Jan Mehrholz, PT, PhD and Marcus Pohl, MD
From the Department of Early Rehabilitation, Klinik Bavaria, Kreischa, Germany
Jan Mehrholz, PT, PhD and Marcus Pohl, MD
From the Department of Early Rehabilitation, Klinik Bavaria, Kreischa, Germany
OBJECTIVES: Although electromechanical-assisted gait training after stroke seems to be effective, in the absence of a direct comparison between electromechanical devices it is not clear which device may be the most effective for recovery of walking. The aim of this study was therefore to compare the effects of different devices used in gait training after stroke.
DATA SOURCES: We searched the Cochrane Stroke Group Trials Register, CENTRAL, MEDLINE, EMBASE, CINAHL, AMED, SPORTDiscus, PEDro, COMPENDEX and INSPEC. In addition, we hand-searched relevant conference proceedings, trials and research registers, checked reference lists and contacted authors to identify further trials.
STUDY SELECTION: Randomized studies were included. Authors independently selected trials for inclusion, assessed trial quality and extracted the data.
DATA EXTRACTION: Data were extracted with the help of a standardized data extraction form.
DATA SYNTHESIS: Data were pooled for meta-analysis. The primary outcome was the proportion of patients walking independently.
RESULTS: We included 18 trials involving 885 patients. We found significantly higher rates of independent walking in end-effector compared with exoskeleton-based training (p = 0.03). Complication rates in both groups were comparable. CONCLUSION: The results suggest that the type of electromechanical-assisted device might influence the outcome of gait rehabilitation after stroke.
Key words: stroke; exercise; walking; rehabilitation.
J Rehabil Med 2012; 44: 193–199
Correspondence address: Jan Mehrholz, Wissenschaftliches Institut, Klinik Bavaria, An der Wolfsschlucht 1-2, DE-01731 Kreischa, Germany. E-mail: jan.mehrholz@klinik-bavaria.de
Submitted July 27, 2011; accepted November 23, 2011
Introduction
Electromechanical-assisted gait training and treadmill training, with and without partial body weight support (1), are used as adjuncts to overground gait training for the rehabilitation of patients after stroke (2). Automated electromechanical gait machines consist either of a robot-driven exoskeleton orthosis (3) or an electromechanical solution with two driven foot-plates simulating the phases of gait (4). The main difference between electromechanical-assisted and treadmill training is that the process of gait training is automated and supported by an electromechanical solution. Electromechanical devices for automated-assistive walking training can be differentiated into end-effector and exoskeleton devices. Examples of end-effector devices are the “G-EO-System” (5), the “Lokohelp” (6), the “Haptic Walker” (7), and the “Gait Trainer GT 1” (4). The definition of an end-effector principle is that a patient’s feet are placed on foot-plates, whose trajectories simulate the stance and swing phases during gait training (5). Examples of the exoskeleton type of device are the “LOPES” (Lower Extremity Powered Exoskeleton) (8) and the “Lokomat” (3). Such exoskeletons are outfitted with programmable drives or passive elements, which move the knees and hips during the phases of gait (5).
Electromechanical devices can be used to give non-ambulatory patients intensive practice (in terms of high repetitions) of complex gait cycles with a reduced effort for therapists, as they no longer need to set the paretic limbs or assist trunk movements (9).
Although there is evidence of a beneficial effect of electromechanical devices for gait rehabilitation after stroke (10), there are no studies that directly compare the effects of different types of electromechanical devices.
The main objective of the present study was to compare the effects of end-effector and exoskeleton devices used in electromechanical-assisted gait training after stroke in a systematic review with pooled analysis.
Methods
This study followed a published systematic protocol (11) and was undertaken according to Cochrane Guidelines (12) and followed the checklist in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (13).
Data sources
We searched the Cochrane Stroke Group Trials Register, CENTRAL, MEDLINE, EMBASE, CINAHL, AMED, SPORTDiscus, PEDro, COMPENDEX and INSPEC (our search words can be found in Appendix I). In addition, we hand-searched relevant conference proceedings, searched trials and research registers, checked reference lists and contacted authors in an effort to identify further published, unpublished and ongoing trials. Our final search was completed on March 2011.
Study selection
The following studies were included: (i) those with participants of any gender over 18 years of age after stroke; (ii) all randomized controlled trials that evaluated electromechanical- and robotic-assisted gait training plus physiotherapy vs physiotherapy (or usual care) for regaining and improving walking after stroke; (iii) studies of automated electromechanical devices used in combination with functional electrical stimulation applied to the legs during gait training. Automated electromechanical devices were defined as any device with an electromechanical solution designed to assist stepping cycles by supporting body weight and automating the walking therapy process in patients after stroke. This category included any mechanical or computerized device designed to improve walking function. The following interventions and trials were excluded: (i) non-weight-bearing interventions, such as non-interactive devices that delivered continuous passive motion only (14); (ii) trials testing the effectiveness of treadmill training or other approaches, such as repetitive task training in physiotherapy or electrical stimulation alone.
The primary outcome was defined as the ability to walk independently at study end.
The ability to walk was measured with the Functional Ambulation Category (FAC) (15). A FAC score of 4 or 5 indicated independent walking over a 15-m surface irrespective of aids used (such as a cane) and were defined as “event”. An “event” therefore represented the ability to walk independently. A FAC score of less than 4 indicates dependency in walking (supervision or assistance, or both, must be given in performing walking) and were defined as a “non-event”. A “non-event” therefore represented the inability to walk independently.
If FAC scores were not reported in the included studies we used alternative indicators of independent walking, such as: a score of 3 on the ambulation item of the Barthel Index (BI) (16); or a score of 6 or 7 for the walking item of the Functional Independence Measure (FIM) (17); or a “yes” response to the item “walking inside, with an aid if necessary (but with no standby help)” or “yes” to “walking on uneven ground” in the Rivermead Mobility Index (RMI) (18). We contacted all study investigators and requested information regarding walking ability status at study onset and study end.
We defined number of drop-outs during the intervention phase of a study as a measure of “acceptability” of use of electromechanical-assisted gait training devices. The risk of patients dropping out during a study was calculated and pooled.
We included only studies that used random assignment. Two review authors independently selected trials for inclusion, assessed trial quality and extracted the data (for a flow-chart see Fig. 1). The primary outcome was the proportion of patients walking independently at follow-up.
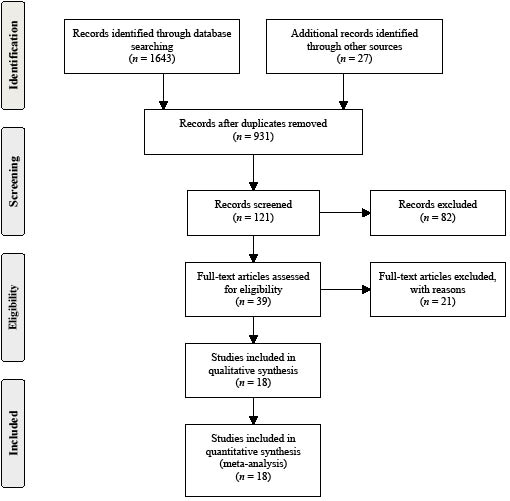
Fig. 1. Search and screening process.
Data extraction
The review authors independently read the titles and abstracts of the identified references and eliminated obviously irrelevant studies and independently ranked these studies as relevant, irrelevant or possibly relevant. Both review authors independently extracted trial and outcome data from the selected trials (as shown in Fig. 2).
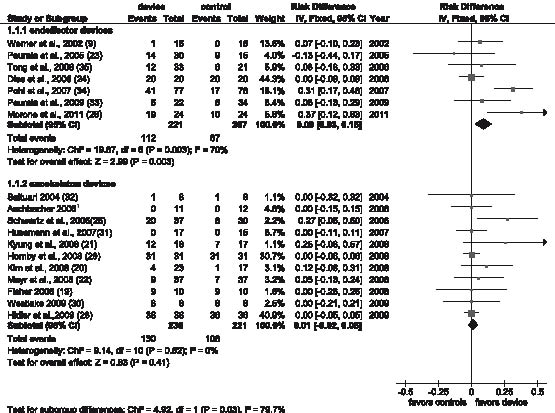
Fig. 2. Independent walking rates (independent walkers were defined as “events”) at the end of the intervention phase and comparison of the effects between electromechanical devices used (end-effector vs exoskeleton devices). CI: confidence interval; df: degrees of freedom.
1Aschbacher B. Comparing gait training in patients after stroke with task oriented physiotherapy or robot-assisted treadmill training a feasibility study. Lokomat-Symposium, 2006, Oktober 6th, Zürich, Suisse, unpublished conference presentation.
We analysed the binary outcomes with an odds ratio (OR) fixed-effect model with 95% confidence intervals (CI). We performed a formal subgroup analysis using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (12), comparing patients after stroke treated with end-effector and exoskeleton devices. As some trials had no events, we calculated risk differences (RD) instead of ORs in this specific situation, with 95% CI.
Results
Description of studies
A total of 18 trials, involving 885 patients, were included in the review. All included studies investigated the effect of automated electromechanical- or robotic-assisted gait training devices in improving walking after stroke. Some of the included studies have only been published as abstracts (19–22) or were presented at conferences (23), but we obtained at least some results for these unpublished trials through correspondence with the trial co-ordinator or principal investigator.
The mean age in the included studies ranged from 53 years (24) to 69 years (25). There were more males than females (63% males), more patients with ischaemic stroke than haemorrhagic stroke lesions (70% ischaemic) and a comparable proportion of patients with left- or right-sided hemiparesis (51% left-sided) included in the studies.
The duration of study ranged from 10 days (20) to 8 weeks (22) or 9 weeks (26, 27); but most studies used a 4-week study period. Nine out of 18 studies included at least some patients who could walk independently at study onset (19–21, 23, 24, 27–30).
Ten studies investigated end-effector based devices, such as the robotic-assisted (exoskeleton) device “Lokomat” as the experimental intervention (20–23, 25, 28–32), 7 studies investigated the (end-effector) device “Gait Trainer GT I” (9, 23, 24, 26, 33–35) and one study the robotic-assisted (exoskeleton) device “AutoAmbulator” (19). An overview of studies and devices used is presented in Table I. A more detailed description of all studies, including primary and secondary outcomes for each trial and methodological quality of studies, has been described elsewhere (10).
| Table I. Overview of included studies | ||||||||
| Studies | Device | Subjects n | Age, years Mean | Time since stroke | Severity | Outcome variable(s) for walking | Treatment duration | Additional therapy |
| End-effector studies (reference) | ||||||||
| Dias et al., 2007 (24) | Gait trainer | 40 | 69 | 47 months | 34 Barthel Index points | FAC, 10-m walking test and gait cycle parameters, Time up and Go test, 6-min walking distance test, Step test | 4 weeks | – |
| Morone et al., 2011 (26) | Gait trainer | 48 | 61 | 20 days | 60 Barthel Index points | FAC | 12 weeks | – |
| Peurala et al., 2005 (23) | Gait trainer | 45 | 52 | 3 years | Scandinavian Stroke Scale 42 points | 10-m walk test, 6-min walk test, FAC | 3 weeks | – |
| Peurala et al., 2009 (33) | Gait trainer | 56 | 68 | 8 days | Unclear | FAC, 10-m walk test, 6-min walk test | 3 weeks | Electrical stimulationa |
| Pohl et al., 2007 (34) | Gait trainer | 155 | 63 | 4 weeks | 37 Barthel Index points | FAC, walking velocity, walking endurance | 4 weeks | – |
| Tong et al., 2006 (35) | Gait trainer | 54 | 68 | 3 weeks | 51 Barthel Index points | FAC, 5-m walking speed | 4 weeks | Electrical stimulationa |
| Werner et al., 2002 (9) | Gait trainer | 30 | 60 | 7 weeks | 38 Barthel Index points | FAC, fast walking speed over 10 m | 2 weeks | – |
| Exoskeleton studies (reference) | ||||||||
| Aschbacher, 20061 | Lokomat | 23 | 61 | ≤ 3 months | Unclear | Walking velocity, step length, endurance, walking ability (FAC) | 3 weeks | – |
| Fisher, 2008 (19) | AutoAmbulator | 20 | Unclear | Less than 12 months | Unclear | Gait test portion of Tinetti’s Balance and Mobility assessment, 3-min walk, 25-foot walk | 24 sessions | – |
| Hidler et al., 2009 (28) | Lokomat | 72 | 57 | 125 days | Unclear | Self-selected walking speed over 5 m, walking distance in 6 min, FAC | 24 sessions | – |
| Hornby et al., 2008 (29) | Lokomat | 62 | 57 | 62 months | Unclear | Self-selected walking speed, 6-min walk test, modified Emory Functional Ambulation Profile | 12 sessions | – |
| Husemann et al., 2007 (31) | Lokomat | 32 | 59 | 12 weeks | 35 Barthel Index points | FAC | 4 weeks | – |
| Kim et al., 2008 (20) | Lokomat | 40 | Unclear | Less than 3 months | Unclear | FAC | 10 days | – |
| Kyung & Kim, 2008 (21) | Lokomat | 35 | Unclear | Unclear | Unclear | FAC, gait speed | 4 weeks | – |
| Mayr et al., 2007 (22) | Lokomat | 74 | Unclear | Between 10 days and 6 months | Unclear | Modified Emory Functional Ambulatory Profile, Hochzirl Walking Aids Profile, Rivermed Motor Index, Mobility Milestones | 8 weeks | – |
| Saltuari, 2004 (32) | Lokomat | 16 | 61 | 12 weeks | Unclear | EU-Walking Scale, RMA, 10-m timed walking speed, 6-min timed walking distance | 2 weeks | – |
| Schwartz et al., 2010 (25) | Lokomat | 67 | 63 | 22 days | 10 points NIHSS at entry | FAC, gait velocity, 2-min walk test, Time Up and Go Test | 6 weeks | – |
| Westlake & Patten, 2009 (30) | Lokomat | 16 | 57 | 40 months | Unclear | Self-selected and fast walking speed, 6-min walk test | 4 weeks | – |
| aIn 2 studies (33, 35) additional functional electrical stimulation of leg muscles during gait training was applied in 1 of the treatment groups. Since functional electrical stimulation was carried out as an adjunct during electromechanical-assisted gait training and the results of these experimental groups did not differ significantly, we combined the results of both experimental groups in 1 (collapsed) group and compared this with the results of the control group. FAC: Functional Ambulation Categories; RMA: Rivermead Motor Assessment Scale. 1Aschbacher B. Comparing gait training in patients after stroke with task oriented physiotherapy or robot-assisted treadmill training a feasibility study. Lokomat-Symposium, 2006, Oktober 6th, Zürich, Suisse, unpublished conference presentation. | ||||||||
Comparison 1: Independent walking at the end of intervention phase, comparison between electromechanical devices used (end-effector vs exoskeleton devices). The characteristics of patients included in studies investigating end-effector and exoskeleton devices are shown in Table II. Seven trials with a total of 428 patients used an end-effector device; 11 trials with a total of 457 patients used an exoskeleton device (see overview of studies included in Table II and Fig. 2).
| Table II. Patient characteristics in included studies. Table shows pooled values, from all included studies; n = (pooled) number of subjects in the different groups | |||||
| End-effector studies | Exoskeleton studies | ||||
| Experimental group | Control group | Experimental group | Control group | ||
| Total, n | 241 | 217 | 243 | 227 | |
| Age, years, mean (SD) | 63.3 (10.0) | 62.6 (10.3) | 59.2 (12.8) | 58.2 (12.8) | |
| Time post-stroke, months, mean (SD) | 11.6 (13.7) | 14.3 (22.7) | 12.9 (10.9) | 14.6 (13.8) | |
| Males, % | 68 | 62 | 64 | 62 | |
| Right-sided paresis, % | 47 | 47 | 59 | 62 | |
| SD: standard deviation. | |||||
In the end-effector subgroup significantly fewer patients were walking independently at study onset compared with the exoskeleton subgroup (78 of 428 patients, 18.2% vs 286 of 457 patients, 62.6%, respectively, χ2 = 179.6, degrees of freedom (df) = 1, p < 0.001). The intensity and frequency of therapy provided in the studies was comparable between the subgroups; this has been as described in detail elsewhere (10).
In the end-effector subgroup the test for an overall effect for achieving independent walking was statistically significant (risk difference, RD = 0.09, 95% CI: 0.03–0.15; Z = 2.99; p = 0.003; as shown in Fig. 2), but in the exoskeleton subgroup the test for an overall effect was not significant (RD = 0.01, 95% CI: –0.02 to 0.05; Z = 0.83, p = 0.41; Fig. 2). The subgroup comparison between end-effector and exoskeleton subgroup showed statistically significant differences (test for subgroup differences: χ2 = 4.92, df = 1, p = 0.03).
Comparison 2: Acceptability of devices during the intervention phase (acceptability defined as the risk of drop-out during intervention phase), comparison between electromechanical devices used (end-effector vs exoskeleton devices). The calculated risk differences for drop-out during intervention phase were not statistically significant (RD = –0.01, 95% CI: –0.05 to 0.03, p = 0.70 and RD = –0.06, 95% CI: –0.15 to 0.03, p = 0.17, respectively). The subgroup comparison showed no statistically significant risk differences between the device groups for drop-out during intervention phase (test for subgroup differences: χ² = 1.08, df = 1, p = 0.30). In addition, in both the end-effector subgroup and the exoskeleton subgroup the risk of adverse events and complications were rare.
Discussion
In the absence of a direct empirical comparison between electromechanical-assisted gait training devices, our results provide evidence that walking recovery after stroke may depend on the types of training devices. While an existing Cochrane Review has demonstrated benefits associated with electromechanical-assisted gait training after stroke (10), we have investigated differences between types of electromechanical training devices.
Our method of secondary analysis of data may be challenged because we pooled together studies comparing an electromechanical device group with a control group, but we believe that, in the absence of a direct empirical comparison between different devices, our results provide the best available evidence.
Secondary analyses of rehabilitation trials are often challenged because the control group does not receive the same intensity of intervention as the experimental group. However, our analysis showed that the intensity (amount of therapy provided) and the frequency (how often training occurred) of gait training were comparable between groups. However, more precise details about intensity of therapy, such as number of steps practiced during training and cardio-respiratory intensity, were not available for all control groups in the studies; therefore this lack of information could overestimate the pooled effect size. Nevertheless, based on the available information it does appear that within both subgroups there were comparable intensity and frequency of therapy between experimental and control groups.
We found differences in the severity of impairment at study start, with fewer patients able to walk in the end-effector subgroup compared with the exoskeleton subgroup. This difference in initial impairment could be argued to be a limitation of this study. However, despite the more severe initial impairment, the end-effector subgroup achieved higher rates of independent walking at study end than the exoskeletal subgroup. This could be viewed as evidence that patients with different levels of impairment may have respond differently to the two types of devices. Alternatively, this finding could be interpreted as occurring due a ceiling effect in the walking recovery of the exoskeleton group.
The majority of patients from the end-effector subgroup came from one trial (34); and it could be argued that this may provide an explanation for the superior effect found in support of end-effectors. Furthermore, because both authors were co-investigators on this multicentre study this may be interpreted as a potential conflict of interest. However, in a sensitivity analysis reported elsewhere (10), excluding this one trial was not found to change the main pooled effect.
The duration of training could also have an important influencing factor on the effect shown. Unfortunately, details of the number of trained steps during rehabilitation were not clearly measured and/or reported in studies, and therefore the precise training intensities cannot be described or compared.
Although our results suggest that an end-effector approach may be more favourable for gait training after stroke, it is not very clear why the device type should provide a difference in walking benefit. Both types of device have their own strengths and weaknesses. It is therefore important to consider the rationale for the two types of device and the purported benefits or disadvantages of each.
The end-effector device might allow the patient to extend their own knee with more freedom, and also the task of maintaining balance might be more demanding (with the degree of balance required dependent on how the harness is set-up and whether the patient holds the hand rails). One advantage of exoskeleton devices might be that gait cycles could be more easily controlled; detailed biomechanical descriptions and a description of limitations of the devices used in this study are provided by Hidler et al. (36) and others (37, 38). Hidler et al. (38), for instance, described an altered electromyography pattern with the potential risk of shearing forces in case of an alignment between the external and internal joint axis. Hidler et al. (38) observed significant differences in spatial and temporal muscle activation patterns across the gait cycle between exoskeleton-assisted and un-assisted walking. Hidler et al. (38) concluded that the gait pattern that occurs within a robotic orthosis that limits the df of leg and pelvis movement (the exoskeleton-assisted approach) might lead to changes in naturally occurring muscle activation patterns.
We are not aware of any studies directly comparing different electromechanical devices for gait rehabilitation in patients with stroke. There is a single case study by Regnaux et al. (27) describing a healthy volunteer and comparing the gait patterns induced with the exoskeleton and end-effector devices. Regnaux’s group (27) found that both types of device impose mechanical constraints that may alter leg accelerations-decelerations during stance and swing phases, as well as during stance duration. This occurred particularly at speed settings that were slower than those found during overground walking.
There are a number of limitations associated with this present study. There was heterogeneity between the trials in terms of trial design, characteristics of the therapy interventions, study duration and patient’s characteristics and also methodological differences in randomization and allocation concealment, blinding and use of intention-to-treat analysis. The results should therefore be interpreted with caution. We found a mean time since stroke of between 11 and 14 months. Therefore, many studies focused on chronic stroke patients. There remains a need for studies that compare the effects of gait training in the acute/subacute and chronic phases after stroke.
The optimum amount of electromechanical-assisted gait training (optimal frequency, optimal duration in the use of assistive technologies and timing of application) and which patients might benefit most remains unclear.
Our primary outcome variable was the proportion of independent walkers at study end, which is often seen as clinically important and relevant. The recovery of walking after stroke is often a priority for many patients and their relatives. The proportion of independent walkers at study end is therefore proposed to be a robust marker of success in gait rehabilitation. Unfortunately, the severity of impairment of patients included in these studies was mixed, with approximately 44% of all included patients walking almost independently at study onset. It could be argued, therefore, that our effect estimate may have been biased by initial walking ability. However, we consider that we are likely to have underestimated, rather than overestimated, the true effect.
Overground gait speed could be viewed as an alternative indicator of improvement in walking function. However, this outcome parameter was used in less than 50% of all included studies. Furthermore, we argue that the number of independent walkers at the study end is more clinically relevant and easily understood.
It is sometimes argued that there are insufficient data or studies, or both, to justify a pooled analysis of published data. However, in the absence of a scientific definition of “the right time” to perform a systematic review with pooled analysis, we believe that 18 studies with 855 patients are sufficient to pool data in a meaningful meta-analysis.
We investigated the clinical question as to which electromechanical device was more beneficial compared with others. We believe that we have provided the first evidence relating to the relative effects of different electromechanical devices for stroke rehabilitation. Although our data suggest a beneficial effect of one type of device, there is insufficient evidence upon which to base a final clinical recommendation. Future research is required that directly compares, within a randomized trial, the exoskeleton and end-effector types of electromechanical device.
In conclusion, repetitive gait training in combination with physiotherapy may improve walking ability in patients after stroke. Our results suggest that the type of electromechanical-assisted device might influence the benefit in terms of gait rehabilitation after stroke.
References
