Po Jung Pan, MD, MS1,3, Ping Huang Tsai, MD2,3,4, Chih Chun Tsai, PhD5, Chen Liang Chou, MD3, Men Tzung Lo PhD6 and Jen Hwey Chiu, MD, PhD 7,8
From the 1Department of Physical Medicine and Rehabilitation, National Yang-Ming University Hospital, 2Department of Neurology, National Yang-Ming University Hospital, Yi-Lan, 3Department of Medicine, National Yang-Ming University, Taipei, 4Graduate Institute of Biomedical Electronics and Bioinformations, National Taiwan University, Taipei, 5Department of Mathematics, Tamkang University, Tamsui, 6Research Center for Adaptive Data Analysis, National Central University, Tao-Yuan, 7Institute of Traditional Medicine, School of Medicine, National Yang-Ming University and 8Division of General Surgery, Department of Surgery, Taipei Veterans General Hospital, Tapei, Taiwan
OBJECTIVE: To determine the influence of mechanical intermittent cervical traction on the autonomic system.
DESIGN: Prospective, cases series study.
SUBJECTS: Sixteen healthy volunteers without contraindications for cervical traction.
METHODS: Subjects received mechanical intermittent cervical traction in a sitting position under two traction forces (10% and 20% of total body weight). Electrocardiographic and neck surface electromyographic signals were recorded and analysed from 3 5-min periods (before, during and after traction). Subjective symptoms, heart rate and heart rate variability parameters, including standard deviation of all normal-to-normal beat intervals, very low-frequency power, low-frequency power, high-frequency power, multiscale entropy, slope of multiscale entropy, and root mean square value of electromyography amplitude were statistically compared. RESULTS: This pilot study showed that using 10% body weight traction force was more comfortable than using 20% body weight. Only subtle perturbation was noted in the autonomic system when using 20% body weight traction force.
CONCLUSION: The response pattern of heart rate variability analysis in this pilot study provides some early information about individual discomfort in cervical traction. The autonomic modulation and the safety of cervical traction with other modality settings or in patients with neck pain require further study.
Key words: neck pain; traction; autonomic nerve; heart rate; physical therapy modality.
J Rehabil Med 2012; 00: 00–00
Correspondence address: Jen Hwey Chiu, Institute of Traditional Medicine, National Yang-Ming University, No. 155, Section 2, Li-Nong Street, Peitou, Taipei, 112, Taiwan. E-mail: chiujh@mailsrv.ym.edu.tw
Submitted February 23, 2011; accepted October 21, 2011
INTRODUCTION
Cervical traction (CT) is widely applied for neck or upper limb pain caused by a disc herniation or degenerative disc disease (with or without cervical root compression), hypomobile facet joints, and cervical muscular dysfunction (1–3). From an anatomical and mechanical viewpoint, CT can separate zygapophyseal joints with intervertebral foramina enlargement, increase intervertebral space, tighten the posterior longitudinal ligament to adjust the adjacent annulus fibrous and stretch muscles and ligaments (1, 4). Depending on the pain physiology, CT can also stimulate the mechanoreceptors and inhibit reflex nuchal muscle guarding (2). However, some possible side-effects of CT, such as dizziness, headache, muscle tenderness and nausea have been noted (5, 6). Few studies have addressed the physiological effects of CT on the cardiovascular system (3, 6). Those studies reported changes in blood pressure during CT and suggested that clinical physicians and therapists notice haemodynamic changes during its use (2, 3, 7). However, the relationship between side-effects and haemodynamic alternation is still unclear. Since cardiovascular homeostasis is controlled mainly by the autonomic system (8, 9), autonomic modulation during CT requires further investigation.
Heart rate variability (HRV) analysis, due to its simplicity and non-invasiveness, is commonly used for characterizing autonomic modulation (10–13). HRV analysis is also used in autonomic intervention in disorders such as anxiety, gastric reflux, bladder dysfunction (14–16), and other therapeutic application, such as biofeedback therapy (17, 18). In numerous HRV analyses, linear fast Fourier transform (FFT) (12, 19) is used to identify the dominant frequency modes in different frequency bands. After using the FFT, HRV was categorized into 3 components: very low-frequency power (VLFP; 0.01–0.4 Hz), low-frequency power (LFP; 0.04–0.15 Hz), and high-frequency power (HFP; 0.15–0.40 Hz). The LFP component or the ratio of LFP to HFP reflects the sympathetic modulation or sympathovagal balance. In contrast, the HFP component is equivalent to respiratory sinus arrhythmia, correlating with vagal control of heart rate (15, 19). Non-linear analyses (20), such as fractal and entropy analysis, have been developed to evaluate the complexity of the electrocardiogram (ECG).
Multiscale entropy (MSE), one of the complexity markers proposed by Costa et al. (21, 22), is a possible method to quantify the complexity of signals over multiple time-scales. MSE quantifies time series at different temporal scales by means of an entropy-based algorithm and describes the embedded complexity structure from the fluctuations of heart beat. It is based on the assumption that a healthy system demonstrates a meaningful complex control to maintain homeostasis. MSE is regarded as a marker of healthy dynamics, and decreased complexity of MSE has been found in many diseases (23, 24). Other features of the MSE curve, such as the slope of the MSE, defined by sample entropy values between different time-scale factors, could assist with clinical classification (21, 22).
The present study was a pilot test via HRV analysis to determine whether mechanical intermittent CT influences the autonomic system. An attempt was made to provide more information about the neurophysiological effects of CT so that clinical practitioners would have evidence-based parameter settings for CT use.
METHODS
The study was approved by the Human Subject Research Ethics Committee of National Yang-Ming University. Healthy subjects without neck pain and who did not have a history of trauma, osteoporosis, pregnancy, diabetes mellitus, arrhythmia, or other major neuropsychological disorders, in which autonomic functions could have been affected by drugs, were recruited. Subjects were clearly informed of the study aim and procedures. All volunteers gave their consent. After a physical examination by a physiatrist, a cervical spine radiograph was taken in anterior-posterior and lateral views to exclude any structural abnormality. Sixteen healthy subjects (8 males and 8 females, mean age 33 years (standard deviation (SD) 7.68)) were recruited for this study. They all received mechanical intermittent CT (20 s on: 10 s off, Digit-Trac 900, Hsin-Tien city, Tapei Hsien, Taiwan) in a sitting position under two traction forces (10% and 20% total body weight). The CTs were one week apart (Table I). Before recording data, subjects were placed in a chair that inclined at 30 degrees to achieve the optimum neck flexion angle. Each subject was then equipped with surface recording electrodes and fitted with a head halter and pulley system. Two sets of EMG electrodes were stuck on the right posterior and lateral neck individually to record the right cervical paraspinal splenius capitis muscle and sternocleidomastoid muscle electromyographic activity. A continuous ECG was recorded via limb leads. Both the electrocardiographic and electromyographic signals were recorded with 1 kHz digitized signals on a data acquisition MP36 (BIOPAC, Guleta, USA). Before recording, the subjects were asked to relax in a sitting position for 3 min. For the entire 20-min study process (5 min before CT, 10 min during CT and 5 min after CT), subjects were asked to maintain the correct sitting position without moving. Five-minute ECG and EMG recordings were taken for 3 time periods: baseline (before CT), during intermittent traction and after CT. Each heartbeat was annotated using an automatic arrhythmia detection algorithm, whereas each annotation was verified by visual inspection. The R-R interval time series for each subject was then computed, and extracted to form a time series of normal-to-normal (N-N) sinus intervals.
| Table I. General characteristics and applied traction forces of study subjects |
| No. | Age, years/gender | Body weight, kg | 10% body weight traction, kg | 20% body weight traction, kg |
| 1 | 38/M | 73 | 7 | 14 |
| 2 | 28/M | 88 | 8 | 16 |
| 3 | 34/M | 65 | 6 | 12 |
| 4 | 27/M | 68 | 7 | 14 |
| 5 | 33/M | 62 | 6 | 12 |
| 6 | 39/M | 60 | 6 | 12 |
| 7 | 28/M | 70 | 7 | 14 |
| 8 | 55/M | 71 | 7 | 14 |
| 9 | 32/F | 62 | 6 | 12 |
| 10 | 29/F | 55 | 5 | 11 |
| 11 | 40/F | 51 | 5 | 10 |
| 12 | 36/F | 50 | 5 | 10 |
| 13 | 26/F | 60 | 6 | 12 |
| 14 | 25/F | 50 | 5 | 10 |
| 15 | 25/F | 56 | 5 | 13 |
| 16 | 33/F | 45 | 5 | 9 |
| M: male; F: female. |
The power spectrums were computed using the FFT technique, where VLF power, LF power, HF power, and total power (TP) are the area below the power spectrum density curve at corresponding frequency bands. MSE was calculated in two steps: (i) coarse-graining the signals into different time-scales; (ii) quantifying the degree of irregularity in each coarse-grained time series using sample entropy (SaEn). This was done to investigate the complex dynamics beneath the continuous R-R intervals and the breakdown of complex dynamics, such as the lower values of SaEn, indicating higher predictability (22). Briefly, this method calculates the negative natural logarithm of the conditional probability that similar sequences for m points remain similar when one more point (m + 1) is added to the sequence. The conditional probability is the frequency of occurrence of similar runs of m within the tolerance r. The coarse-grained time series at scale 1 is identical to the original signals. For scale n, the original data were segregated into blocks, where each block contains n data points. The mean of n data points in each block then forms the coarse-grained time series at scale n. Slopes of MSE (scale 1–5) were estimated by the least-squares method. This study used m = 2 and r = 15% of SD for the N-N interval. The ECG signals were analysed using self-designed programs with Matlab 7.0 on a Personal Computer.
The amplitudes of surface EMG signals were analysed with root mean square (RMS) values. RMS values derived from 0.03-second interval sampling rates in 5-min periods (before, during and after traction) under both traction force conditions were compared (25, 26).
The study process was halted if the subject felt undue discomfort. These points of discomfort were noted in the CT record after the traction processes.
Data were processed with statistical software SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA). The categorical variables of related symptoms were compared between the two traction weight groups with the Fisher’s exact test. HRV parameters included linear and non-linear parameters. The linear parameters included the standard deviation of all normal to normal intervals (SDNN), total power, VLFP, LFP, HFP and LFP/HFP. The non-linear parameters were MSE n (n = 1–5), the slope of MSE presented as a mean, and a 95% confidence interval. The differences in the 3 time periods, before, during, and after traction, were statistically compared with a general linear model with repeated measurement. Statistical significance was assumed at p < 0.05.
RESULTS
After the CT, any discomfort the subjects felt was recorded. Discomfort included neck tightness, dizziness, pain, sleepiness, and a cold-sweat sensation. The incidence of neck tightness discomfort under the two traction conditions was significantly higher under 20% body weight traction (Table II). One subject felt undue discomfort with temporomandibular joint pain during 20% body weight traction and the study process was halted. The subject completed the traction one week later.
| Table II. Incidence of related symptoms between two traction force conditions (n = 16) |
| Variables | 10% body weight % (n) | 20% body weight % (n) | p-value |
| Neck tightness | 4 (1) | 50 (8) | 0.015* |
| Dizziness/giddiness | 4 (1) | 25 (4) | 0.333 |
| Pain | 6.25 (1) | 18.75 (3) | 0.600 |
| Sleepiness | 12.5 (2) | 0 (0) | 0.484 |
| Cold sweat | 0 (0) | 6.25 (1) | 1.000 |
| Feeling discomfort | 25 (4) | 75 (12) | 0.012* |
| Fisher’s exact test, *p < 0.05. |
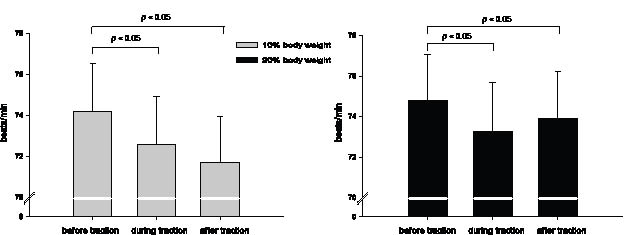
Under the 10% body weight traction force condition, heart rates declined significantly both during and after the CT periods successively (Fig. 1). The heart rates also showed a significant decrease during the CT period in the 20% body weight traction condition. However, during the period following the CT, heart rates did not slow any further. On the contrary, a slight increase occurred under the 20% body weight traction condition.
Fig. 1. Heart rate before, during and after cervical traction with 10% and 20% body weight traction forces. n = 16, mean ± standard error. General linear model with repeated measurement.
Table III list the HRV measurements for the before, during, and after CT periods and the differences within the periods under both traction force conditions. The SDNN displayed a significant reduction during the after CT period in the 20% body weight force condition. The total power in the 20% body weight force condition increased during the CT period and decreased significantly after the CT period. The VLFP in both CT conditions decreased, while the LFP in the 10% body weight force condition increased significantly during the after CT period compared with the CT period. HFP showed no clear change during the different periods under both CT force conditions. The MSE5 decreased significantly for during and after CT periods compared with the before CT period under the 20% body weight traction condition. The slope showed a significant increase only in the after CT period under the 20% body weight traction condition compared with the before and during CT periods.
| Table III. Linear and non-linear parameters of heart rate variability, (n = 16) |
| | Before traction Mean (95% CI) | During traction Mean (95% CI) | After traction Mean (95% CI) | p-value |
| During vs before | After vs before | After vs during |
| Lineal parameters |
| 10% body weight | | | | | | |
| SDNN (ms) | 41.42 (33.51–49.33) | 41.91 (34.37–49.45) | 41.52 (33.44–49.60) | 0.83 | 0.97 | 0.78 |
| Total power (ms2) | 1,679.33 (1,073.60–2,285.06) | 1,718.22 (1,095.10–2,341.35) | 1,648.08 (999.00–2,279.16) | 0.85 | 0.88 | 0.53 |
| VLFP (ms2) | 498.28 (232.41–764.15) | 534.13 (279.52–788.75) | 386.13 (212.49–559.78) | 0.71 | 0.18 | 0.02* |
| LFP (ms2) | 189.43 (138.47–240.39) | 157.00 (108.81–205.19) | 261.75 (160.44–363.06) | 0.13 | 0.14 | 0.01* |
| HFP (ms2) | 148.41 (75.42–221.40) | 155.52 (91.75–219.29) | 177.15 (77.72–276.58) | 0.53 | 0.31 | 0.45 |
| LFP/HFP | 2.67 (1.34–4.00) | 1.87 (0.87–2.86) | 2.38 (1.45–3.31) | 0.11 | 0.61 | 0.06 |
| 20% body weight | | | | |
| SDNN (ms) | 39.75 (35.17–44.34) | 43.88 (37.78–49.98) | 39.48 (33.58–45.37) | 0.05 | 0.89 | 0.02* |
| Total power (ms2) | 1,458.25 (1,141.520–1,774.97) | 1,823.07 (1,344.06–2,302.09) | 1,480.24 (1,075.02–1,885.46) | 0.04* | 0.89 | 0.04* |
| VLFP (ms2) | 416.49 (291.01–541.96) | 563.42 (370.98–755.85) | 404.82 (274.91–534.72) | 0.09 | 0.83 | 0.04* |
| LFP (ms2) | 177.01 (138.80–215.23) | 208.30 (158.48–258.12) | 212.08 (142.13–282.03) | 0.30 | 0.30 | 0.90 |
| HFP (ms2) | 128.90 (83.46–174.34) | 135.16 (90.51–179.80) | 123.48 (80.642–166.31) | 0.66 | 0.75 | 0.40 |
| LFP/HFP | 2.01 (1.23–2.79) | 1.90 (1.43–2.38) | 2.00 (1.50–2.51) | 0.73 | 0.98 | 0.65 |
| Non-lineal parameters |
| 10% body weight | | | | | | |
| MSE1 | 1.26 (1.11–1.42) | 1.31 (1.17–1.45) | 1.26 (1.12–1.41) | 0.39 | 0.98 | 0.16 |
| MSE2 | 1.78 (1.62–1.94) | 1.89 (1.72–1.96) | 1.78 (1.64–1.92) | 0.36 | 0.95 | 0.24 |
| MSE3 | 1.85 (1.67–2.03) | 1.92 (1.79–2.05) | 1.97 (1.66–2.29) | 0.27 | 0.48 | 0.73 |
| MSE4 | 1.82 (1.62–2.02) | 1.97 (1.80–2.14) | 1.97 (1.72–2.23) | 0.21 | 0.36 | 0.97 |
| MSE5 | 1.78 (1.62–1.94) | 1.87 (1.70–2.04) | 1.76 (1.55–1.97) | 0.39 | 0.89 | 0.39 |
| Slope | 0.13 (0.07–0.18) | 0.12 (0.06–0.18) | 0.11 (0.06–0.15) | 0.84 | 0.42 | 0.68 |
| 20% body weight | | | | | | |
| MSE1 | 1.30 (1.16–1.43) | 1.24 (1.14–1.34) | 1.28 (1.18–1.38) | 0.25 | 0.73 | 0.43 |
| MSE2 | 1.84 (1.72–1.96) | 1.82 (1.71–1.92) | 1.83 (1.70–1.96) | 0.66 | 0.85 | 0.87 |
| MSE3 | 1.85 (1.75–1.94) | 1.80 (1.67–1.94) | 1.91 (1.75–2.07) | 0.58 | 0.43 | 0.28 |
| MSE4 | 1.90 (1.74–2.05) | 1.77 (1.63–1.09) | 1.94 (1.69–2.19) | 0.11 | 0.70 | 0.18 |
| MSE5 | 1.93 (1.78–2.07) | 1.65 (1.48–1.83) | 1.62 (1.47–1.77) | 0.03* | 0.00* | 0.76 |
| Slope | 0.08 (0.03–0.13) | 0.08 (0.03–0.13) | 0.13 (0.08–0.18) | 0.93 | 0.04* | 0.01* |
| *p < 0.05. SDNN: standard deviation of all NN interval; VLFP: very low-frequency power; LFP: low-frequency power; HFP: high-frequency power; CI: confidence interval, general linear model repeated measurement; MSE: multiscale entropy, n indicates time-scale, slope of MSE (scale 1–5) were estimated by least-squares method. |
During cervical traction, no apparent surface electromyographic activity change was seen in the right sternocleidomastoid in either the 10% or 20% body weight CT conditions (Fig. 2). Electromyographic activity recorded on the right posterior neck surface showed a fluctuating recruitment pattern occurring with the traction off-on cycle in the 20% body weight condition. In addition, the RMS values derived from during the traction period in the 20% body weight condition increased moderately compared with the before traction period (Fig. 3).
DISCUSSION
No consensus currently exists regarding the correct amount of CT force to use in treatment (5). The CT force is set subjectively in relation to the body weight and the subjective responses of the patient. No quantitative parameter that could reliably avoid side-effects has been proposed. Our study was the first to attempt to access the autonomic response as such an indicator in clinical CT therapy.
The surface electromyographic activity recorded at the right sternocleidomastoid did not display significant change during both 10% and 20% body weight CT trials (Fig. 2). The sternocleidomastoid muscle may have undergone stretching relaxation rather than tonic contraction during CT. Therefore, the baroreceptor of the carotid sinus beneath the sternocleidomastoid muscle might have been stimulated intermittently (6). However, the parameters of SDNN, VLFP, LFP, HFP, LFP/HFP and MSE 1–4 assessed from the HRV analysis also showed no significant change during CT under both traction force conditions, compared with the before CT period (Table III). These data indicate that CT using 10% or 20% body weight traction force is only a minor stimulation for the autonomic system in healthy subjects. Therefore, the decrease in the heart rate during both traction periods could be attributed mainly to the decreased demand on cardiac output due to the comfortable sitting position. In addition, the combinative effects of a slight increase in vagal activities and a slight decrease in sympathetic activities, especially under the 10% body weight condition, instead of a significant enhancement of vagal effect or an inhibition of sympathetic tone alone could provide another explanation. In addition, each subject’s autonomic modulation response to traction stimulation may have been different. The traction force that was suitable for one subject may have been too strong for another. Thus, the overall change in the parameters analysed via HRV measurement would not be statistically apparent.
Fig. 2. A demonstration of electrocardiography (upper panel) and surface electromyography recorded at right posterior neck splenius capitis muscle (middle panel) and right sternocleidomastoid muscle (lower panel) during cervical traction with 10% (left part) and 20% (right part) body weight traction forces. The mean heart rates derived from electrocardiography and root mean square values derived from surface electromyography in three 5-min periods (before, during and after traction) were shown.
The 10% body weight condition could be considered as a mild or even comfortable stimulation, thus the vagal-sympathetic interaction would not manifest itself. The heart rate declined continuously during the 5 min following CT due to the additional residual effect of baroreflex. Thus, the rebounded sympathetic activity that was reflected in the elevation of LFP manifested itself during the after CT period (Table III). This rebound phenomenon helped maintain physiological homeostasis.
Under the stronger traction force condition, the posterior neck muscles, such as the splenius capitis and upper trapezius, contracted to prevent over-stretching of the neck (Fig. 3). Thus, the sympathetic plexus surrounding the vertebral arteries may be stretched and stimulated (27). This stimulated the actions of the stretch-activated mechanoreceptive muscle afferent fibres (28, 29) and maintained sympathetic activity. This action was in contrast to the continuous slight decline in activity under the lighter traction condition. The increase in heart rate was less during the after CT period. The significant changes in MSE 5, slope, SDNN and total power revealed the influence of autonomic modulation in the 20% body weight CT condition (Table III). Thus, posterior neck muscle contraction with autonomic perturbation account for the higher incidence of discomfort in CT with 20% body weight. Thus, if the heart rate does not decline in the post-CT period, and the slope of MSE changes significantly, the subject will tend to feel discomfort. For the clinical therapist treating such cases, traction force should not be increased quickly or should be increased more carefully in the next CT course.
Fig. 3. The root mean square (RMS) values derived from the amplitude of surface electromyography recording at right posterior neck splenius capitus muscle in 5-min periods (before, during and after traction) in 10% and 20% body weight traction conditions. n = 14, mean ± standard error. General linear model with repeated measurement. *During vs before, p = 0.06.
In this study, the correlation between the HR and linear FFT was inconsistent because no simple physiological counterpart was related to these markers and an intrinsic error existed in the linear HRV analytical algorithm. The autonomic system may be too complex and individualized to be characterized by a few HRV markers. Hence, more studies are necessary in order to find physiological markers with better clinical correlation. The non-linear analysis method with MSE and slope calculations was a method used to evaluate the interactions within the autonomic system, including the balance of sympathetic and parasympathetic nerves and the coupling of the cardio-respiratory system. In a previous study (23), the slope of MSE 1 to 5, as in this study, was flat in healthy, elderly patients, and negative in severely autonomic dysfunctional patients, such as those with congestive heart failure (23). In this study, elevated slope and mild elevation with decreased MSE 5 occurred in the patients receiving heavy traction, meaning that the autonomic interaction was interrupted by the external stimulation and responsive to it.
The autonomic nervous system consists of sympathetic and parasympathetic nerves, which play an important role in the regulation of the physiological processes during normal and pathological conditions (30). If the sympathetic and parasympathetic tones reach a balance, the process of homeostasis is subconscious or only occurs with mild discomfort without symptoms. The autonomic symptoms would not occur. On the other hand, if the modulation does not compensate for the response caused by the stimulation, severe discomfort with various symptoms will occur. In our study, although differences in heart rates in during and before CT periods occurred under both traction force conditions, they were within the normal physiological range. For all the parameters of the autonomic system, only MSE 5 and the slope of MSE 1–5 showed some difference, which means that only a mild interaction occurred in the 20% body weight CT condition. That was compatible with the higher incidence of discomfort and without severe symptom in the 20% body weight CT condition. Following the same reasoning, the relative safety of mechanical intermittent CT used clinically in the sitting position with a force of 10% and 20% body weight in healthy people was revealed in this study. This was compatible with the observation in the study by Tsai et al. (31).
However, those who arrange to receive CT therapy often have some co-morbidity in other body systems, such as cardiovascular or neuropathic disorders. Each patient must be treated as an individual. Moreover, autonomic changes differ with respect to different CT positions, such as the supine position, and different traction approaches, such as using continuous force. Heart rates did not change significantly in the lying position in a study by Akinbo et al. (3), but they changed significantly in the sitting position in a study by Utti et al. (6). Because of the small space requirement, the sitting position in CT is frequently applied in Taiwan, especially in out-patient services. In this pilot study, the sitting position was investigated first and altered heart rates were also found. However, the sample size is small and the subjects are only healthy people. The heart rate response and autonomic modulation in other CT variations remain to be proved with larger sample size in further research.
Considering the confounding effect of the sphygmomanometer and the stability of ECG signals, blood pressure was not measured in this pilot study. Thus haemodynamic information was lacking and could not be compared with previous studies (3, 6). A new design is required in future research to prevent signal interference when using the tourniquet.
The results of the current study show that intermittent CT in a sitting position using 10% body weight traction force is more comfortable than using 20% body weight traction force. Using 20% body weight traction force causes more subtle perturbation in the autonomic system and is accompanied by a higher incidence of discomfort. The response pattern of heart rate and increased slope of the non-linear parameters of the HRV analysis may provide early indication of this discomfort. However, the autonomic modulation and the safety of CT with other modality settings or in patients with neck pain or co-mobility diseases requires further research with larger samples.
ACKNOWLEDGEMENTS
This study was supported by grants from National Yang-Ming University Hospital (RD2009-017).
REFERENCES