Marjolein M. van der Krogt, PhD, Caroline A. M. Doorenbosch, PhD, Jules G. Becher,
MD, PhD and Jaap Harlaar, PhD
From the Department of Rehabilitation Medicine, Research Institute MOVE, VU University Medical Center, Amsterdam, The Netherlands
Marjolein M. van der Krogt, PhD, Caroline A. M. Doorenbosch, PhD, Jules G. Becher,
MD, PhD and Jaap Harlaar, PhD
From the Department of Rehabilitation Medicine, Research Institute MOVE, VU University Medical Center, Amsterdam, The Netherlands
OBJECTIVE: To quantify dynamic spasticity, i.e. the coupling between muscle-tendon stretch velocity and muscle activity during gait, of the gastrocnemius and soleus muscles in children with spastic cerebral palsy.
DESIGN: Prospective, cross-sectional study.
SUBJECTS: Seventeen ambulatory children with cerebral palsy with spastic calf muscles, and 11 matched typically developing children.
METHODS: The children walked at 3 different speeds. Three-dimensional kinematic and electromyographic data were collected. Muscle-tendon velocities of the gastrocnemius medialis and soleus were calculated using musculoskeletal modelling.
RESULTS: In typically developing children, muscles were stretched fast in swing without subsequent muscle activity, while spastic muscles were stretched more slowly for the same walking speed, followed by an increase in muscle activity. The mean ratio between peak activity and peak stretch velocity in swing was approximately 4 times higher in spastic muscles, and increased with walking speed. In stance, the stretch of muscles in typically developing children was followed by an increase in muscle activity. Spastic muscles were stretched fast in loading response, but since muscle activity was already built up in swing, no clear dynamic spasticity effect was present.
CONCLUSION: Spastic calf muscles showed increased coupling between muscle-tendon stretch velocity and muscle activity, especially during the swing phase of gait.
Key words: cerebral palsy; spasticity; gait; biomechanics.
J Rehabil Med 2010; 42: 656–663
Correspondence address: Marjolein M. van der Krogt, VU University Medical Center, Department of Rehabilitation Medicine, PO Box 7057, NL-1007 MB Amsterdam, The Netherlands. E-mail: mmvanderkrogt@gmail.com
Submitted February 17, 2009; accepted April 27, 2010
Introduction
Spastic paresis is the most common motor disorder in children with cerebral palsy (CP), occurring in 85% of all children with CP (1). In this group of children, spasticity is one of the main symptoms of disturbed muscle function. Although different definitions of spasticity exist throughout the literature, the most commonly used definition is that of Lance (2), stating that spasticity is a velocity-dependent increase in muscle tone, resulting from hyperexcitability of the stretch reflex. Spasticity is thought to lead to gait deviations, and in the long term to muscle contractures and bone deformities. In clinical practice, spasticity is measured in physical examination using passive muscle tests, such as the (Modified) Ashworth Scale or the (Modified) Tardieu Scale (3).
For patient care, it is of particular importance to understand the effect of spasticity, not only during passive muscle testing, but also during functional tasks such as gait. Determining the effect of spasticity on gait is also essential for accurate treatment planning and evaluation. However, due to the interplay with other impairments in CP, such as muscle contractures, weakness, bony deformities, and diminished selective motor control, it is difficult to determine the precise effect of spasticity on gait. Therefore, little is known about the clinical significance of spasticity during functional tasks such as gait (4).
Insight into the effect of spasticity on gait can be gained by studying the relationship between spasticity measured during physical examination and gait parameters (e.g. 5–8). These studies yielded ambiguous results, possibly due to the different measures of spasticity used, and the fact that gait parameters are often assessed at joint level rather than muscle level. Moreover, the expression of spasticity during dynamic tasks such as walking may differ from that during passive tests in physical examination (9–11).
Spasticity can also be assessed directly during gait, by studying the relationship between muscle stretch velocity and muscle activity. This concept was introduced by Crenna et al. (9, 12, 13) and termed dynamic spasticity. Crenna (9) found that in children with CP this coupling between muscle lengthening velocity and muscle activity is often increased compared with normal. The increased coupling can be present either in terms of a decreased “threshold” (velocity at which muscle activity is evoked) or increased “gain” (change in muscle activity relative to change in stretch). Crenna (13) showed that, in spastic hamstrings muscles, the stretch velocity threshold for muscle activation is decreased and muscle activation is increased compared with normal during the swing phase of gait. However, Crenna (9) also discusses that differences exist in the expression of spasticity during gait, between gait phases and between muscles.
Dynamic spasticity of the calf muscles has never been studied systematically in a group of children with CP. Therefore, the aim of this study was to explore the relationship between muscle-tendon stretch velocity and muscle activity of gastrocnemius and soleus muscles, in a group of children with CP with spastic calf muscles. It was hypothesized that in spastic muscles the coupling between stretch velocity and muscle activity is increased, and that spastic muscles will not stretch fast without concomitant excessive muscle activity.
Methods
Subjects
Seventeen children with spastic CP and 11 typically developing (TD) children, matched for age, height and weight, participated in this study. The characteristics of the children with CP were (mean and standard deviation (SD)): age 8.9 years (SD 2.1) (range 6–12 years); height 136 cm (SD 13), and weight 33 kg (SD 10). The characteristics of the TD children were: age 8.2 years (SD 1.8) (range 6–12 years); height 134 cm (SD 12), and weight 32 kg (SD 13). All children with CP were clinically diagnosed with spastic CP (13 bilateral, 4 unilateral), were able to walk independently without walking aids, were classified on the gross motor function classification scale (GMFCS) as level I–II (14), had no prior orthopaedic surgery, selective dorsal rhizotomy or baclofen treatment, and had no prior botulinum toxin treatment within the previous 16 weeks. All children showed spasticity in the calf muscles of their affected legs, as measured by a standard physical examination (15), except for one leg, which was excluded. All affected legs showed an equinus gait pattern or an early heel rise (higher than normal plantar flexion of the foot at mid-stance) at comfortable and/or fast walking speed. All children and their parents provided informed consent. The study protocol was approved by the medical ethics committee of the VU University Medical Center.
Design
The children underwent a standard clinical gait analysis, at 3 different walking speeds. They all walked at self-selected comfortable walking speed (CWS), followed by SLOW speed (instruction to walk between 65 and 75% of CWS) and FAST speed (instruction to walk between 125 and 135% of CWS), in random order. Walking speed was varied in order to be able to control for differences in walking speed between patients and controls, and in order to modulate the velocity-dependent effect of spasticity. Walking speed was recorded online, and controlled by giving instant feedback to the children. Six successful trials were collected for each speed condition, divided over 2 separate sessions. Data of 2 sessions were included as part of a larger study, and because this resulted in a larger and more reliable dataset than when data of a single session were used. In this way, some of the variability in electromyographic (EMG) data was reduced. This variability has been shown to be relatively high in children, with variance ratios in the gastrocnemius of 0.43 and 0.47 for intrasession and intersession variability, respectively; where 0 is perfect and 1 is poor repeatability (16). The 2 sessions took place 18 ± 12 days apart at the same time of day, and no interventions were performed in the intersession interval. For logistic reasons, 2 children could be measured only once.
Three-dimensional (3D) kinematic data were collected during the walking trials using a motion capture system (Optotrak, Northern Digital, Waterloo, Ontario, Canada) for the trunk, pelvis, upper and lower legs, and feet. The movement of each segment was tracked using technical clusters of 3 markers, which were anatomically calibrated using virtual anatomical markers (17).
EMG data were collected for the gastrocnemius medialis (GM) and soleus (SO) muscles (Noraxon Telemyo, Noraxon U.S.A Inc., Scottsdale, USA). Surface electrodes were placed according to the SENIAM (Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles) guidelines (18). EMG data were collected at 1000 Hz and online high-pass filtered at 20 Hz to remove artefacts.
Analysis
3D kinematic data were analysed with open source Matlab® software (www.BodyMech.nl). Initial contact (IC) and toe-off (TO) values were calculated from the forward foot velocity, and defined as the moments at which this velocity became lower (IC) or higher (TO) than 20% of its maximal value (19). From each trial, one successful stride (IC to IC) was selected, for both the left and the right leg of the CP subjects, and for only the right leg of the TD subjects. For one patient, data on only one leg were available for technical reasons, resulting in a total of 28 affected legs in the CP group, and 11 legs in the TD group.
Actual walking speed during the successful strides was calculated as the average forward velocity of the pelvis markers over the full stride, and non-dimensionalized by √ g · lleg (20), with lleg the leg length, calculated as the summed length from trochanter major to lateral epicondyle to lateral malleolus.
Muscle-tendon lengths of GM and SO were calculated with SIMM musculoskeletal modelling software (Musculographics, Inc., Chicago, IL, USA) (21, 22). The SIMM standard generic model of the lower extremities was used, consisting of pelvis, thigh, shank, and foot segments, and all major individual leg muscles. The generic model was scaled to the individual subject sizes, using 3D kinematic data from the anatomical landmarks. The individually scaled models were made to match the subjects’ gait kinematics by tracking the individual marker trajectories. Varus-valgus motion of the model’s knee was allowed, and the maximal ankle plantarflexion range was increased to 75° to allow ankle motion as observed in CP subjects (23). GM and SO muscle-tendon lengths during gait were then calculated by the SIMM software according to the muscle attachment sites and moment arms around the joints.
Muscle-tendon lengths were low-pass filtered using an 8 Hz low-pass symmetrical filter, and differentiated, in order to obtain muscle-tendon velocities. Muscle-tendon velocities were non-dimensionalized by √ g · lref, with Lref the anatomical reference length with all joint angles set at zero, as calculated with SIMM. EMG signals were rectified and low-pass, symmetrically filtered at 5 Hz. Next, EMG signals were normalized to the peak value during the stride at CWS for CP; and to the peak value at SLOW speed for TD, which turned out to be similar absolute speeds (see Results). Muscle-tendon velocities and EMG data were time-normalized to 100% gait cycle.
To evaluate dynamic spasticity, the coupling between muscle-tendon stretch velocity and muscle activity was compared between the affected CP limbs (CP; n = 28) and the control limbs of the TD subjects (TD; n = 11). First, dynamic spasticity was assessed qualitatively, by simultaneously plotting the time series of EMG activity and stretch velocity averaged over all CP and TD subjects, and by plotting EMG activity vs stretch velocity, for the stance and swing phase separately.
Secondly, dynamic spasticity was assessed quantitatively, for those gait phases where the muscles were stretched and muscle activity should normally be absent, which is the case in the swing phase (24). For this phase we calculated:
An analyses of variance (ANOVA) for repeated measures, with Bonferroni adjustment for multiple comparisons was used to test the effects of group (CP and TD) and speed condition (SLOW, CWS, FAST) on actual achieved non-dimensional walking speed, peak stretch velocity in swing, peak EMG activity following stretch in swing, and EMG-velocity ratio. Mean data over the 6 trials per subject were input to the statistical analysis. A Student’s t-test was used to compare comfortable walking speed in CP with slow walking speed in TD; as well as fast walking speed in CP with comfortable walking speed in TD.
Results
Table I shows non-dimensional walking speeds as well as stride times for the CP and TD groups. The CP subjects walked significantly slower than the TD subjects (p < 0.001). Comfortable walking speed in CP was similar to slow speed in TD (p = 0.25), and fast speed in CP was close to comfortable walking speed in TD (p = 0.85). Thus, to eliminate the effect of absolute differences in walking speed, comparisons between the 2 groups were made at comparable walking speeds, i.e. CWS in CP and SLOW in TD; and FAST in CP and CWS in TD.
| Table I. Spatiotemporal parameters (means (SD)) | |||
| Parameter | Condition | CP | TD |
| Nondimensional walking speed | SLOW | 0.26 ( 0.06) | 0.36 (0.03) |
| CWS | 0.39 ( 0.07) | 0.51 (0.04) | |
| FAST | 0.51 ( 0.07) | 0.66 (0.05) | |
| Stride time (s) | SLOW | 1.27 ( 0.21) | 1.11 (0.11) |
| CWS | 0.97 (0.10) | 0.92 (0.08) | |
| FAST | 0.84 (0.08) | 0.81 (0.08) | |
| Walking speed increased with speed condition (as imposed; p < 0.001). and was lower in CP than in TD (p < 0.001). Stride time decreased with speed condition (p < 0.001) and was lower in CP than in TD for the same absolute walking speed (p = 0.001). TD: typically developing; CP: cerebral palsy; CWS: comfortable walking speed; SD: standard deviation. | |||
Fig. 1 shows both the muscle-tendon stretch velocity and the EMG activity for the GM (means and SD), for all speed conditions, for CP and TD. Since the main effects occurred around initial contact, the horizontal scale in Fig. 1 runs from 0 to 150% gait cycle. At matched walking speed (i.e. compare Fig. 1B with D, or C with E), the GM velocity pattern in spastic muscles differed from the velocity pattern in TD subjects. In CP, muscles showed a fast peak stretch velocity in early stance and a slower stretch in swing. In TD, GM showed the fastest peak in swing and a slower stretch in mid-stance.
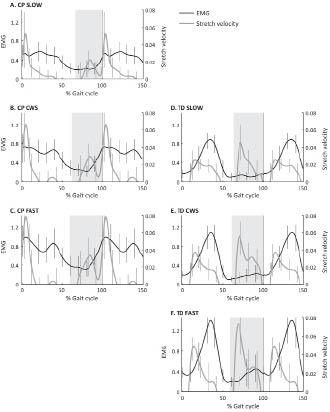
Fig. 1. Gastrocnemius electromyographic (EMG) activity and muscle-tendon stretch velocity vs the gait cycle, for spastic cerebral palsy (CP) and typically developing (TD) children. Because the main effects occurred around initial contact, the x-axis runs from 0 to 100 and again to 50% of the gait cycle. For better comparison, data at matched speeds are presented in the same rows. EMG data is normalized to peak EMG activity during the stride at comfortable walking speed (CWS) for CP, and at SLOW speed for TD. Stretch velocity is non-dimensionalized by dividing by √g · lref. Grey areas indicate swing phase.
When simultaneously examining the EMG and velocity patterns, the most notable effect shown in Fig. 1 is that in TD subjects GM stretched fast in swing without a subsequent increase in muscle activity. In contrast, in CP a slower stretch of spastic muscles was seen in swing, followed after a short delay by an increase in muscle activity. In stance, a totally different pattern occurred. Here the stretch phase in TD subjects was followed almost instantly by an increase in muscle activity. In CP, the fast stretch in early stance coincided with a peak in EMG activity, but was not followed by a clear additional increase in EMG activity following stretch.
Fig. 2 shows the same graphs for SO. A similar pattern was seen in SO as in GM: in TD a fast stretch occurred in swing without concomitant muscle activity, while in CP the stretch was much slower, and followed, with some delay, by an increase in muscle activity. In stance, the stretch in TD was followed by muscle activity, while in CP a fast stretch was seen in early stance, coinciding with, but not followed by, additional muscle activity.
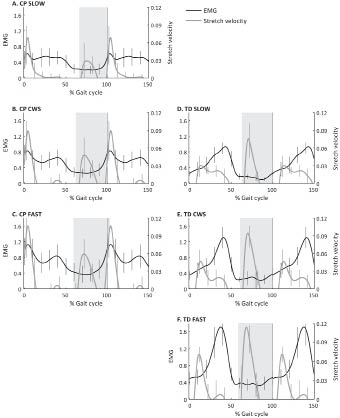
Fig. 2. Soleus electromyographic (EMG) activity and muscle-tendon stretch velocity vs the gait cycle, for spastic cerebral palsy (CP) and typically developing (TD) children. Because the main effects occurred around initial contact, the x-axis runs from 0 to 100 and again to 50% of the gait cycle. For better comparison, data at matched speeds are presented in the same rows. EMG is normalized to peak EMG during the stride at comfortable walking speed (CWS) for CP, and at SLOW speed for TD. Stretch velocity is non-dimensionalized by dividing by √g · lref.. Grey areas indicate swing phase.
Fig. 3 shows loops of the muscle activity vs muscle-tendon stretch, as proposed by Crenna (9). Graphs are shown for GM, for the matched CP CWS and TD SLOW speeds. The graphs are separated into stance and swing stretch phases. CP muscles in swing showed a decreased stretch velocity and an increased muscle activity compared with TD muscles in swing, which show high velocity without concomitant muscle activity. TD muscles showed a similar loop in stance, while in spastic muscles activity was already built up during swing, and no loop was present in stance.
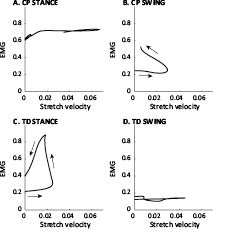
Fig. 3. Gastrocnemius electromyographic (EMG) activity vs muscle-tendon stretch velocity for spastic cerebral palsy (CP) and typically developing (TD) children, at matched speed (CP CWS and TD SLOW). Note that the graphs contain the same data as Fig. 1B and D, but presented differently. EMG is normalized to peak EMG during the stride; stretch velocity is non-dimensionalized by dividing by √g · lref.
Peak stretch velocity and peak EMG activity as built up in swing (to exclude possible effects of the fast stretch in stance), as well as their ratio are shown in Fig. 4 for both GM and SO. At matched speed, spastic muscles were approximately one-third slower in swing than muscles in TD. Also, spastic muscles showed about 3 times higher EMG activity in swing compared with muscles in TD. This resulted in a ratio between peak EMG activity and peak stretch velocity in swing that was approximately 4 times higher in CP than in TD.
To gain an indication of the time interval between stretch velocity and EMG activity, the time difference was calculated between peak stretch velocity and peak EMG activity as built up in swing, and Table I gives absolute stride times as a reference for Figs 1 and 2. The mean time interval between peak stretch velocity and peak EMG activity in swing over all trials was 155 ± 80 ms for GM, and 236 ± 64 ms for SO, and did not differ between groups (GM: p = 0.82; SO: p = 0.36).
With increasing walking speed, both peak stretch velocity and peak EMG activity in swing increased, for both groups (Fig. 4A–D; p < 0.001 for all). The ratio between the peak EMG activity and peak stretch also increased with walking speed (Fig. 4E and F; p = 0.015 for GM and p = 0.025 for SO). The time interval between peak stretch and peak EMG activity was constant with speed for GM (p = 0.40), while it slightly decreased in SO from 265 (SD 67) at slow speed to 209 (SD 46) ms at fast speed (p < 0.001).
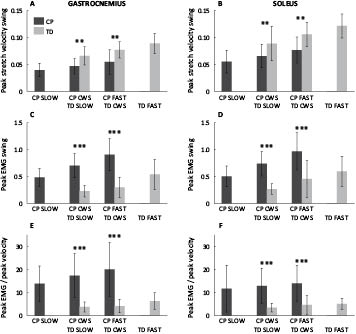
Fig. 4. (A and B) Peak stretch velocities in swing; (C and D) peak electromyographic (EMG) activity in swing; and (E and F) ratio of peak EMG activity and peak stretch velocity; for increasing matched walking speeds. The group effect was evaluated for the 2 matched speeds only, **p < 0.01; ***p < 0.001. All outcomes increased significantly with walking speed (p < 0.001 for peak stretch velocity and peak EMG; p < 0.05 for peak EMG/peak stretch ratio). CWS: comfortable walking speed; CP: cerebral palsy; TD: typically developing.
Discussion
This study investigated dynamic spasticity, i.e. an increased coupling between muscle-tendon stretch velocity and muscle activity during gait, of spastic calf muscles in children with CP. It was found that spastic muscles showed an increased coupling between stretch and activity in swing: in TD subjects, muscles were stretched fast in swing without subsequent muscle activity, while in CP subjects muscles were stretched slower, followed by an increase in muscle activity. The ratio between peak EMG activity and peak stretch was 4 times higher in spastic CP muscles on average, and increased with walking speed.
The results as found in swing, are in line with the concept of spasticity: a velocity-dependent increase in muscle activity. Both a decreased stretch velocity and an increased muscle activity were observed in spastic muscles. These 2 can be logically related: more dynamically spastic muscles already show an increased activity at low stretch velocity, which slows down the movement and results in low peak velocity. Although most of the literature on spasticity effects during gait in calf muscle focuses on the stance phase (e.g. 9, 26), our results show the clearest effect of dynamic spasticity in swing.
Our findings are also in line with literature on stretch reflex activity during gait. Hyperexcitability of the stretch reflex is assumed to be one of the main underlying causes of spasticity (2). Several studies have investigated the strength and modulation of the stretch reflex during normal and CP gait. Sinkjaer et al. (25) tested the stretch reflex during gait by mechanically perturbing the soleus length. They showed that in normal walking, the amplitude of the soleus stretch reflex is high during stance, and low during swing. This allows a fast stretch in swing without evoking muscle activity, as observed in TD subjects in the present study. During stance, the GM and SO activity following stretch in healthy subjects may, for a large part, be attributable to stretch reflex activity (25). Hodapp et al. (27) tested H-reflexes during gait in CP. These H-reflexes generally are representative of stretch-reflexes, at least in terms of basic rhythmic modulation (28). Hodapp et al. (27) showed that in spastic calf muscles in CP, the H-reflex is amplified during the entire stride compared with normal. Hence, contrary to control subjects, the stretch of spastic muscles in swing is likely to evoke a stretch reflex and subsequent muscle activity. This is in line with our results, as illustrated by the 4 times higher ratio between EMG activity and stretch velocity in CP compared with TD.
Furthermore, the ratio between peak EMG activity and peak velocity increased with walking speed (Fig. 4E and F), indicating that the effect of dynamic spasticity in swing is enhanced at faster walking speed. Interestingly, the ratio of EMG activity and stretch also increased with faster walking speed in TD subjects. A similar increase in EMG activity in swing with walking speed was found by Schwartz et al. (29), who studied the effect of walking speed on gait in a large group of children. This increase in EMG activity in swing is likely not an artefact, since cross-talk of other muscles to the GM or SO is limited (30). The increase in EMG activity in TD children with speed indicates that stretch-related muscle activity in swing may also play a role at faster speed in normal gait. This is in line with increased stretch reflex amplitudes in swing at faster walking speed in healthy subjects, as reported by Sinkjaer et al. (25). In contrast, Hodapp et al. (28) found a decrease in H-reflexes in swing in healthy children walking at faster speed, and an increase in stretch-reflex modulation compared with slow speed. However, both their slow and fast speeds (1.2 km/h and 3.0 km/h) were slow compared with the speeds imposed in the present study or the study by Schwartz et al. (29). Therefore, the increase in EMG activity and possible underlying stretch-reflexes may especially play a role at faster than normal speeds in TD children.
In stance, the fast stretch in CP muscles during loading response coincided with muscle activity, but contrary to our hypothesis, this stretch was not followed by an additional increase in muscle activity (Figs 1 and 2). This discrepancy may be due to the fact that the muscles were already active at the onset of the second stretch peak. Therefore, the muscle belly was contracting and, as a consequence, the muscle belly may not have been lengthening at a similar rate to that of the muscle-tendon complex. Muscle force can be expected to be increasing in this phase to support body weight, resulting in lengthening of the tendon, possibly accounting for most of the muscle-tendon stretch velocity. In voluntary toe walking, the muscle belly has even been shown to be shortening in this phase of the gait cycle, using ultrasound measurements (31). Since reflex activity is evoked by stretch of the muscle spindles (i.e. stretch in the muscle belly) rather than the muscle-tendon complex, this may explain why no further muscle activity is evoked following the fast stretch in early stance. In swing, where the muscle force is initially low, the muscle belly length follows muscle-tendon length more closely. In voluntary toe walking, peak contractile element (CE) stretch velocity in swing of gastrocnemius muscles has been shown to occur slightly later than peak muscle-tendon stretch, but at a similar rate (31). To accurately estimate stretch reflex activity during stance, future study should quantify CE velocity in relation to muscle activity, for example using ultrasound or modelling studies.
From the present data we could not precisely quantify a stretch-reflex delay, which would have given further indication as to whether or not the EMG activity in swing could be attributed to stretch-reflex activity. As expected, some time difference was found between the peaks in muscle-tendon stretch velocity and EMG activity; 155 ms (SD 80) for GM, and 236 ms (SD 64) for SO, respectively. Stretch reflex latencies during walking vary between approximately 40 and 120 ms for short- and long-latency reflexes (32). This difference can be attributed partly to the fact that we determined the time difference from peak stretch velocity to peak EMG activity, which occurs considerably later than the onset of the EMG activity. In order to calculate a stretch-reflex delay, information about the stretch velocity threshold value and onset of EMG activity would be needed, both of which were difficult to determine from the present data. Furthermore, as described above, the peak stretch of the muscle belly may occur somewhat later in time than the peak stretch of the muscle-tendon complex. Moreover, the time intervals were comparable to the interval between peak stretch and peak activity during stance in TD muscles, where stretch-reflex activity is thought to play a role as well (25). Taken together, the time intervals were within reasonable limits to attribute the rise in EMG activity to reflex activity. The relatively large standard deviations found for the time intervals were mainly due to variability in EMG activity patterns. This is in line with the literature, showing large variability ratios for EMG activity between trials, especially in children (16), and especially at slow walking speeds (33).
Although the present data are in line with the concept of spasticity and stretch reflex modulation during gait, it cannot be concluded with certainty that spasticity is the only cause of the low muscle stretch velocity and increased muscle activity in swing in spastic muscles. First, spastic muscles also showed higher baseline EMG activity in initial swing (Figs 1 and 2), which can partly explain why spastic muscles were slower in swing. Passive stiffness is also higher in spastic muscles (e.g. 34), which can lead to slower muscles in swing. Secondly, if healthy subjects walk on their toes voluntarily, they also show increased muscle activity in terminal swing, to prepare for toe-landing (35, 36). However, the ankle and knee kinematic data in these studies differed between voluntary and CP toe walking, and no data were available on muscle-tendon velocities during voluntary toe walking. Moreover, stretch reflex modulation may be altered during voluntary toe walking, so that reflexes can be used to initiate GM and SO activity in terminal swing to support the body during stance in the equine position.
Our results are in line with the study by Crenna (13), who found an increased ratio between hamstrings activity and stretch velocity in swing. For the calf muscles, Crenna (9) reported somewhat different results from ours. Although Crenna did not present data on a group of subjects, he did show EMG activity vs lengthening velocity curves during stance, for the soleus of one child with diplegia and 1 healthy control subject. For the diplegic child, he found a looped curve similar to the curves found in swing in our spastic muscles (Fig. 3B). The difference may be due to the fact that only one example muscle was shown, which may have been less affected compared with our subjects. Nevertheless, the concept of dynamic spasticity as proposed by Crenna (9, 13) was similar to our results.
The ratio of EMG activity and muscle-tendon stretch velocity during swing may be a good and simple measure for dynamic spasticity of the calf muscles during gait. First, it is in line with the concept of spasticity, defined as a velocity-dependent increase in muscle tone, since it incorporates both stretch velocity and muscle activity. Secondly, it distinguishes clearly between spastic and non-spastic muscles (approximately 4 times higher in CP, on average). Thirdly, since muscle force is low and few dynamic effects (e.g. due to body weight support) are present in swing, it clearly shows the increased coupling between muscle stretch and activity, with few possible confounding factors.
To what extent the observed dynamic spasticity correlates with spasticity as measured in passive spasticity tests during physical examination remains to be determined. In the present study, spasticity was measured clinically. In order to correlate dynamic spasticity during gait with passively measured spasticity, future research should quantify spasticity in a more objective, quantitative, and sensitive manner using instrumented tests.
The effect on the gait pattern of the evoked muscle activity in terminal swing is that it will limit further elongation of the calf muscles in terminal swing, thereby limiting knee extension and ankle dorsiflexion at initial contact, preventing accurate foot positioning. Because of electromechanical delay, most of the resulting muscle force will occur in early stance, thereby supporting the ankle extension in the equine position after landing. The high muscle activity in loading response and consequent high muscle force will then prevent further stretching of the muscle, supporting body weight in the equine position, consequently leading to heel rise or toe walking in early and mid stance. Due to the relatively higher increase in EMG activity with faster walking speed, this effect becomes more pronounced as walking speed increases, leading to a more severe toe-walking pattern at faster speed. Walking with limited knee extension and limited ankle dorsiflexion in terminal swing and a toe-walking gait pattern could thus, at least partly, be attributed to dynamic spasticity effects in the spastic calf muscles, which effects are already initiated in swing.
In general, we can conclude that the most prominent effect of dynamic spasticity in calf muscles occurred during the swing phase of gait: spastic calf muscles showed decreased stretch velocity combined with subsequent increased muscle activity, compared with non-spastic muscles, and this effect increased with walking speed.
References
