OBJECTIVE: To investigate the feasibility and effects of a physical exercise programme on functioning and health-related quality of life in adults with myotonic dystrophy type 1.
DESIGN: A randomized controlled trial.
SUBJECTS: Thirty-five adults with myotonic dystrophy type 1.
METHODS: After stratification for level of functioning, study participants were assigned by lot to either a training group or a control group. Training-group participants attended a 60-minute comprehensive group-training programme, Friskis&Svettis® Open Doors, twice a week for 14 weeks. The six-minute walk test was the primary outcome measure and the timed-stands test, the timed up-and-go test, the Epworth sleepiness scale and the Short Form-36 health survey were secondary outcome measures.
RESULTS: Intention-to-treat analyses revealed no significant differences in any outcome measures, except for an increased between-group difference after intervention in the Short Form-36 mental health subscale and a decrease in the vitality subscale for the control group. The programme was well tolerated and many training-group participants perceived subjective changes for the better. No negative effects were reported.
CONCLUSION: The Friskis&Svettis® Open Doors programme was feasible for adults with myotonic dystrophy type 1 who had been screened for cardiac involvement, had distal or mild-to-moderate proximal muscle impairment, and no severe cognitive impairments. No beneficial or detrimental effects were evident.
Key words: exercise; neuromuscular disease; physical education and training; physical therapy; rehabilitation.
J Rehabil Med 2011; 00: 00–00
Correspondence address: Marie Kierkegaard, Department of Physical Therapy, R1:07 Karolinska University Hospital, SE-171 76 Stockholm, Sweden. E-mail: marie.kierkegaard@karolinska.se
Submitted January 31, 2011; accepted April 29, 2011
Introduction
People with progressive neuromuscular diseases are less active than healthy people (1). This may lead to an increased risk of secondary disorders and disabilities that are not caused by the disease itself, but rather by inactivity. Regular physical activity is an important factor for general health and disease prevention (2). Furthermore, being physically active is associated with better health-related quality of life, and in older adults with better cognitive function and a reduced risk of falling (2). There is accumulating evidence for prescribing physical activity in the treatment of a number of chronic diseases (2). The scientific evidence supporting recommendations about physical activity and exercise in neuromuscular disease is, however, limited.
Myotonic dystrophy type 1 (DM1) is a slowly progressive neuromuscular disease with an estimated European prevalence of 5–20 per 100,000 (3). It is caused by an unstable expansion of a CTG-repeat on chromosome 19 and is inherited in a dominant pattern. DM1 can be subdivided into several forms based on the clinical presentation and the CTG repeat size, as follows: congenital, childhood, classic adult, and mild adult forms. Characteristic symptoms are myotonia, and muscle weakness and wasting in neck and facial muscles, and in a distal-to-proximal progression order in the limb muscles. In addition to the muscles, other organs and systems are affected, and common problems include excessive daytime sleepiness, and impairments in cognitive executive functions and in the functions of the cardiovascular and respiratory system.
Exercise programmes, in which at least 5 people with DM1 have participated, consist of resistance training (4–8), aerobic training (9, 10) and qigong (11, 12). Major shortcomings of these studies are the inclusion of persons with various neuromuscular diseases (4, 5, 9, 11, 12), small sample sizes, i.e. less than 20 persons with DM1 (4, 5, 7–12) and the lack of a randomized controlled trial design (4, 5, 7–10). Thus, it is difficult to assess to whom the findings can be generalized. There are, as far as we know, no studies evaluating the effects of a more comprehensive training programme available outside the healthcare system.
When promoting physical activity and exercise for people with DM1, reference settings outside the healthcare system are important. Friskis&Svettis® is a Swedish non-profit sports association and has, in cooperation with the Swedish Association of People with Mobility Impairments (DHR), the Swedish Association for Persons with Neurological Disabilities (NHR) and the Swedish Association for Survivors of Accident and Injury (RTP), developed a special form of training programme for persons with disability called “Öppna dörrar” (Open Doors). This form of exercise seemed to be appropriate for people with DM1. However, before referring them to Open Doors classes, an evaluation of the feasibility and effects of the programme was required. Thus, the present objectives were to investigate the feasibility and effects of the physical exercise programme, the Friskis&Svettis® Open Doors, on functioning, in particular walking capacity, lower-extremity performance, mobility and balance, excessive daytime sleepiness, and health-related quality of life, in adults with DM1.
Methods
Participants
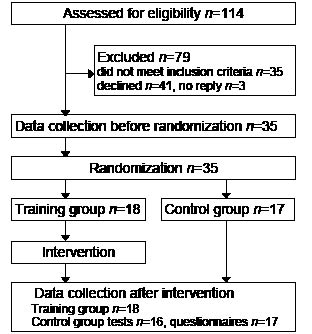
Participants were recruited from a register of adults with DM1, most of whom were identified in connection with a previous study (13), held at the Karolinska University Hospital, Stockholm, Sweden. Inclusion criteria were: diagnosed DM1; living in the Stockholm County Council area; aged 18 years or older; ability to walk 50 m without assistance; permission from a cardiologist to take part in an exercise programme; and classified as grade 2–5 on the muscular impairment rating scale (MIRS) (14). The MIRS is a disease-specific 5-point scale for monitoring major stages of disease progression, ranging from 1 = no muscular impairment to 5 = severe proximal weakness. Exclusion criteria were: inability to communicate in Swedish; clinically obvious severe cognitive impairment; and other diagnoses that could interfere with participation. Of 114 eligible persons, 35 did not fulfil the inclusion criteria (24 were unable to walk 50 m, 4 were classified as MIRS 1, 4 had severe cardiac arrhythmia, 2 had other concurrent diagnoses and 1 did not speak Swedish). Information about the study was sent by post, and followed up by a telephone call. Three persons did not reply and 41 declined to participate, some giving spontaneously the following reasons: lack of time (n = 15), already exercising (n = 8), too far to travel (n = 4), and not interested (n = 4). Thus, 35 persons with genetically confirmed DM1 were included in the study. Both oral and written information were provided, and all participants gave their signed informed consent before enrolment. The study was approved by the Regional Ethical Review Board in Stockholm and procedures were conducted in accordance with the Declaration of Helsinki.
Design and procedures
This study was a randomized controlled trial with data collections before and after the intervention by two independent experienced physiotherapists, blinded to group allocation and each assessing the same participants on both occasions. Stratification for level of functioning was based on the six-minute walk test (6MWT) (15). The median value of the 6MWT results from data collection before the intervention was used to divide participants into two strata, from which they were assigned by lot to either a training group or a control group. The lots were drawn by a person who was not involved in any other part of the study. Since participants were recruited before randomization, concealed allocation procedures were applied. The study design is shown in Fig. 1.
Measures
Anthropometric measures and information on level of physical activity were collected for descriptive purposes. Body mass index (BMI) was calculated and waist circumference was measured. Published reference values and cut-off points were used for classifying adult underweight, normal weight and overweight, and an increased risk of metabolic complications, respectively (16). Physical activity levels during the previous summer and winter half-years were rated by participants on a 6-graded scale (17), which has been modified and also consider household activities (18), ranging from hardly any physical activity to heavy or very heavy exercise regularly several times a week.
For feasibility evaluation purposes, the exercise self-efficacy scale (ESES) questionnaire (19) was used to assess self-efficacy beliefs related to confidence in performing an exercise programme despite potential barriers, e.g. work schedule, physical fatigue, boredom related to exercise, minor injuries, other time demands, and family and home responsibilities. The scale consists of 6 items and possible scores range from 6 to 60, higher scores indicating higher confidence. The scale has been used in a Swedish primary care setting and is considered sufficiently reliable (19).
A study-specific questionnaire, developed by the first author (MK), was used to evaluate perceived experience and effects of the exercise intervention and was answered by participants in the training group after the training period. Perception of the form and intensity of the exercise programme was rated on 5-grade scales, ranging from 1 = very bad to 5 = very good, and from 1 = much too easy to 5 = far too strenuous, respectively. The participants were also asked whether they would recommend the exercise programme to others with DM1. Self-rated changes in fitness, strength, flexibility and daytime sleepiness were also rated on 5-grade scales ranging from 1= much worse to 5 = much better.
The primary outcome measure was the 6MWT (15), a performance-based test of walking capacity and exercise tolerance (20), administered according to the ATS guidelines (21) on a 30-m track in a corridor, as described elsewhere (22). Post-walk perceived rate of exertion was rated on the Borg’s rating of percieved exertion (RPE) scale® (23), which ranges from 6 (nothing at all) to 20 (maximal). The 6MWT is considered valid and reliable in various populations and is used as an outcome measure for evaluation of exercise programmes (20, 22, 24). A difference above or equal to 6% in distance walked between occasions was judged as a minimally clinically important change (22).
The timed-stands test (TST) (25) was used as a measure of lower-extremity performance, and is considered as a proxy for lower-extremity strength. Following a practice trial, the time taken to rise and sit down 10 times as quickly as possible, without using the arms, from a chair without armrests (0.45 m seat height) was recorded. The test is reportedly valid and reliable for lower-extremity function (25, 26).
The timed up-and-go test (TUG) (27) was used as a measure of mobility and balance. Following a practice trial, the time taken to complete the task of standing up from a chair with armrests (0.45 m seat height), walk 3 m, turn, walk back and sit down, was recorded. The persons were instructed to walk at their preferred self-selected speed and timing was begun when their back left the back of the chair and stopped when the buttocks touched the seat of the chair again. The test is common in rehabilitation and has been tested for validity and reliability (20, 27).
The Epworth sleepiness scale (ESS) (28) was used for evaluating excessive daytime sleepiness. The ESS consists of 8 items about how likely the respondent is to fall asleep/doze off in different situations. Possible scores range from 0 to 24, a higher score indicating more sleepiness. Scores above 10 (≥ 11) indicate the presence of excessive daytime sleepiness. The scale is considered reliable in DM1 (29).
The Short Form-36 (SF-36) health survey (30) was used for evaluating health-related quality of life (HRQoL). The scale consists of 36 items and all but one, change in health status, are grouped into 8 health subscales: physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), general health perceptions (GH), vitality (VT), social functioning (SF), role limitations due to emotional problems (RE) and mental health (MH). Possible scores for each subscale range from 0 to 100, a higher score indicating a better health state. The GH, VT and MH subscales are bipolar: their middle values represent no negative evaluation of the personal health, lack of symptoms of tiredness, and absence of psychosocial disability, respectively. The SF-36 is a generic questionnaire and is considered both valid and reliable (30).
Intervention
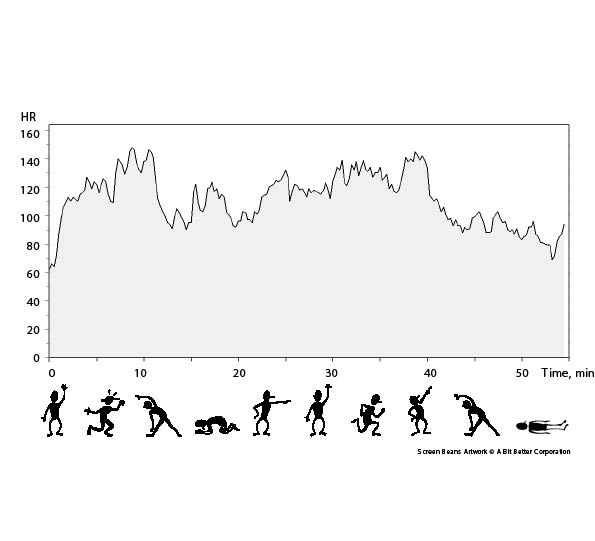
The intervention consisted of the Friskis&Svettis® Open Doors programme, a comprehensive group exercise training programme supported by music. All sessions were supervised by a specialized physiotherapist (MK) and the training set-up consisted of 9–10 min warm-up exercises followed by 3–4 min flexibility exercises. Strength exercises for arms, back and abdomen were then performed on all fours, and prone and supine, for 6–7 min. This was followed by balance exercises in standing for 3–4 min. Aerobic activities followed for 11–12 minutes, and after 9–10 min cool-down exercises the programme was rounded off with stretching and relaxation (Fig. 2). The programme could be adapted to the individual’s level of physical capability and capacity; for example, by performing the exercises with different pace and lever arms, and in varied positions, if necessary. Persons in the training group were asked to participate in the 60-min training programme twice a week for 14 weeks at the Department of Physical Therapy, Karolinska University Hospital, and they had two midday and two afternoon classes every week to chose between. The intensity during the aerobic parts of the programme was intended to represent 60–80% of maximum heart rate and actual heart rate was measured with a portable heart-rate recorder (Polar S610i™) on several occasions during the training period. Maximum heart rate was defined as a heart rate (in beats/min) of 220 minus the person’s age in years.
Fig. 2. Group exercise programme and heart rate (HR) measured during a training session for 1 of the participants, a 43-year-old man.
Acceptable adherence was set to 75% attendance, i.e. participation in at least 21 of the required 28 group training sessions. The training group participants were also offered a 48-h electrocardiogram (ECG) examination during the intervention period to ascertain that there were no negative cardiac effects. They were also to take at least one brisk 30-min walk every week, and to document these in an exercise diary. The control group participants were advised to live their normal lives and not to change their physical activity behaviour during the study period.
Data analysis
Drop-out analyses were performed with a Fisher’s exact test for sex and unpaired t-test for age. Descriptive statistics were used to present mean and standard deviation (SD) or median and interquartile range (IQR), and minimum and maximum values. Attendance at Open Doors classes, the exercise diaries, heart rate recordings, the 48-h ECG recordings, information from the study-specific questionnaire on perceived experience and the ESES were used to explore the feasibility of the exercise regime. The 6MWT was chosen as primary outcome measure and the TST, TUG, ESS and SF-36 as secondary outcome measures for evaluation of effects of the exercise regime. The study-specific questionnaire on perceived effects was used to evaluate self-rated changes in fitness, strength, flexibility and daytime sleepiness in the training group.
Descriptive statistics and Spearman’s rank correlation were used to explore associations between exercise self-efficacy beliefs (ESES scores) and attendance. An intention-to-treat analysis approach was applied and a linear mixed-model repeated-measurement analysis using the compound symmetry covariance structure was used for evaluating within-group, between-group and interaction effects for 6MWT, TST and TUG data. Residual and normality plots were used to examine the assumptions of the fitted model. Borg RPE ratings of perceived exertion, ESS and SF-36 subscale scores did not fulfil the requirements for this statistical model and the Wilcoxon matched-pairs signed-rank test was therefore used for within-group analyses and the Mann–Whitney U test for between-group analyses after the intervention. The level of significance was set at p < 0.05. All the analyses were performed using IBM SPSS Statistics 18.
Results
There was no significant difference in sex or age between participants in the study and the 44 non-participants. The training group comprised 10 women and 8 men, mean age 44 years, standard deviation (SD) 11, range 20–60 years. The control group comprised 10 women and 7 men, mean age 41 years, SD 15, ranging from 20 to 65. Participant characteristics including personal and environmental factors are shown in Table I.
| Table I. Participant characteristics, personal and environmental factors, before intervention (training group n = 18, control group n = 17) |
| | Training group | Control group |
| Form of DM1, classic adult/childhood, n | 17/1 | 12/5 |
| Inheritance, maternal/paternal/not known, n | 5/10/3 | 7/7/3 |
| MIRS grade, n | | |
| 2/3, n | 3/1 | 1/6 |
| 4/5, n | 12/2 | 10/0 |
| Medicines, n | | |
| CNS stimulants (Modiodal®, Ritalin®), n | 10 | 3 |
| Levothyroxine (Levaxin®), n | 3 | 3 |
| BMI, kg/m2, mean (SD) | 24.0 (5.2) | 21.8 (4.3) |
| Underweight, n | 2 | 3 |
| Normal weight, n | 9 | 10 |
| Overweight, n | 7 | 4 |
| Waist circumference, cm, mean (SD) | 89 (17) | 83 (13) |
| Increased risk metabolic complications, n | 7 | 5 |
| Smoking/snuffing habits, smokers/snuffers, n | 4/1 | 4/3 |
| Education level, university, n | 5 | 5 |
| Civil status, cohabiting with partner, n | 9 | 9 |
| Employment status, working full-/part-time, n | 6/2 | 2/4 |
| Aids, ADL/orthotic/mobility, n | 7/5/1 | 5/3/1 |
| Physical activity level summer, n | | |
| Mostly sitting to light | 10 | 13 |
| Moderate 1–2 h/week | 6 | 3 |
| Moderate > 3 h/week to hard | 2 | 1 |
| Physical activity level winter, n | | |
| Mostly sitting to light | 13 | 12 |
| Moderate 1–2 h/week | 4 | 4 |
| Moderate > 3 h/week to hard | 1 | 1 |
| DM1: myotonic dystrophy type 1; MIRS: muscular impairment rating scale; CNS: central nervous system; BMI: body mass index; SD: standard deviation; ADL: activities of daily living. |
Three persons had missing data of perceived exertion from the data collection before the intervention and one person in the control group did not attend the data collection after the intervention. He did, however, at that time-point, complete the questionnaires posted to him. Three training group participants with low attendance did not perform the 48-h ECG examination and another two did not include a training session during the recording.
Feasibility
Eleven of 18 persons in the training group had at least 75% attendance (participated ≥ 21 times), i.e. acceptable adherence to the Open Doors programme. Three participated between 15 and 17 times and another 4 between 3 and 5 times. Exercise diaries showed that 8 persons, of whom 7 had acceptable adherence, took additional 30-min walks in at least 11 of the required 14 weeks. Another 7 took between 2 and 10 walks, and 3 persons did not complete the exercise diaries.
Pooled data from 16 persons’ heart-rate recordings during training sessions showed that the median time was 32 (IQR 20–44) min per session, at a heart rate over or equal to 60% of calculated maximum heart rate. Analyses of 48-h ECG recordings revealed that one person had periods of atrial arrhythmia, however not during or in connection with the training. This person was assessed by a cardiologist, received medical treatment and was allowed to complete the study. No other adverse effects were reported
The training programme was labelled as “good” or “very good” by 16 of the 18 persons in the training group, and 17 would recommend this form of physical exercise for others with the same disease. The intensity was perceived as sufficient by 13, but too strenuous by 4 persons with proximal muscle impairment, MIRS 4–5.
The exercise self-efficacy scores were: median 35 (IQR 23–44) in the training group and median 25 (IQR 21–35) in the control group before the intervention. Corresponding values after the intervention were: median 30 (IQR 21–42) and median 31 (IQR 20–34) in the training and control group, respectively. Exercise self-efficacy scores in the training group correlated positively with attendance, non-significantly before the intervention (rs = 0.38, p = 0.117) and significantly afterwards (rs = 0.75, p < 0.001).
Effects
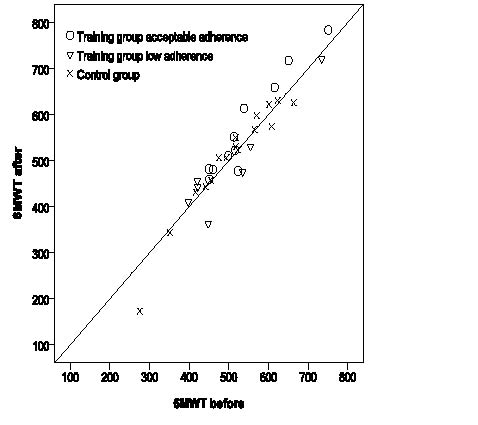
Data from tests are presented in Table II. The mean 6MWT distance increased by 9 m in the training group and decreased by 2 m in the control group. The linear, mixed-model, repeated-measurement analysis revealed no significant differences in mean 6MWT distance, TST time or TUG time within-group or between-groups, or any interaction effects (p-values ranging from 0.18 to 0.85). Pair-wise comparisons are for descriptive purposes presented in Table III. Six persons, of whom 5 had acceptable adherence, in the training group and 2 in the control group increased their 6MWT distance by ≥ 6%. Fig. 3 presents 6MWT distances from tests before and after the intervention for the training-group participants with acceptable and low adherence, and for the control group.
| Table II. Data from tests before and after the exercise intervention |
| | Training group, n = 18 | Control group, n = 17 |
| Mean (SD) | Min–max | Mean (SD) | Min–max |
| Six-minute walk test, m | | | | |
| Before | 527 (103) | 398–752 | 507 (100) | 276–665 |
| After | 536 (116) | 363–783 | 505a (119) | 173–630 |
| Timed-stands test, s | | | | |
| Before | 23.8 (6.6) | 9.9–39.1 | 22.7 (6.7) | 14.7–36.1 |
| After | 23.2 (7.2) | 8.5–36.3 | 23.1a (9.9) | 12.5–50.8 |
| Timed up-and-go test, s | | | | |
| Before | 7.9 (2.2) | 4.0–12.3 | 7.1 (1.5) | 4.8–10.2 |
| After | 7.4 (2.2) | 4.2–13.0 | 7.1a (1.9) | 5.1–12.4 |
| SD: standard deviation. an = 16. |
| Table III. Pair-wise comparisons, result of simple main effects from mixed-model analyses for the six-minute walk test, the timed-stands test and the timed up-and-go test |
| | Mean difference | SE | 95% CI | p-value |
| Six-minute walk test, m | | | | |
| Before: training – control group | 20 | 37 | –55–95 | 0.58 |
| After: training – control group | 31 | 37 | –43–106 | 0.40 |
| Training group: after – before | 9 | 9 | –9–27 | 0.33 |
| Control group: after – before | –2 | 10 | –22–17 | 0.82 |
| Timed-stands test, s | | | | |
| Before: training – control group | 1.1 | 2.6 | –4.1–6.3 | 0.67 |
| After: training – control group | 0.3 | 2.6 | –4.9–5.5 | 0.91 |
| Training group: after – before | –0.6 | 1.4 | –3.4–2.2 | 0.67 |
| Control group: after – before | 0.2 | 1.5 | –2.7–3.2 | 0.88 |
| Timed up-and-go test, s | | | | |
| Before: training – control group | 0.8 | 0.7 | –0.5–2.2 | 0.22 |
| After: training – control group | 0.3 | 0.7 | –1.1–1.6 | 0.69 |
| Training group: after – before | –0.5 | 0.3 | –1.1–0.03 | 0.06 |
| Control group: after – before | 0.03 | 0.3 | –0.6–0.6 | 0.93 |
| SE: standard error; CI: confidence interval. |
Fig. 3. Six-minute walk test (6MWT) distance from tests before and after the intervention (training group acceptable adherence n = 11, training group low adherence n = 7, control group n = 16).
Post-walk ratings of perceived exertion and data from questionnaires are presented in Table IV. There were no significant between-group differences after the intervention, except for the MH subscale in favour of the control group (see Table IV). Within-group analyses showed no significant differences except for a decrease in the VT subscale in the control group (p = 0.027).
| Table IV. Borg rating of percevied exertion ratings after the six-minute walk test (6MWT) and data from questionnaires before and after the exercise intervention, p-values from between-group analyses after the intervention |
| | Training group, n = 18 Median [IQR] (min–max) | Control group, n = 17 Median [IQR] (min–max) | p-valuea |
| Borg RPE score: 0–20 | | | |
| Before | 15b [13–15] (13–17) | 13c [12–13] (9–16) | |
| After | 14 [13–15] (11–17) | 15d [13–17] (9–18) | 0.117 |
| ESS score: 0–24 | | | |
| Before | 12 [8–15] (4–18) | 8 [4–13] (1–18) | |
| After | 12 [8–16] (2–19) | 9 [6–14] (0–21) | 0.297 |
| SF-36, score: 0–100 | | | |
| Physical functioning (PF) | | | |
| Before | 63 [48–76] (10–95) | 65 [40–90] (15–95) | |
| After | 60 [45–84] (20–95) | 60 [48–70] (20–95) | 0.868 |
| Role limitations, physical (RP) | | | |
| Before | 50 [0–100] (0–100) | 75 [38–100] (0–100) | |
| After | 50 [25–100] (0–100) | 100 [13–100] (0–100) | 0.286 |
| Bodily pain (BP) | | | |
| Before | 73 [39–85] (20–100) | 74 [41–100] (31–100) | |
| After | 62 [41–88] (12–100) | 62 [41–92] (31–100) | 0.689 |
| General health perceptions (GH) | | | |
| Before | 54 [34–73] (10–87) | 62 [38–72] (10–92) | |
| After | 55 [38–72] (10–82) | 52 [40–70] (10–90) | 0.869 |
| Vitality (VT) | | | |
| Before | 55 [39–61] (5–80) | 55 [40–68] (5–90) | 0.791 |
| After | 45 [39–58] (10–75) | 40 [25–63] (5–80) | |
| Social functioning (SF) | | | |
| Before | 94 [72–100] (25–100) | 88 [69–100] (50–100) | |
| After | 75 [59–100] (0–100) | 88 [63–100] (38–100) | 0.471 |
| Role limitations, emotional (RE) | | | |
| Before | 67 [33–100] (0–100) | 100 [67–100] (0–100) | |
| After | 83 [33–100] (0–100) | 100 [33–100] (0–100) | 0.872 |
| Mental health (MH) | | | |
| Before | 80 [68–89] (44–100) | 72 [66–92] (48–96) | |
| After | 72 [67–85] (40–92) | 84 [80–92] (36–100) | 0.041 |
| ESS: Epworth sleepiness scale, SF-36: Short Form-36 health survey. ap-values from Mann–Whitney U tests after the intervention; bn = 17; cn = 15; dn = 16. |
The study-specific questionnaire on perceived effects showed that 1 person reported deterioration in muscle strength, and another a worsening in daytime sleepiness. Improvements in fitness, muscle strength, flexibility and excessive daytime sleepiness were reported by 14, 7, 10 and 8 persons, respectively.
Discussion
Knowledge of the feasibility and effects of different physical exercise programmes in neuromuscular diseases is required in order to provide evidence-based recommendations. The main findings of this study contribute to that body of knowledge: a comprehensive group exercise training programme such as the Friskis&Svettis® Open Doors scheme is feasible for persons with DM1, and no detrimental effects were shown.
Intention-to-treat analyses revealed no evidently beneficial or harmful effects. The latter is an important finding when the target population has a progressive disease. The only significant between-group difference was found in the SF-36 MH subscale. A finding of uncertain clinical value, since this subscale is bipolar and any value above 50 indicates absence of psychosocial disability. The lack of statistically significant differences does not per se mean that there were no effects, but merely that an effect of zero cannot be ruled out (31). Furthermore, significant differences are not the same as clinically important or relevant differences. That almost all training-group participants who improved their 6MWT walking distance ≥ 6% had at least 75% attendance implies that a possible dose-response relationship might explain some of the results.
Adherence is a possible threat to the validity and outcome of any intervention study, especially when persons with DM1 are involved, since cognitive and behavioural problems are common aspects of the disorder (3). Supervised training sessions are therefore stressed (7) and bring about better adherence (11, 12). We used various strategies to improve exercise adherence, but the time and place for the training sessions might have negatively influenced attendance. The motivation level for the Open Doors classes was generally high, and the most common reported reason for not attending was illness. However, 2 of the 4 persons who came to only a few training sessions had severe proximal muscle impairment, and perhaps they needed a special introduction or another form of physical exercise. A benefit of the group-training set-up was, besides being cost-effective, the opportunity for participants to meet others with the same disease, acknowledged as an important factor (11). At the same time, this can be bothersome and might have been the reason for low attendance of a newly-diagnosed person with few symptoms of the disease. Nevertheless, most participants in the training group seemed to appreciate the access to social support, which in the shape of social persuasion and comparison is known to influence self-efficacy beliefs (32).
Self-efficacy, i.e. a person’s judgment of his or her capability to do particular things, correlates with participation in physical activity and is a determinant of exercise adherence in adults (33). Although exercise self-efficacy scores in the training group correlated positively with attendance, the strongest association was with scores rated after the intervention. Perhaps training-group participants became more aware of their abilities to engage in exercise under varying conditions and made more realistic assessments after the intervention. It is important to examine exercise self-efficacy in conjunction with contextual factors in order to identify both persons likely to adhere and potential barriers. Specific barriers to exercise in persons with neuromuscular disease have been reported (34). These, and other contextual factors, should be explored in the DM1 population in order to identify possible targets to increase exercise adherence.
The Friskis&Svettis® Open Doors programme was a well-rounded physical activity programme as recommended for general health and well-being in adults with chronic disease (2). The combination of frequency, intensity and duration of exercise provides the overload stimulus that is necessary for producing training effects. Pooled data from heart-rate recordings indicated that the desired intensity during the aerobic parts of the programme was achieved. It can, however, be argued that both frequency and intensity were too low to generate training effects in the variables evaluated. At the same time, the aim was to study the feasibility and effects of a programme that would apply in the real world, and reflect a frequency level that persons with DM1 could incorporate and maintain in their daily lives. Important findings were that no negative cardiological effects were found, and that the training programme appeared to be well tolerated.
A major problem in clinical trials of rare diseases is sample selection and sample size, and there is a risk of selection bias in intervention studies involving physical exercise. People who are willing to participate are often younger and/or have a better functional level than the entire relevant population (35).This might also be true for the present study, although the study-participants’ reported physical activity levels and the examples of reasons for declination imply that both physically active and inactive persons were represented. Efforts were made to include as many persons with DM1 as possible. However, only 35 of those fulfilling the criteria for participation were interested. This resulted in a heterogeneous group with respect to onset form and muscular impairment. This may be a shortcoming, but it reflects reality and the DM1 population. The consequence of a small and heterogeneous sample is usually an underpowered study. However, these studies are not without value since their results can contribute to the larger body of evidence (36).
The selection of primary outcome measure was based on the assumption that it would mirror endurance and functional exercise capacity. Although the 6MWT is both feasible and reliable in DM1 (22), there are some issues to consider. An increase in walking distance implies that walking speed is increased, achieved by a change in stride length and/or cadence. Crucial for accomplishing this is distal lower-limb muscle strength, which correlates significantly with both walking speed and the rate of stumbles and falls in persons with DM1 (1). Hence, a person with DM1 who cannot increase his or her distal lower-limb muscle strength might have difficulty increasing their walking speed without stumbling and falling. Furthermore, as with all performance-based tests, motivation and cooperation are important factors for maximal performance. As lack of initiative and motivation, and apathetic attitudes can be features of DM1, special precautions are needed when administering the tests. Each evaluator emphasized to the participants that they were to achieve their best possible performance. The order, instructions, and encouragement were, however, standardized according to a study protocol, which was the same for both data collection occasions, to avoid biasing the results. All participants understood and completed the tests, but it can be speculated that some might not have performed at their maximal level.
As far as we know, the present study is the first to evaluate a comprehensive group exercise programme, also available outside the healthcare system, for persons with DM1. The small study sample made it difficult to draw any conclusions concerning the effects, and multi-centre studies are required in order to overcome this shortcoming. Despite the lack of obvious improvements in the outcome measures, a majority of participants in the training group perceived subjective positive effects and reported changes for the better in fitness, strength, flexibility and excessive daytime sleepiness. Furthermore, many characterized the Friskis&Svettis® Open Doors programme as “very good” and would recommend it to others with DM1, indicating that this type of exercise regime may be appropriate. However, persons with severe proximal muscle impairment might need more individualized forms of physical exercise, perhaps within a healthcare setting.
In conclusion, the group exercise training programme, the Friskis&Svettis® Open Doors, was well tolerated and feasible for persons with DM1 who had been screened for cardiac involvement, had distal or mild-to-moderate proximal muscle impairment and no severe cognitive impairments. No beneficial or detrimental effects were evident.
Acknowledgements
This research was supported by grants from the Einar Belvén Foundation, the Norrbacka-Eugenia Foundation, Stockholm, the Swedish Association of Registered Physiotherapists and the Swedish Association for Persons with Neurological Disabilities (NHR). Financial support was also provided through the strategic research programme in Care Sciences (SFP-V), Karolinska Institutet, and the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet. The authors thank Lisbet Broman for research and statistical consultation. We also thank Margareta Jonsson, Susanne Littorin and Ulla Persson for assistance in data collection and Nawzad Saleh for help with evaluation of cardiac function.
References