Tae-Du Jung, MD1*, Jun-Yeon Kim, MD1*, Yang- Soo Lee, MD1, Dong-Hyup Kim, MD1,
Jae-Jun Lee, MS2, Jee-Hye Seo, MS2, Hui Joong Lee, MD3 and Yongmin Chang, PhD2,3,4
From the 1Department of Physical Medicine and Rehabilitation, 2Department of Medical & Biological Engineering,
3Department of Diagnostic Radiology and 4The Department of Molecular Medicine, Kyungpook National University
Hospital/Kyungpook National University School of Medicine, 200 Dongduk-Ro Jung-Gu Daegu, Korea.
*Both authors contributed equally to this work.
OBJECTIVE: We report here the case of a 52-year-old Korean woman who was initially diagnosed with non-fluent/global crossed aphasia.
Methods and results: Initial computed tomography of the brain revealed a haematoma of approximately 40 ml in the right basal ganglia area and cavitation around the right lateral ventricle. Three years after onset the aphasia was resolved to a conduction aphasia and she had an ongoing left-sided gait disturbance. Follow-up anatomical magnetic resonance imaging found no recurrence of haemorrhage. Language functional magnetic resonance imaging was examined before and after repetitive transcranial magnetic stimulation treatment. A 90-mm round coil stimulator was used and the repetitive transcranial magnetic stimulation treatment location was P3 on the 10–20 International electrode placement system (1 Hz, 20 min per day for 10 days over a 2-week period). Functional magnetic resonance imaging results before repetitive transcranial magnetic stimulation treatment showed no significant activity in either the ipsilesional or contralesional hemispheres for noun generation and sentence completion paradigms (p < 0.001, cluster size 128). Compared with the pre-treatment phase, following repetitive transcranial magnetic stimulation treatment the data from functional magnetic resonance imaging revealed significant activations in the right inferior frontal lobe (Broca’s area), posterior temporal gyrus (Wernicke’s area), and parietal lobe for both the noun generation and sentence completion tasks (p < 0.001, cluster size 128).
CONCLUSION: This functional magnetic resonance imaging case study is the first to suggest the use of repetitive transcranial magnetic stimulation for improving language outcome in a patient with crossed aphasia. In addition, we report the value of language functional magnetic resonance imaging before and after repetitive transcranial magnetic stimulation treatment for determining the effect of treatment and the underlying neurobiological mechanism of functional recovery following repetitive transcranial magnetic stimulation treatment.
Key words: crossed aphasia; functional magnetic resonance image; repetitive transcranial magnetic stimulation.
J Rehabil Med 2010; 42: 973–978
Correspondence address: Yongmin Chang, Department of Molecular Medicine, Kyungpook National University Hospital, 700-412 Daegu, South Korea. E-mail: ychang@knu.ac.kr
Submitted March 1, 2010; accepted September 21, 2010
Introduction
Improvement in the fluent command of language in patients with post-stroke aphasia is one of the most important issues in neuroscience; however, our understanding of the recovery mechanism and treatment of post-stroke aphasia is incomplete (1, 2). Previous functional imaging studies have provided conflicting evidence regarding whether ipsilesional or contralesional neuronal reorganization of the cortex responsible for language can help improve fluent language command in patients with aphasia (3, 4). Belin et al. (5), Rosen et al. (6), Naeser et al. (7) and Martin et al. (8) have suggested that contralesional overactivation might be maladaptive in chronic, non-fluent aphasia patients. Improved naming and propositional speech in non-fluent aphasia patients have been observed when repetitive transcranial magnetic stimulation (rTMS) was used to suppress the right pars triangularis portion of the right Broca’s homologue (contralesional hemisphere) (9–12). A recent functional magnetic resonance imaging (fMRI) study (13) observed that improved naming and phrase length in a chronic non-fluent aphasia patient was associated with new activation in the left hemisphere (ipsilesional) following suppression of the right pars triangularis with 1 Hz rTMS for 20 min a day, for 10 days.
Right hemisphere language dominance in a person with right-handedness is a rare phenomenon. Since the first description of crossed aphasia in 1899, many aetiologically different cases of crossed aphasia have been reported (14); however, until now, few reports have described functional imaging of crossed aphasia caused by a lesion in the right hemisphere, in a right-handed patient (16). Furthermore, to the best of our knowledge, no studies have examined the possible benefit of rTMS in the functional recovery of chronic crossed aphasia. In the present study, we report a case of global crossed aphasia patient with right subcortical haemorrhage in the basal ganglia whose language deficits resolved to conduction aphasia at the chronic stage (3 years after stroke onset) and investigate the effect of contralesional inhibition of rTMS, using fMRI.
Case report
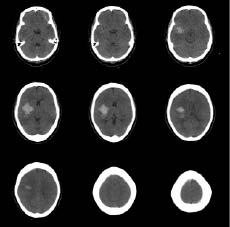
A 52-year-old woman with right-hand dominance and no significant medical history was brought to the emergency room following the sudden onset of left-sided weakness and loss of consciousness. The patient initially scored 11 points in the Glasgow Coma Scale (GCS): 4 for eye blinking, 5 in response to verbal command, and 2 for motor function; the Manual Muscle Test (MMT) revealed trace muscle movement in the upper and lower extremities. Computed tomography (CT) of the brain revealed a haematoma of approximately 40 ml in the right basal ganglia area and collapsed brain around the right lateral ventricle (Fig. 1). Burr-hole aspiration was performed to treat the haematoma. The patient was started on nimodipine (a calcium channel blocker) and depakote chrono (valproic acid, an anti-epileptic drug) to prevent seizures. The patient’s GCS score improved to 15, but MMT showed a poor grade for all extremities. Repeated serial CT scans showed complete resolution of the haematoma and no recurrence of bleeding. The patient was released from hospital without multidisciplinary rehabilitation treatment due to financial difficulties, with the intention of obtaining treatment at a public social facility.
Fig. 1. Initial computed tomography scan of brain disclosed about 40 ml haematoma at right basal ganglia and brain collapse around right lateral ventricle.
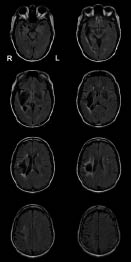
Three years after onset, the patient still had communication problems. She had left-sided hemiparesis; however, it was not her major concern. She visited a rehabilitation medicine outpatient clinic and began multidisciplinary rehabilitation treatment, which included cognitive and speech evaluation and treatment. Follow-up anatomical MRI revealed no recurrence of haemorrhage (Fig. 2). Her family reported she was strongly right-handed, showing a laterality quotient of +100 on the Edinburgh Handedness Inventory (17). There was no history of left-handedness in her family. Neuropsychological tests performed included the Korean version of the Mini-Mental State Examination (MMSE) (18) and Memory Assessment Scale (MAS) (19). In addition, the Korean version of the Western Aphasia Battery (WAB) (20) was performed for speech evaluation. The patient received rTMS intervention, and all outcome measurements were tested once before and once after rTMS. The patient received no speech therapy for the duration of this study.
Fig. 2. Three years from onset, T2 fluid attenuated inversion recovery magnetic resonance imaging disclosed that there is no recurrence of haemorrhage and new lesion of infarction.
Methods
rTMS stimulation
A 90 mm round coil stimulator (Magstim® Rapid2, Magstim Co. Ltd, UK) was used with the handle pointed posteriorly and stimulated approximately perpendicular to the left lateral sulcus (P3, defined by International Ten-Twenty (10–20) electrode placement system) with 90% of motor threshold (Fig. 3). We assessed the motor threshold of the first dorsal interosseous muscle of the right hand by visual inspection as a gross measure of cortical excitability. We determined the stimulator intensity that produced a visually detectable minimal muscle contraction in the right first dorsal interosseus muscle, in at least 5 of 10 trials, when the TMS stimulus was applied to the left M1 area on the scalp. We then treated at 90% of this motor threshold, with a frequency of 1 Hz, 20 min a day, for 10 days over a 2-week period, as described in the protocol of Naeser et al. (9, 10).
Fig. 3. The round stimulation coil is placed approximately perpendicular to the left lateral sulcus (P3) while the patient is seated in a chair.
Experiment 1: Noun generation
While undergoing fMRI, the patient performed a noun-generation task consisting of two conditions: noun generation and rest. The stimuli were projected using a video projector onto a translucent screen mounted on the table of the scanner. Stimuli were seen via mirrors on the head coil. We used a video projector placed in the control room. The visual angle was approximately 30°. The patient’s head was immobilized and noise protection was provided. Syllables were presented visually with a stimulus presentation time of 3000 ms. For the activation condition, the patient was instructed to think (i.e. without speaking aloud) of nouns that correspond to the presented syllable written in the Korean alphabet. That is, a syllable, which is a part of a word was presented and the patient was requested to make a more than 2-syllable word including the presented one. For the rest condition, the patient was asked to look at a fixation point on the screen. Each run consisted of task/rest cycles, with 10 syllables presented per task cycle. Total scanning time was 4 min and 12 s for 4 runs of each task.
Experiment 2: Sentence completion task
The patient was shown sentences with a word missing in the middle. She was asked to silently choose an appropriate word to complete the sentence from two words presented to her. After choosing an appropriate word, the patient pressed 1 of 2 buttons, corresponding to the selected word with her dominant (right) hand. Sentences were presented in blocks of fixed difficulty, determined by the range of suitable words suggested by the sentence context. Each block lasted 30 s and included 3 sentences. Sentences were presented for a period of 10 s, and the patient could respond at any time until the next sentence appeared. During the resting block, which lasted 30 s, the patient was requested to press 1 of 2 buttons when viewing a fixation white cross sign on a black background for a period of 10 s.
fMRI data acquisition
Blood oxygenation level dependent (BOLD) contrast was collected for each subject using a 3.0 T EXCITE scanner (GE Medical Systems, Milwaukee, WI, USA) equipped with a transmit–receive body coil and a commercial 8-element head coil array. T2*-weighted echo planar imaging was used for fMRI acquisition. The following acquisition parameters were used in the fMRI protocol: echo time (TE) = 40 ms, repetition time (TR) = 3000 ms, field of view (FOV) = 24 cm, flip angle = 90°, acquisition matrix = 64 × 64. A mid-sagittal scout image was used to position 31 contiguous axial slices of 5 mm thickness along the anterior–posterior commissure (AC–PC) plane, to cover the entire brain. The first 3 acquisitions were discarded due to T1 saturation effects. A 3D T1-weighted anatomical scan was obtained for structural reference. The first fMRI was performed before rTMS treatment and second fMRI session 3 days after treatment.
fMRI data analysis
Functional images were analysed using Statistical Parametric Mapping software (SPM2, Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Motion correction of the first functional scan was performed using a 6-parameter rigid-body transformation. The mean of the motion-corrected images was co-registered to the co-planar MRI using mutual information, followed by co-registration of the co-planar, high-resolution structural MRIs, and then spatially smoothed by an isotropic 8-mm full-width half-maximum (FWHM) Gaussian kernel. Statistical analysis was performed using the general linear model approach, as implemented in SPM. Estimates of head movement from the realignment stage of pre-processing were included as additional regressors. Contrast was constructed to examine the active condition vs rest. The SPM{t}s were thresholded at p < 0.001 and cluster size of 128. Activation maps were superimposed on the individual T1-weighted images.
Results
The results of fMRI performed before rTMS treatment showed no significant activity in either the ipsilesional (right) or contralesional (left) hemispheres during the noun generation and sentence completion paradigms. These initial fMRI results are in good agreement with the results of Joanette et al. (15) regarding patients diagnosed with crossed aphasia. We began contralesional (left) inhibitory rTMS over posterior in the parietal lobe (P3), as described in our protocol. We then repeated cognitive evaluation, speech evaluation, and fMRI of 3 days after the last rTMS. There was no improvement in the results of cognitive evaluation, whereas improvement was observed in the results of speech evaluation (excluding some reading and writing components of the evaluation) (Table I). Among items for language evaluation, the major area of improvement after rTMS treatment was in the naming task. However, due to few testing points, statistical significance could not be assessed on the WAB measures. Post-rTMS treatment fMRI data (Fig. 4; Table II) revealed statistically significant activations in the right inferior frontal lobe (Broca’s area), posterior temporal gyrus (Wernicke’s area), and parietal lobe, in both the noun generation and sentence completion tasks (p < 0.001). In the contralesional (left) hemisphere, there were activations in sensorimotor area and posterior occipital lobe.
| Table I. Cognition and language test |
| Test | Pre- rTMS | Post- rTMS |
| MMSE | 26 | 26 |
| MAS | 72 | 72 |
| WAB |
| Fluency | 11/20 | 11.5/20 |
| Comprehension | 161/200 | 176/200 |
| Repetition | 22/100 | 22/100 |
| Naming | 54/100 | 64/100 |
| Reading | 25/100 | 25/100 |
| Writing | 17/100 | 17/100 |
| Aphasia quotient | 54.2 | 57.8 |
| Language quotient | 43.1 | 46.1 |
| rTMS: repetitive transcranial magnetic stimulation; MMSE: Mini-Mental State Examination; MAS: Memory Assessment Scale; WAB: Western Aphasia Battery. |
Fig. 4. Statistical Parametric Mapping (SPM{t}) results of noun generation and sentence completion tasks from the patient for before and after repetitive transcranial magnetic stimulation (rTMS) treatment. All activation voxels are significant at p < 0.001 with 128 contiguous voxels.
| Table II. Brain activations of noun generation and sentence completion after repetitive transcranial magnetic stimulation treatment |
| | Side | Cluster size | Coordinates (mm) | Peak T-value |
| x | y | z |
| Noun generation | | | | | | |
| Superior temporal gyrus (Wernicke’s area) | R | 882 | 54 | –13 | 9 | 4.02 |
| Inferior frontal gyrus (Broca’s area) | R | 1472 | 30 | 43 | 14 | 4.48 |
| Parietal cortex | R | 942 | 24 | –54 | 20 | 4.34 |
| Sentence completion |
| Superior temporal gyrus (Wernicke’s area) | R | 809 | 46 | –14 | 9 | 9.75 |
| Inferior frontal gyrus (Broca’s area) | R | 168 | 39 | 19 | –4 | 5.16 |
| Parietal cortex | R | 1855 | 16 | –54 | 25 | 7.89 |
| | L | 1335 | –16 | –59 | 29 | 6.46 |
Discussion
The case study described here revealed interesting findings in a patient with crossed aphasia following right basal ganglia haemorrhage. Initial language fMRI data before rTMS treatment showed a lack of activation in bilateral hemispheres. In the initial fMRI before rTMS treatment, the language paradigms (noun generation and sentence completion) did not activate the expressive or receptive language regions in the right hemisphere. No homologous activations were found in the left hemisphere. However, it should be mentioned that no brain activation before rTMS treatment in the patient is possibly due to the conservative threshold chosen in statistical analysis of fMRI data. To be more rigorous and remove spurious signals from artefacts, we chose p < 0.001 for both conditions (before and after treatment). At this level there was no activation before treatment due to small fMRI signal changes. However, if we allowed a lower statistical threshold, the patient showed brain activations even before rTMS treatment.
A previous positron emission tomography (PET) study (21) reported earlier that, in a patient with persistent crossed aphasia, brain glucose metabolism was reduced not only in the right hemisphere, but also in the structurally unaffected left hemisphere.
There are few functional neuroimaging studies with chronic patients with aphasia who have received rTMS treatment (13). To the best of our knowledge, the current case study is the first to use fMRI to investigate whether rTMS stimulation affects language performance in a right-handed chronic post-stroke patient experiencing crossed aphasia. After contralesional inhibitory rTMS treatment, fMRI results showed that rTMS increased brain activity in the ipsilesional (right) hemisphere. Activated brain areas in the right hemisphere included the inferior frontal lobe, posterior temporal gyrus, and parietal lobe. These activated areas were in good agreement with the brain areas reported by Vandervliet et al. (22) in a patient with crossed aphasia. The increase in activations in the language-related areas of the ipsilesional hemisphere corresponded to improvement in speech evaluation after rTMS treatment. In the present study, we conducted contralesional inhibitory rTMS around the Sylvian fissure, based on a previously reported improvement in language function following contralesional inhibition of rTMS in a chronic patient with severe global aphasia. Another rTMS study (10) demonstrated that rTMS suppression of the right posterior pars triangularis portion of the right Broca’s homologue led to improved picture naming in a patient (6.5 years after a left basal ganglia bleed) with severe non-fluent global aphasia. In this study a round coil was used. Thus, although located at P3, there was no stimulation occurring at the centre of the coil and the stimulation can be more diffuse with a round coil compared with a figure-of-8-shaped coil. In addition, we chose contralesional inhibitory rTMS because there was an increased risk of seizure by electrical stimulation itself, and this patient was treated with an anti-epileptic drug to reduce the risk of epilepsy.
Although the underlying neurobiological mechanism of language improvement after contralesional inhibitory rTMS treatment in crossed aphasia is currently unknown, our study demonstrated that suppression of contralesional (left) hemisphere language areas by application of rTMS led to an improvement in language ability in a patient with chronic crossed aphasia. One possible explanation for our observation is that inhibitory rTMS promotes suppression in contralesional language areas, which in turn promotes a better modulation throughout the bi-hemispheric neurofunctional network for language. That is, initial fMRI before rTMS treatment showed no activations in language-related brain areas in both hemispheres. After application of rTMS to the contralesional (left) hemisphere, sensorimotor and posterior occipital activations were found in the left hemisphere, but activations were found on language-related brain areas in the right hemisphere. Therefore, the changes in fMRI activities from noun generation and sentence completion tasks before and after rTMS treatment corresponded with improvements in the language behaviour on speech evaluation after rTMS treatment. In particular, the increase in Wernicke’s area activity in the sentence completion task compared with the noun generation task seems to suggest that the sentence completion task is more suitable to evaluate the receptive aspect of speech. In the current case study we did not observe overactivation in the undamaged hemisphere, which was reported in the previous low-frequency rTMS study (13). Although no overactivation was seen on the pre- rTMS fMRI scans, the notion of using 1 Hz to suppress activation in the undamaged hemisphere still appeared to be effective in our case. A previous combined rTMS and PET study reported that the compensatory potential of contralesional (non-dominant) language areas was less effective in patients who recover language function in the dominant hemisphere, although in some post-stroke aphasia patients, activations in the contralesional language areas play a role in residual language function (22). Accordingly, we speculate that activation of contralesional language areas is not compensatory, but rather maladaptive, and agree with this notion as previously suggested by others (5, 6, 9, 10, 13). The use of rTMS to suppress an area in the contralesional P3 appears to modulate the bi-hemispheric neural network for naming.
Finally, the possible limitations of the current study are behavioural data measurement and practice effect resulting from administering the WAB 2 weeks apart. In the current study, there was no behavioural test session. Therefore, it is difficult to know what the patient did during the scanning. Although we did not measure behavioural data during the scanning session and outside the scanner, the patient underwent a brief training session inside the scanner before fMRI data acquisition and was asked to press a button when she performed a given task. In addition, after fMRI session, we asked the patient whether she performed a given task during the scanning; both the visual inspection on pressing a button and verbal inquiry after scanning revealed that she did.
Acknowledgement
This work was supported by the Ministry of Health & Welfare (A092106) in Korea.
References