OBJECTIVE: To compare the effects of unilateral and bilateral training on upper limb function after stroke with regard to two key factors: severity of upper limb paresis and time of intervention post-stroke.
DESIGN: Systematic review and meta-analysis of randomized controlled trials.
METHODS: Two authors independently selected trials for inclusion, assessed the methodological quality and extracted data. Study outcomes were pooled by calculating the (standardized) mean difference ((S)MD). Sensitivity analyses for severity and time of intervention post-stroke were applied when possible.
RESULTS: All 9 studies involving 452 patients showed homogeneity. In chronic patients with a mild upper limb paresis after stroke a marginally significant SMD for upper limb activity performance (SMD 0.34; 95% confidence interval): 0.04–0.63), and marginally significant MDs for perceived upper limb activity performance (amount of use: MD 0.42; 95% confidence interval: 0.09–0.76, and quality of movement: MD 0.45; 95% confidence interval: 0.12–0.78) were found in favour of unilateral training. All other MDs and SMDs were non-significant.
CONCLUSION: Unilateral and bilateral training are similarly effective. However, intervention success may depend on severity of upper limb paresis and time of intervention post-stroke.
Key words: rehabilitation; stroke; upper limb; systematic review; CIMT; bilateral arm training.
J Rehabil Med 2012; 44: 106–117
Correspondence address: Lex van Delden, Faculty of Human Movement Sciences, VU University Amsterdam, Van der Boechorststraat 9; NL-1081 BT Amsterdam, The Netherlands. E-mail: l.van.delden@vu.nl
Submitted July 13, 2011; accepted October 31, 2011
INTRODUCTION
Stroke is a leading cause of long-term disability. The World Health Organization (WHO) estimates the world-wide incidence of persons with stroke at 15 million per year (1, 2). Although prospective epidemiological studies are lacking, it is estimated that only one-third of patients with a flaccid upper limb (UL) early post-onset regain some UL function using conventional rehabilitation programmes (3). The surplus value of most of these conventional therapies beyond spontaneous recovery early after stroke is unclear (4). Consequently, recent developments in post-stroke UL rehabilitation have provided therapists with a broad choice of treatment types for the paretic UL (see also (5)). Remarkably, within this collection two therapeutic concepts figure prominently that stand in stark contrast. On the one hand, there are therapies that prevent the use of the non-paretic UL, such as constraint-induced movement therapy (CIMT) (6). On the other hand, there are therapies that dictate utilization of the non-paretic UL to enhance motor function in the paretic limb, such as bilateral arm training with rhythmic auditory cueing (BATRAC) (7). Therefore, the main goal of the present review was to compare the effects of unilateral and bilateral training on UL function.
In unilateral UL training, such as CIMT, modifications of CIMT (mCIMT), and Forced Use, training is restricted to the most affected arm. The theoretical framework for unilateral training was derived from Edward Taub’s basic research with deafferented monkeys and is based on the behavioural theory of “learned non-use” of the affected limb (8). This learning phenomenon refers to a conditioned suppression of movement. From this conditioning point of view it should be possible to reverse the phenomenon or even to prevent it from happening. Positive results in this regard motivated the introduction of this particular conceptual framework and associated techniques in stroke rehabilitation in humans (8, 9).
Reviews on unilateral UL training have been largely confined to (m)CIMT. Since its introduction, the application of CIMT has been heterogeneous and not all components of the signature protocol (8–10) have always been integrated. Modified versions of CIMT are generally characterized by a reduced amount of time and/or intensity of training, as well as less time during which the non-paretic UL is restrained. In a number of reviews, systematic searches and meta-analyses were performed. Whereas most systematic reviews found positive effects of (m)CIMT (5, 11–14), others reported that results were insufficient to draw conclusions on the effectiveness of (m)CIMT in improving arm function (15–17).
Bilateral UL training after stroke is based on the premise that movement of the non-paretic UL may support movement of the paretic UL when performed simultaneously. This type of therapy has a relatively short history and arose partly serendipitously (18, 19) and partly from insights gleaned from the motor control literature. In this literature, coupling (or interaction) effects between the two ULs have been investigated extensively in rhythmic interlimb-coordination studies involving healthy subjects (20–24). It is well established that humans show a basic tendency towards in-phase (i.e. symmetrical movements) or anti-phase (i.e. alternating movements) coordination, with a prevalent 1:1 frequency locking mode for UL bilateral movements (24). The tendency towards these patterns reflects the coupling between the ULs. In bilateral UL training, this coupling is exploited using interactions between both sides of the central nervous system through intact connecting structures, such as the corpus callosum (25, 26). (For other possible structures see (27).)
In 5 systematic reviews on bilateral UL training meta-analyses determining the effect of bilateral UL training were performed (5, 28–31). Two of these systematic reviews found strong evidence in support of bilateral UL training after stroke (28, 31). However, in these meta-analyses, outcomes from the 3 levels of the WHO International Classification of Functioning, Disability and Health (ICF) (32) ((i) body functions and structure, (ii) activity, and (iii) participation), as well as outcomes from kinematic performance were pooled, thereby assuming that they refer to the same underlying construct (33, 34). In addition, the included studies compared bilateral arm training (sometimes assisted by robotic devices or electro-stimulation) with a range of other treatments or no treatment at all, rendering it difficult to draw unambiguous conclusions. For example, without comparison with a control treatment, it is impossible to tell whether improvements after bilateral training were indeed induced by the bilateral aspect of the training (35). Another review, that also included non-randomized besides randomized clinical trials (RCTs), was more reticent in its conclusions than the previous two (30). The remaining two systematic reviews concluded that bilateral training may be no more (or less) effective than other treatments (5, 29).
By definition, unilateral and bilateral UL training represent conflicting therapeutic concepts with, ultimately, the same goal, i.e. improvement in UL function after stroke. Conceptual and practical differences aside, there are also similarities between unilateral and bilateral UL training: in both types of training patients must use their most affected arm, both induce plastic changes in the central nervous system (CNS) (35–44), and both result in changes (i.e. improvements) in kinematic measures of motor control (18, 45–47).
As the effects of a therapy depend on patient characteristics, it is conceivable that differential effects between unilateral and bilateral training vary as a function of such characteristics. In this review two key factors are therefore considered. The first is the severity of the UL paresis. Patients with a higher level of distal functioning have a higher probability of regaining UL function (48, 49). The success of CIMT is often ascribed to its very stringent inclusion criteria (patients possess at least 20º of active wrist extension and 10º of active extension of each finger of the involved UL (8, 50)), which are only met in relatively mildly impaired stroke survivors (51). In later studies these criteria have been adjusted to examine the effects of CIMT in patients with poorer functioning (6, 52). Nevertheless, in all CIMT studies a certain degree of active extension of the wrist and fingers of the paretic UL was required. Hence, the presence of residual distal UL function in terms of control of finger extension at therapy onset may be conditional for a positive effect of CIMT (53–55). Essential for motor control of the distal part of the UL is the integrity of the corticospinal tract (CST) (56–59), which is a strong predictor of functional outcome post-stroke (60). Stoykov & Corcos (51) suggested that patients in the chronic phase post-stroke who retain a degree of corticospinal integrity (as reflected by, for example, active finger movements) should receive unilateral training, and that those with little or no distal movement might benefit more from bilateral training (61). Their suggestion was based on a modification of an algorithm proposed by Stinear et al. (60) that predicts that patients in the chronic phase with functional CST integrity will partly or fully recover when the most affected UL is used intensely in training, targeting the ipsilesional hemisphere; for those without CST integrity, targeting the contralesional hemisphere using bilateral training is expected to be more appropriate, although the functional gains are expected to be small.
Thus far, the differential effects of bilateral training and unilateral training in relation to UL impairment have not been systematically reviewed, even though both have been applied to patients with different levels of severity. Therefore, the first focus in the present review is on the training effects in relation to severity of UL paresis before the intervention.
The second focus of the present review is on the training effects related to the time of intervention post-stroke. Initially, CIMT studies recruited patients in the chronic phase post-stroke (9, 62). However, the interest in the application of CIMT earlier post-stroke has grown (63–66). Bilateral UL training has, from its recent emergence in stroke rehabilitation literature, been applied to patients in both acute and chronic phases post-stroke (7, 18, 19, 67). Nevertheless, what the differential effects of unilateral and bilateral training are in relation to the time of intervention post-stroke remains unclear.
Thus, although reviews for both unilateral and bilateral arm training are available, no systematic review of studies directly comparing both types of training has been published to date. The purpose of the present systematic review and meta-analysis was: (i) to identify and summarize studies that compared unilateral with bilateral UL training after stroke, (ii) to assess the methodological quality of these studies, and (iii) to compare the differential effects of both types of training in terms of improvements of UL motor function related to the two key factors: (1) severity of UL paresis before intervention, and (2) time of intervention post-stroke.
METHODS
Definitions
Stroke. We included trials of participants with a clinical diagnosis of stroke as defined by the WHO: “a syndrome of rapidly developing symptoms and signs of focal, and at times, global, loss of cerebral function lasting more than 24 hours or leading to death, with no apparent cause other than that of vascular origin” (68).
Severity. A plethora of measures is used for the classification of severity of UL paresis. For the purpose of the present review the classifications as used in the included studies were followed and when no classification was given, two independent reviewers (AEQ, GK) classified the severity of the UL paresis of the included patients as “severe”, “moderate” or “mild”. These classifications are further specified in the Results section. In case of disagreement of the classification, an independent researcher (CEP) would make the final decision. However, such disagreements did not arise.
Time of intervention post-stroke. Patients starting the intervention less than 1 month post-stroke were identified as acute, those starting between 1 and 6 months post-stroke as subacute, and those starting more than 6 months post-stroke as chronic.
Therapy. The included studies had to investigate both unilateral and bilateral UL training as intervention alternatives. Unilateral UL training was defined as an exercise treatment involving the hemiparetic UL to the exclusion of the contralateral UL (e.g. CIMT). In case of bilateral UL training, both ULs had to perform the same motor task simultaneously. For a fair comparison (and following the definitions), we only included studies in which active exercise of the UL was the single focus. Hence, we excluded trials that investigated unilateral or bilateral training using robot assistance, electrical augmentation (i.e. electromyography biofeedback and electrostimulation), visual illusions (i.e. mirror therapy and virtual reality), and intramuscular injections of botulinum toxin. Note that some consider BATRAC as a robot-assisted treatment (69). However, during BATRAC it is the participant who actively executes the movements on an imposed rhythm using a device that only constrains movement direction. Consequently, studies comparing unilateral training with BATRAC were included in this review. We included interventions of any intensity and duration. In case it was unclear whether intervention alternatives were a type of unilateral or bilateral training (e.g. in cases in which we were uncertain about the description of a “control treatment” or “usual care”) the original trial authors were contacted for clarification. We included controlled trials in which participants had been randomly assigned to one of the alternative treatments.
Study identification
Potentially relevant literature was identified through computerized and manual searches. The following electronic databases were systematically searched through June 2011: PubMed, EMBASE, Cochrane Central Register of Controlled Trials, CINAHL, Physiotherapy Evidence Database (PEDro), SportDiscus, and OT-Seeker. The databases were searched using a study identification strategy that was formulated in PubMed and adapted to the other databases.
The following MeSH headings and key words were used:
• cerebrovascular disorder$, cerebrovascular accident, CVA, stroke, hemiparetic stroke, paresis, hemiparesis, hemiplegia (patient type);
• upper extremit$, upper limb$, arm$, forearm$, wrist$, hand$, finger$ (body part);
• bilateral$, bimanual$, BATRAC, unilateral$, constraint induced, Forced Use (intervention type);
• randomized controlled trial, controlled clinical trial, randomized, randomly, trial, placebo, groups (study type);
The articles had to be written in English.
Bibliographies of review articles, empirical articles, and abstracts published in proceedings of conferences were also examined. In further iterations, references from retrieved articles were examined to identify additional relevant trials that met the inclusion criteria. The full search strategy is available on request.
Two reviewers (AEQ, GK) independently selected studies based on title and abstract, after which the full-text articles were screened and compared against our inclusion and exclusion criteria. In case of disagreement of the selection, an independent researcher (CEP) would make the final decision. However, such disagreements did not arise.
Methodological quality
The methodological quality of each RCT was assessed by two independent reviewers (AEQ, GK) using the PEDro scale (70, 71). The reviewers were not blinded to authors, journals, and outcomes. PEDro is an 11-item scale, in which the first item relates to external validity and the other 10 items assess the internal validity of a clinical trial. One point was given for each criterion that was satisfied (except for the first item, which was allocated a YES or NO), yielding a maximum score of 10. The higher the score accumulated, the better the quality of the study. Two items of the PEDro scale (viz. blinding of participants and blinding of therapists) could not be met in the included trials. Consequently the maximum score was reduced to 8 points. PEDro scores of ≥ 4 points were classified as “sufficient quality”, whereas studies with ≤ 3 points were of “insufficient quality”(11). A point for a particular criterion was awarded only if the article explicitly reported that the criterion had been met. In case of disagreement, consensus was sought, but when disagreement persisted, a third independent reviewer (CEP) was available to make the final decision. However, such disagreements did not arise.
Quantitative analysis
The extracted data (numbers of patients in the unilateral and bilateral treatment groups and the means and standard deviations (SDs) of pre- and post-intervention scores for each intervention group) were checked independently by two reviewers (AEQ, GK). For each outcome variable, we pooled the results by calculating the mean difference (MD) and 95% confidence interval (CI) in case all outcomes were reported on the same scale. A standardized mean difference (SMD) was calculated when outcome variables were reported on different scales but nonetheless measured the same underlying construct. The differences in pre- to post-intervention means and the post-intervention SDs were used to calculate the MD or the SMD. The χ2 test (Cochran’s Q) was used to test for homogeneity, set at a significance level of 10%. Because the χ2 test tends to underestimate heterogeneity in meta-analyses, I2 was calculated as well to provide an estimate of the percentage of variability due to heterogeneity rather than chance alone (72). In addition, I2 does not depend on the number of studies. The threshold for I2 was set at 50%: I2 values < 50% indicate homogeneity, allowing a fixed effects model, and I2 values ≥ 50% indicate heterogeneity, requiring a random effects model. Because for all the dependent variables I2 values < 50% were found (see Results section) fixed effects models were applied (72). For all outcome variables, the critical value for rejecting H0 was two-tailed and set at a level of 0.05. The software package Review Manager 5 was used to calculate the MDs or SMDs and to visualize the results by using forest plots.
Since the focus of the present review is on the differential effects of unilateral and bilateral UL training related to the two key factors (viz. severity of UL paresis at baseline and time of intervention post-stroke), a sensitivity analysis was applied in which MDs and SMDs are reported separately for the severity of paresis (i.e. severe, moderate and mild), and time post-stroke (i.e. acute, subacute and chronic). Studies that used cross-over designs were considered as randomized clinical trials up until the point of cross-over, whereas studies with 3 arms (e.g. two experimental and one control groups) were adjusted for the numbers that actually participated in the experimental groups, ensuring that each patient was counted once in the meta-analyses.
RESULTS
Study identification
The search strategy yielded 990 citations. Duplicate articles were excluded, leaving 429 potentially relevant articles for further screening. A flow chart showing the selection process of the articles is presented in Fig. 1. First, based on title and abstract, 414 studies were excluded. Reasons for exclusion were that studies had been conducted in a different patient population, that studies used more than plain exercise therapy, or that studies had an inappropriate study design (i.e. not an RCT). Of the remaining 15 full-text articles, 6 were excluded because the studies were sub-studies of an RCT included in this review (40, 73–75), some subjects participated twice in a similar study (76), and the study was not an RCT (77). Screening of references did not yield any further studies.
Fig. 1. Flowchart of study identification. RCT: randomized controlled trial.
A total of 9 studies were included in this systematic review (35, 42, 45, 67, 78–82), comprising 452 participants divided over unilateral and bilateral training groups. In 2 studies (45, 78) an additional 42 participants received a third intervention, not specified as unilateral or bilateral, as control treatment. Since the purpose of the present review is to compare the effects of unilateral and bilateral training, data of these 42 participants were not used in the meta-analyses. One study compared mCIMT with functional bilateral arm training (79), 2 studies compared distributed CIMT (here referred to as mCIMT) with functional bilateral training and a neuro-developmental treatment-based (NDT) (83) control treatment (45, 78), 1 study compared CIMT with NDT-based bilateral arm training (81), 1 study compared Forced Use therapy (here referred to as CIMT) with functional bilateral arm training (82), 3 studies compared functional unilateral training (but not CIMT) with functional bilateral training (42, 67, 80), and 1 study compared NDT-based unilateral arm training with BATRAC (35).
Within all RCTs, treatment regimens were designed to ensure that both groups received an equal amount of therapy; however, participants receiving (m)CIMT were encouraged to also wear the restraining mitt or splint on their less affected arm and train the most affected arm in functional tasks out of therapy-hours. The main characteristics of the included studies are shown in Table I.
| Table I. Characteristics of the studies included in this review |
| Study, year | Length
of therapy, weeks | Interventions | Outcome measures | Times of assessment | Authors’ conclusion |
| Hayner et al., 2010 (79) | 2 | Unilateral: mCIMT (6 h/day,
5 days/week; restraint: compliance was not required) Bilateral: fBAT (6 h/day,
5 days/week) | WMFT, COPM | Weekday before intervention, weekday after intervention, and 6 months after post-test | High-intensity occupational therapy using a CIMT or a bilateral approach can improve upper limb function in people with chronic upper limb dysfunction after CVA. |
| Lin et al., 2009 (78) | 3 | Unilateral: mCIMT (2 h/day,
5 days/week; restraint:
6 hours/day) Bilateral: fBAT (2 h/day,
5 days/week) | FMA, FIM, MAL, SIS 3.0 | Before and after the 3-week intervention period (specific times not reported) | Functional BAT was superior to mCIMT in improving motor function of the proximal UL; mCIMT demonstrated larger gains in functional use of the affected UL in daily life and improved functional independence and quality of life. |
| Morris et al., 2008 (67) | 6 | Unilateral: fUAT (20 min/day,
5 days/week) Bilateral: fBAT (20 min/day,
5 days/week) | ARAT, RMA, 9HPT, MBI, NHP, HADS | Before and after the 6-week intervention period (specific times not reported), and 18 weeks after intervention start | Bilateral training was no more effective than unilateral training. In terms of overall improvement in dexterity, the bilateral training group improved significantly less. |
| Stoykov et al., 2009 (80) | 8 | Unilateral: fUAT (1 h/day,
3 days/week) Bilateral: fBAT (1 h/day,
3 days/week) | MSS, MAS | 1–3 weeks before intervention and within 1 week after intervention | Both bilateral and unilateral training are efficacious for moderately impaired chronic stroke survivors. Bilateral training may be more advantageous for proximal arm function. |
| Summers et al., 2007 (42) | 1 | Unilateral: fUAT (50trials/day,
6 days/week) Bilateral: fBAT (50 trials/day,
6 days/week) | MAS Additional: TMS | 1 day before and 1 day after intervention | A short-term bilateral training intervention may be effective in facilitating upper limb motor function in chronic stroke patients. |
| Suputtitada et al., 2004 (81) | 2 | Unilateral: CIMT (6 h/day,
5 days/week; restraint: encouraged to wear at home) Bilateral: NDT-BAT (6 h/day,
5 days/week) | ARAT Additional: dynamometry | 3–5 days before and 3–5 days after intervention | CIMT has an advantage for chronic stroke patients and may be an efficacious technique for improving motor activity. |
| Van der Lee et al., 1999 (82) | 2 | Unilateral: FU (6 h/day,
5 days/week; restraint: encouraged to wear at home) Bilateral: NDT-BAT (6 h/day,
5 days/week) | FMA, ARAT, MAL, RAP | 2 weeks and 3–5 days before intervention, 3 and 6 weeks after intervention start, and 6 months and 1 year after intervention start | The effect of FU therapy was clinically relevant in the subgroups of patients with sensory disorders and hemineglect, respectively. |
| Whitall et al., 2011 (35) | 6 | Unilateral: NDT-UAT (1 h/day, 3 days/week) Bilateral: BATRAC (1 h/day,
3 days/week) | FMA, WMFT, SIS 2.0 Additional: fMRI dynamometry | At 2 baseline times separated by 6 weeks, after 6 weeks of intervention, and 4 months after the intervention | BATRAC is not superior to NDT-based unilateral training with equal intensity, but both rehabilitation programs durably improve motor function for individuals with chronic upper limb hemiparesis and with varied deficit severity. |
| Wu et al., 2011 (45) | 3 | Unilateral: mCIMT (2 h/day,
5 days/week; restraint: 6 h/day) Bilateral: fBAT (2 h/day,
5 days/week) | WMFT, MAL Additional: kinematics | Before and after the 3-week intervention period (specific times not reported) | BAT is a better option if improvement in force generation is the treatment goal, and mCIMT is more appropriate for improving functional ability and use of the affected arm in daily life. |
| WMFT: Wolf Motor Function Test; COPM: Canadian Occupational Performance Measure; CVA: cerebrovascular accident; (m)CIMT: (modified) constrain-induced movement therapy; fBAT: functional bilateral arm training; FMA: Fugl-Meyer motor assessment Arm; FIM: Functional Independence Measure; MAL: Motor Activity Log; SIS: Stroke Impact Scale; UL: upper limb; ARAT: Action Research Arm Test; RMA: Rivermead Motor Assessment; 9HPT: Nine Hole Peg Test; MBI: Modified Barthel Index; NHP: Nottingham Health Profile; HADS: Hospital Anxiety and Depression Scale; fUAT: functional unilateral arm training; MAS: Motor Assessment Scale; MSS: Motor Status Scale; TMS: transcranial magnetic stimulation; NDT-BAT: bilateral arm training based on neuro-developmental treatment; RAP: Rehabilitation Activities Profile; FU: Forced Use; NDT-UAT: unilateral arm training based on Neuro-Developmental Treatment; fMRI: functional magnetic resonance imaging. |
Severity classification
Severity of UL paresis in 4 studies was classified as mild for the present review: two studies recruited patients with a Brunnstrom stage above III for proximal and distal parts of the UL (45, 78), and two studies recruited patients with a minimum of 20º of active wrist extension and 10º of active finger extension (81, 82).
Two studies recruited patients with a moderate UL paresis: Stoykov et al. (80) recruited patients with a Fugl-Meyer motor assessment of the arm (FMA) score between 19 and 40 points, and Whitall et al. (35) recruited patients who could flex the paretic arm 3 inches from a neutral position and reported mean FMA scores of 32.3 (SD 14.1) and 31.0 (SD 14.8) for bilateral and unilateral treatment groups, respectively, at baseline.
Two studies stratified the recruited patients based on severity of UL paresis at baseline. Hayner et al. (79) stratified into two severity subgroups (for the present review classified as moderate and mild subgroups) as determined by the Wolf Motor Function Test functional ability scale (WMFT-FAS) intake score (i.e. a score below and above 36 points on the WMFT-FAS, respectively). Morris et al. (67) divided patients into 3 severity subgroups based on baseline Action Research Arm Test (ARAT) and Nine Hole Peg Test (9HPT) scores: subgroup 1 had ARAT scores 0–3 and no pegs in 9HPT; subgroup 2 had ARAT scores 4–28 and no pegs in 9HPT; subgroup 3 had ARAT scores 29–56 and some or all pegs in 9HPT. For the present review subgroups 1, 2 and 3 were classified as severe, moderate and mild, respectively.
Summers et al. (42) recruited patients who had most components of movement present in the most-affected UL, but impairment of function relative to the less-affected side. Based on the pre-test modified Motor Assessment Scale (MAS) scores, 4 patients in the unilateral group and 2 patients in the bilateral group were considered as having mild UL paresis (MAS hand movements scores 5 to 6), 1 patient in the unilateral group and 3 patients in the bilateral group were considered as having moderate UL paresis (MAS hand movements scores 2 to 3), and 1 patient in the unilateral group and 1 patient in the bilateral group were considered as having severe UL paresis (MAS hand movements scores 0).
Time of intervention
All studies recruited patients in the chronic phase post-stroke except one (67), in which patients in the acute phase post-stroke were recruited. None of the studies recruited patients in the subacute phase post-stroke. The numbers of patients starting the intervention in the acute phase post-stroke with a severe, moderate and mild UL paresis were 35, 39 and 23, respectively. The numbers of patients starting the intervention in the chronic phase post-stroke with a severe, moderate and mild UL paresis were 2, 126 and 226, respectively. Demographics of included participants are shown in Table II.
| Table II. Demographics of the participants included in this review |
| Study, year | Number of
participants at post-
test (randomized) | Age, years Mean (SD) | Gender (F/M) | Time since stroke Mean (SD) | Side of stroke,
right/left |
| Hayner et al., 2010 (79) | 12 (13) | Unilateral: 54.0 (11.6) Bilateral: 59.5 (11.8) | Unilateral: 4/2 Bilateral: 3/3 | Unilateral: 642.3 days (421.1) Bilateral: 2039.0 days (925.3) | Not reported |
| Lin et al., 2009 (78) | 40 (40) [20 (20) in control treatment] | Unilateral: 55.3 (9.3) Bilateral: 51.6 (8.7) [Control: 50.7 (13.9)] | Unilateral: 9/11 Bilateral: 8/12 [Control: 9/11] | Unilateral: 21.3 months (21.6) Bilateral: 18.5 months (17.4) [Control: 21.9 months (20.5)] | Unilateral: 8/12 Bilateral: 11/9 [Control: 12/8] |
| Morris et al., 2008 (67) | 97 (106) | Unilateral: 67.8 (9.9) Bilateral: 67.9 (13.1) | Unilateral: 23/27 Bilateral: 22/34 | Unilateral: 23.2 days (5.7) Bilateral: 22.6 days (5.6) | Most affected side (right/left) Unilateral: 23/27 Bilateral: 29/27 |
| Stoykov et al., 2009 (80) | 24 (24) | Unilateral: 64.8 (11.1) Bilateral: 63.8 (12.6) | Unilateral: 5/7 Bilateral: 3/9 | Unilateral: 10.2 years (10.1) Bilateral: 9.5 years (5.4) | Not reported |
| Summers et al., 2007 (42) | 12 (12) | Unilateral: 59.8 (14.0) Bilateral: 63.5 (15.9) | Unilateral: 3/3 Bilateral: 2/4 | Unilateral: 4.0 years (3.4) Bilateral: 6.3 years (5.7) | Unilateral: 1/4 (1 right and left) Bilateral: 2/4 |
| Suputtitada et al., 2004 (81) | 69 (69) | Unilateral: 60.1 (4.8) Bilateral: 58.7 (4.2) | Unilateral: 11/22 Bilateral: 11/25 | Unilateral: 81.8% 1–3 years; 6.1% 3–5 years; 3.0% 5–7 years; 9.1% 7–10 years Bilateral: 80.6% 1–3 years; 16.6% 3–5 years; 2.8% 5–7 years | Most affected side (right/left) Unilateral: 30/3 Bilateral: 34/2 |
| van der Lee et al., 1999 (82) | 62 (66) | Unilateral median: 59 (IQR 52–60) Bilateral median: 62 (IQR 51–67) | Unilateral: 12/19 Bilateral: 15/16 | Unilateral median: 3.4 years (IQR 2.1–7.0) Bilateral median: 2.7 years (IQR 1.6–4.4) | Most affected side (right/left) Unilateral: 26/5 Bilateral: 25/6 |
| Whitall et al., 2011 (35) | 92 (111) | Unilateral: 57.7 (12.5) Bilateral: 59.8 (9.9) | Unilateral: 26/24 Bilateral: 16/26 | Unilateral: 4.1 years (5.2) Bilateral: 4.5 years (4.1) | Unilateral: 25/25 Bilateral: 23/18 (1 right and left) |
| Wu et al., 2011 (45) | 44 (44) [22 (22) in control treatment] | Unilateral: 51.9 (11.9) Bilateral: 52.2 (10.7) [Control: 55.2 (2.5)] | Unilateral: 7/15 Bilateral: 4/18 [Control: 6/16] | Unilateral: 14.9 months (12.0) Bilateral: 15.9 months (13.7) [Control: 17.7 months (12.4)] | Unilateral: 8/14 Bilateral: 12/10 [Control: 10/12] |
| SD: standard deviation; F/M: female/male; IQR: interquartile range. |
Methodological quality
Table III shows the methodological quality scores of the included studies, according to the PEDro scale. The PEDro scores ranged from 5 to 8 points, with a mean score of 6.44 points (SD 0.88). The assessment of the methodological quality using the 10-item PEDro scale resulted in a Cohen’s κ of 0.88 between the 2 independent review authors. All studies scored more than 4 points on the PEDro scale.
| Table III. Methodological quality of the included trials, as assessed using the PEDro scale |
| Study, year | Eligibility criteria specified (yes/no) | 1. Random allocation | 2. Concealed allocation | 3. Comparable at baseline | 4. Blind subjects | 5. Blind therapists | 6. Blind assessors | 7. Adequate follow-up | 8. Intention to treat analysis | 9. Between group comparisons | 10. Point estimate and variability | PEDro total score (0–10) |
| Hayner et al., 2010 (79) | Yes | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Lin et al., 2009 (78) | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Morris et al., 2008 (67) | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Stoykov et al., 2009 (80) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Summers et al., 2007 (42) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Suputtitada et al., 2004 (81) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| van der Lee et al., 1999 (82) | Yes | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Whitall et al., 2011 (35) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
| Wu et al., 2011 (45) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Item 1–10: 1: criterion was satisfied; 0: criterion was not satisfied. |
Quantitative analysis
Based on the categorization of the WHO ICF, the present review focused on: (i) outcome measures of UL impairment associated with body functions and structure, and (ii) measures of UL performance on the level of activity. Pooling of results with SMDs was possible for: (i) UL impairment, measured with the Fugl-Meyer motor assessment of the arm (FMA) and the Motor Status Score (MSS); and (ii) UL activity performance, measured with the ARAT and WMFT. The results of the FMA and MSS were pooled, since both tests measure the same underlying construct (84). The same applies to the ARAT and the WMFT (85). Although results for the MAS were also available, they were not pooled with the ARAT and WMFT results, because we did not find any proof of them sharing the same underlying construct. In our analyses we pooled the ARAT scores with the Functional Ability Scale (FAS) of the WMFT. Pooling of outcomes with MDs was possible for: (iii) UL activity performance, measured with the MAS, and (iv) perceived UL activity performance, measured with the Motor Activity Log (MAL) for amount of use (AOU) and quality of movement (QOM). None of the analyses showed heterogeneity (all I2 values < 50%), and therefore fixed effects models were used. Note that with this small number of studies investigating publication bias (using rank correlation tests, regression methods, fail-safe N methods and visual inspection of funnel plots) is inapt (72, 86).
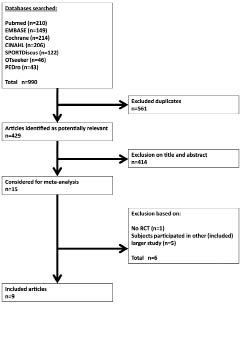
Fugl-Meyer motor assessment of the arm and Motor Status Score. Three studies assessed the FMA (35, 78, 82) and one study assessed the MSS (80). All recruited patients were in the chronic phase post-stroke. For none of the severity classifications a differential effect of unilateral and bilateral UL training was found on the body functions and structure level of the WHO ICF (p-values > 0.05), as can be seen in Fig. 2.
Fig. 2. Forest plot of the pooled Fugl-Meyer motor assessment of the arm and Motor Status Score scores. SD: standard deviation; CI: confidence interval; df: degrees of freedom; std: standardized.
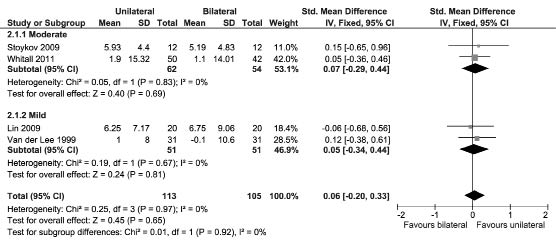
Action Research Arm Test and Functional Ability Scale of the Wolf Motor Function Test. Three studies assessed UL activity performance using the ARAT (67, 81, 82), and 3 studies used the WMFT (35, 45, 79). Five studies recruited patients in the chronic phase post-stroke (35, 45, 79, 81, 82) and one in the acute phase post-stroke (67). Fig. 3 shows a significant SMD in favour of unilateral UL training in patients with a mild UL paresis (SMD [fixed], 0.30; 95% CI: 0.02–0.58; Z = 2.11; p = 0.03; I2 = 0%), and this effect increases when only studies recruiting patients in the chronic phase post-stroke were included in the analysis (SMD [fixed], 0.34; 95% CI: 0.04–0.63; Z = 2.24; p = 0.03; I2 = 0%). All 4 studies that recruited patients with a mild UL paresis in the chronic phase post-stroke applied (m)CIMT as unilateral UL training (45, 79, 81, 82). For the severity classifications moderate and severe, no significant SMDs were obtained. For none of the severity classifications a differential effect of unilateral and bilateral UL training was found in patients in the acute phase post-stroke.
Fig. 3. Forest plot of the pooled Action Research Arm Test and Wolf Motor Function Test scores. SD: standard deviation; std: standardized;
CI: confidence interval; df: degrees of freedom.
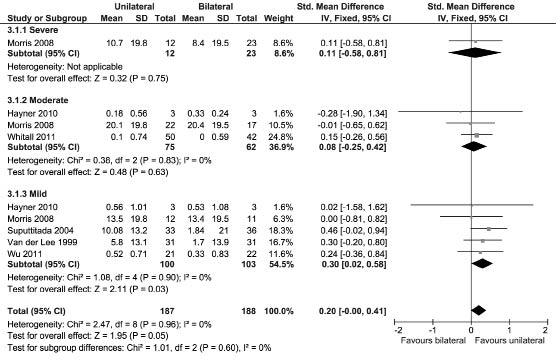
Motor Assessment Scale. Two studies assessed UL activity performance using the MAS (42, 80). Both studies recruited patients in the chronic phase post-stroke. Since the samples of patients with severe, moderate, and mild UL paresis were too small, no subgroup MDs were estimable for the study by Summers et al. (42). Fig. 4 shows that pooling the results of the 2 studies that assessed the MAS yielded a non-significant MD.
Fig. 4. Forest plot of the Motor Assessment Scale scores. SD: standard deviation; CI: confidence interval.
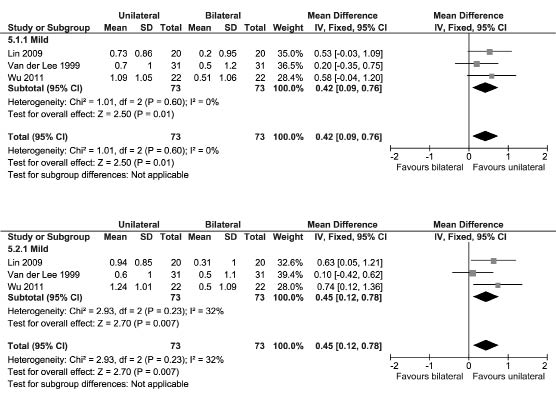
Amount Of Use and Quality Of Movement of the Motor Activity Log. Three studies assessed the MAL (45, 78, 82). These studies recruited patients with a mild UL paresis in the chronic phase post-stroke. Fig. 5 shows a significant MD for the AOU in favour of unilateral UL training (MD [fixed], 0.42; 95% CI: 0.09–0.76; Z = 2.50; p = 0.01; I2 = 0%) and a significant MD for the QOM also in favour of unilateral UL training (MD [fixed], 0.45; 95% CI: 0.12–0.78; Z = 2.70; p = 0.007; I2 = 32%). All 3 studies that assessed the MAL used (m)CIMT as unilateral UL training.
Fig. 5. Forest plot of the Motor Activity Log (MAL) scores (top: amount of use; bottom: quality of movement). CI: confidence interval;
df: degrees of freedom; SD: standard deviation.
DISCUSSION
In this systematic review 9 RCTs of sufficient quality were identified, with a total of 452 participants, comparing the differential effects of unilateral and bilateral UL training after stroke. The effects of unilateral and bilateral UL training were related to two key factors that may determine the outcome: severity of UL paresis and time of intervention post-stroke. No differential effects in terms of UL impairment and (perceived) UL activity performance were found within any of the 3 severity levels in patients starting the intervention in any of the 3 phases post-stroke, except for one combination. For patients classified with a mild UL paresis starting the intervention in the chronic phase post-stroke a marginally positive effect was found on UL activity performance (as assessed with the ARAT and WMFT, but not the MAS) and perceived UL activity performance (MAL) in favour of unilateral training. However, the obtained effects were small (e.g. 5.4 points when transformed back to the ARAT scale; i.e. 9.5% of the maximum score) and below the conventional threshold judged as clinically meaningful (82, 87–90).
Nevertheless, the results of the present review suggest that intervention success depends on severity of upper limb paresis and time of intervention post-stroke. The studies contributing most to the favourable effects of unilateral training used (m)CIMT as experimental intervention (45, 78, 81, 82). It is still unclear which was the essential component or combination of components of (m)CIMT leading to this difference in functional recovery in chronic patients with a mild UL paresis (17, 91, 92). The common denominator in the (m)CIMT applications of the included studies was the fact that patients were trained unilaterally and were encouraged to continue training unilaterally for several hours in addition to therapy sessions, by wearing a restraint. In all probability the latter has led to more practice with the most affected limb in the (m)CIMT groups than in the control groups (cf. 13). Therefore, intensity of the treatment, or more specifically, additional time spent practicing, may have confounded the results and may be a far more important determinant of treatment success than the method of training used. In addition, the bilateral interventions that were compared with (m)CIMT in the included studies were control (i.e. not experimental) interventions in 3 (79, 81, 82) out of 5 studies. Two (81, 82) of these 3 were based on obsolete NDT principles (93). Hence, the suggested hypothesis, that unilateral training is best suited for stroke survivors with mild-to-moderate impaired distal UL function, can partly be confirmed from the results of the present review; however, with apt reticence.
The present systematic review had some limitations. First, the inclusion criteria in combination with the relatively short history of bilateral training protocols resulted in a small number of included studies, preventing a thorough sensitivity analysis to investigate characteristics that may influence the relative effect of unilateral and bilateral UL training. Treatment outcome may also have been influenced by other characteristics besides the severity of UL paresis at baseline and the timing of intervention post-stroke, such as sensory disorders, cognitive impairments, and visual impairments. All included studies recruited patients with no severe cognitive impairment, while only one study excluded patients with visual field deficits (80). Some studies excluded patients with neglect and sensory disorders (67, 80, 81), while in another study these conditions were of particular interest and proved to be relevant for the effect of treatment (82). Secondly, heterogeneity of training types of the included studies may have interfered with the interpretation of the results. Next to the obvious differences between unilateral and bilateral training, there were also discrepancies between unilateral training types and between bilateral training types. In some studies this resulted in situations where patients in one group had more possibilities to train tasks involving distal control, whereas the other group did not have these possibilities. For example, in the study by Whitall et al. (35), the BATRAC-group performed tasks requiring proximal control, while the unilateral group had the possibility to also train distal control. As stated previously, distal control is essential for functional improvement. Furthermore, we cannot rule out publication bias. In particular, small RCTs with negative (for the experimental treatment), non-significant or inconclusive results are less likely to be submitted or accepted for publication. Because of the small number of studies and the large variety in types of unilateral and bilateral training, caution is required in interpreting these results.
In general, but surely when comparing (m)CIMT with an alternative intervention, it should be ensured that all types of training in RCTs are provided dose-matched (i.e. with equal intensity or number of repetitions applied in the control group compared with the training protocol used in the experimental group) and that there are equal possibilities to practice distal control. Since both unilateral and bilateral training improve UL function, it is even more important to know exactly what it is that patients learn from unilateral and bilateral training and how these processes work. More insight into learning processes, coordination, and degrees of restitution and compensation will make it easier to tailor the therapy to the individual goals of a patient. This means that measuring changes in outcomes of impairment, activity performance and activities of daily living are not sufficient, but that measures of kinematics, timing, and neural reorganization also have to be incorporated.
Acknowledgements
The authors would like to thank Marijke Mol for her cooperation in the literature search.
References