = 0.88; and pre vs post 2, p = 0.012, r = –0.78) after DAVS.
The DAVS group demonstrated a significant decrease in the MAS score for elbow flexor muscles compared with the Rest group (post 1, p = 0.008, r = –0.59). It also demonstrated significant decreases in the MAS scores for elbow flexor muscles compared with the Stretch group (post 1, p = 0.008, r = 0.56), and for wrist flexor muscles compared with the Rest group (post 1, p < 0.001, r = –0.82; and post 2, p = 0.005, r = –0.69) and the Stretch group (post 1, p = 0.004, r = –0.65; post 2, p = 0.014, r = –0.50).
Discussion
We compared the efficacy of DAVS with that of stretching the spastic muscles or the resting condition using randomized controlled methods. Changes in spasticity were assessed using F-wave parameters and MAS scores. Our results showed reductions in F-wave amplitude, F/M ratio and F-wave persistence after DAVS, as well as improvements in the MAS scores for elbow and wrist flexor muscles. There were statistically significant differences between the DAVS group and the two control groups. Meanwhile, the anti-spastic effect of DAVS in the MAS scores for elbow flexor muscles weakened after 30 min, whereas the effect on F-wave parameters and the MAS scores for wrist flexor muscles remained after 30 min. The Stretch group showed a tendency to decrease in F-wave parameters immediately after stretching, but this tendency disappeared after 30 min.
One-point reductions in the MAS scores, as shown by the DAVS group in the present study, were previously reported to improve the motor functions in post-stroke patients (26). Furthermore, the F-wave parameters showed equivalent reductions to those in our previous pilot study, which indicated improved motor function (i.e. finger tapping or active range of motion at wrist) (14). These findings suggest that the anti-spastic effects of DAVS in the present study had clinical significance.
The current randomized, controlled trial investigated the anti-spastic effects of DAVS. Stretching is commonly used in the physical management of spasticity, and there is evidence to support its immediate effects (27). Prolonged stretching (such as standing on a tilt table or splinting) has a particularly notable effect (22, 28). However, the anti-spastic effects of stretching in the present study were observed only immediately after treatment, probably due to the short duration (5 min) of the intervention. These findings indicated that the effects of DAVS were not attributable to the benefits of stretching.
We used F-wave parameters and MAS scores to measure spasticity. In the methodology of F-wave evaluations, it remains controversial whether F-wave parameters recorded from the APB indicate the spasticity of the whole upper limb. Generally, F-wave parameters are recorded from the APB in cases of median nerve stimulation and from the abductor digiti minimi muscle in cases of ulnar nerve stimulation (20, 29). The current study used methodologies of previous studies, for example, investigations into the effects of physiotherapy (30), drugs (31) and various physiological conditions (32). On the other hand, although the MAS is frequently used to assess spasticity, the adequacy of this is not conclusive. Some studies have revealed strong associations between MAS scores and objective measures of resistance to passive movement (28, 33). Others do not recommend using this assessment because of an absence of validity and reliability (34). In the current study, the first outcome measure for the assessment of spasticity was the electromyographic examination using F-wave parameters and the second outcome measure was MAS.
In this study, we observed significant correlations between the F-wave parameters and the MAS scores (0.201 < r < 0.568, p < 0.05). However, F-wave parameters showed a tendency to decrease in the stretch group immediately after stretching, but this was not observed in the MAS scores. A difference in sensitivity is therefore likely between F-wave assessments and MAS scores.
DAVS treatment is intended to apply multiple vibratory stimuli simultaneously to the fully stretched spastic muscles of upper limbs. The vibratory stimuli initially produce intense contraction (known as TVR) of the spastic muscles. After continuous stimulation for several minutes, the contraction derived from TVR disappears and the spasticity is suppressed for more than 30 min without stimuli (14). However, care must be taken, because some patients can experience increased muscle tonus caused by the vibratory stimuli, especially during the initial period of treatment. In addition, there is a possibility that prolonged stimulation might cause burns to the skin due to friction heat. In the present study, none of the subjects reported excessive vibratory stimulation or discomfort. We therefore believe that by using a comfortable intensity and frequency of vibration, and optimizing the duration of stimulation, the potential side-effects can be minimized.
The mechanism by which DAVS might suppress TVR and reduce spasticity is unclear. In a previous study, the T-reflex and H-reflex were inhibited by the spinal mechanism of presynaptic inhibition induced by vibratory stimulation in healthy subjects (11). However, muscle vibration had little or no effect on the monosynaptic reflex in spastic limbs, because presynaptic inhibition was depressed in these patients (35). It was therefore unlikely that presynaptic inhibition was the primary origin of the DAVS suppression.
In addition, changes to motor-evoked potentials (MEPs) elicited by TMS were reported when vibration was applied to peripheral muscles, which suggested increased motor cortex excitability (36). The alteration of motor cortex excitability could thus potentially be involved in the anti-spastic activity of DAVS.
Several limitations of the present study should be acknowledged. First, it included only a small number of participants, therefore future studies with a larger number of participants are needed to confirm our results. Secondly, only assessments for spasticity were made in this study, thus future studies should evaluate motor function, activity limitation and changes in quality of life to explain the contribution to stroke rehabilitation. Finally, it is unclear how long anti-spastic effects last after DAVS. We showed that F-wave persistence and MAS scores for elbow flexor muscles did not extend beyond 30 min, but further investigations are necessary to confirm the duration of DAVS in a long-term follow-up study.
In conclusion, the results of this study provide good evidence of the anti-spastic effects of vibratory therapy in the hemiplegic upper limbs of post-stroke patients, and indicate that the efficacy of DAVS cannot be attributed to the effects of stretching.
REFERENCES