OBJECTIVE: To test the hypothesis that dry needle stimulation of a myofascial trigger point (sensitive locus) evokes segmental anti-nociceptive effects.
DESIGN: Double-blind randomized controlled trial.
SUBJECTS: Forty subjects (21 males, 19 females).
METHODS: Test subjects received intramuscular dry needle puncture to a right supraspinatus trigger point (C4,5); controls received sham intramuscular dry needle puncture. Pain pressure threshold (PPT) readings were recorded from right infraspinatus (C5,6) and right gluteus medius (L4,5S1) trigger points at 0 (pre-needling baseline), 1, 3, 5, 10 and 15 min post-needling and normalized to baseline values. The supraspinatus and infraspinatus trigger points are neurologically linked at C5; the supraspinatus and gluteus medius are segmentally unrelated. The difference between the infraspinatus and gluteus medius PPT values (PPTseg) represents a direct measure of the segmental anti-nociceptive effects acting at the infraspinatus trigger point.
RESULTS: Significant increases in PPTseg were observed in test subjects at 3 (p = 0.002) and 5 (p = 0.015) min post-needling, compared with controls.
CONCLUSION: One intervention of dry needle stimulation to a single trigger point (sensitive locus) evokes short-term segmental anti-nociceptive effects. These results suggest that trigger point (sensitive locus) stimulation may evoke anti-nociceptive effects by modulating segmental mechanisms, which may be an important consideration in the management of myofascial pain.
Key words: myofascial trigger point; anti-nociception; acupuncture; algometry; pain pressure threshold.
J Rehabil Med 2010; 42: 463–468
Correspondence address: John Z. Srbely, Department of Human Health and Nutritional Science, University of Guelph, Guelph, Ontario, N1G 2W1 Canada. E-mail: jsrbely@uoguelph.ca; jsrbely@rogers.com
Submitted December 20, 2008; accepted December 22, 2009
INTRODUCTION
Musculoskeletal pain is a significant and common medical condition. Up to 85% of the general population will experience at least one episode of musculoskeletal pain during their lifetime (1). Myofascial pain is one of the most common examples of musculoskeletal pain; an accumulating body of evidence suggests that unique hypersensitive loci, named myofascial trigger points in the literature, are intimately associated with the pathophysiology and clinical manifestation of myofascial pain (2).
Trigger point injections with local anesthetics have been performed to alleviate musculoskeletal pain since the early 1930s. No significant differences in subjective pain have been reported when comparing the effects of lidocaine injection with dry needle stimulation of trigger points (3), suggesting that the site-specific needling of the trigger point may be exclusively responsible for the observed anti-nociceptive effects (4). Similarly, acupuncture, which has been practiced in Asia for over 2000 years, involves the site-specific application of needles to specific points (acupoints) on the body (5) to evoke a broad range of systematic therapeutic effects (6–9).
Whereas the literature demonstrates the importance of site-specific stimulation, the neurophysiologic mechanisms responsible for these effects have yet to be characterized adequately. Furthermore, these points have been named differently (trigger point, acupoint) to reflect the different paradigms under which they are considered. Historical nomenclature describes acupoints as distinct anatomic coordinates lying along parallel longitudinal arrays of energy channels (meridians) in the body. Needle stimulation of these points is believed to facilitate the flow of energy along these meridians; however, this concept is still under scientific scrutiny (10). Research shows that acupoints are anatomically unique, possessing a greater density of large, myelinated fibers compared with normal (non-acupoint) tissue (11). A myofascial trigger point, on the other hand, is defined as a localized, hyperirritable nodule nested within a palpable taut band of skeletal muscle or fascia (2) and have been reported in both humans (2) and animals (12); several theories exist to explain their origin (13). Commonly accepted diagnostic criteria for trigger points have been reported in the literature (2) and, while several of these criteria have demonstrated moderate reliability, the reliability of trigger point detection is still being challenged (14).
When compared for spatial distribution and pain referral patterns (15), research has demonstrated a remarkable 71% correlation between trigger points and acupuncture points (acupoints), leading researchers to believe that they may be identical physiologic phenomena governed by similar neurophysiologic mechanisms (15). Accordingly, we have introduced a new term, the “secondary hyperalgesic locus” (SHL), to represent the trigger point/acupoint phenomenon based on our novel hypothesis of trigger point pathophysiology. Under this hypothesis of trigger point formation, the “neurogenic hypothesis”, trigger points are discrete secondary peripheral neurogenic manifestations of central sensitization caused by a primary pathology within the common neuromeric field. If this hypothesis holds true, SHL mechanisms and pathophysiology must necessarily be governed, at least in part, by segmental spinal mechanisms.
The aim of this study was to investigate the neurophysiologic mechanisms of SHL stimulation by testing the hypothesis that site-specific dry needle stimulation of a SHL evokes systematic segmental anti-nociceptive effects (segmental neuromodulation). In light of the documented systematic effects of SHL stimulation, elucidating and describing these mechanisms in contemporary neurophysiologic terms may provide important insight into the development of novel therapeutic approaches in musculoskeletal pain management.
METHODS
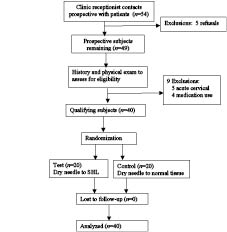
This study was approved by the University of Guelph Ethics Committee. Each participant provided signed informed consent prior to participating in the study and none of the subjects withdrew from the study. Based on previously reported data (16), a power analysis determined that a sample size of 20 subjects per group (test, control) provides a minimum of 90% power to detect an effect magnitude of 1.6 standard deviation (SD) at an alpha of 0.05. A total of 40 volunteers (21 males, 19 females) participated in this study. The demographic profile of subjects is listed in Table I and subject allocation is illustrated in Fig. 1.
Fig. 1. Subject recruitment process and allocation. SHL: secondary hyperalgesic locus.
| Table I. Summary of the demographic profile of test and control groups used for this study. Values are expressed as mean (standard deviation) |
| | Test (n=20) | Control (n=20) |
| Age (years) | 48.2 (15.2) | 45.4 (17.8) |
| Sex (% males) | 45 | 60 |
| Height (cm) | 172.5 (9.1) | 163.3 (41.7) |
| Weight (kg) | 71.8 (12.9) | 76.1 (19.7) |
The main inclusion criterion was the presence of an active SHL (trigger point) within each of the supraspinatus, infraspinatus and gluteus medius muscles on the right side. There are 2 types of clinically identifiable SHL; active and passive. Active SHL are symptomatic at rest, whereas latent SHL are asymptomatic at rest and require stimulation to evoke pain (17). The primary diagnostic feature used to identify a SHL in this study was a distinct hypersensitive locus palpable within the myofascial tissues of the respective muscles. This locus is characteristically associated with a nodule that is nested within a taut band of skeletal muscle. Sustained pressure on the nodule (10–20 s) elicits diffuse, achy, referred pain, which is recognizable to the subject (pain recognition) (18). To improve reliability of detection and emphasize hypersensitivity in the diagnostic process, only SHL with a baseline (pre-intervention) pain pressure threshold (PPT) value of 35 N or less were used in this study (19). The precise location of each SHL was marked on the skin using a non-toxic marker for ease of follow-up identification; follow-up identification did not require 10–20 s of sustained pressure.
Two clinicians, each with over 15 years of clinical experience in rehabilitation medicine, were involved in patient recruitment and data collection. Prospective subjects were selected randomly from the patient roster of an outpatient rehabilitation clinic specializing in the treatment of myofascial pain syndrome and chronic pain. All prospective subjects presented with the diagnosis of myofascial pain, either regional or generalized.
The clinic receptionist randomly selected prospective subjects by file number from the clinic patient roster and contacted them to provide details of the study and to arrange a consultation with the first (assessing) clinician. A total of 54 prospective subjects were contacted during the recruitment process; of these, 5 people chose not to participate.
The assessing clinician was responsible for examining all prospective subjects for active SHL in each of the supraspinatus, infraspinatus and gluteus medius muscles using the aforementioned criteria. If active SHL were present, prospective subjects were then asked to complete a health history questionnaire and undergo a physical examination by the assessing clinician to identify exclusion criteria, such as neurologic conditions (neuropathy, myopathy), use of medication (antidepressants, opioids) and/or acute cervico-thoracic injury (whiplash, facet irritation, acute discopathy) that could directly influence normal somatosensory processing at the C5 segment. At this stage, 9 subjects were excluded, 5 due to the presence of acute cervico-thoracic conditions and 4 due to long-term use of medication. Each qualifying patient’s file number was then given to the receptionist; none of the qualifying subjects chose to withdraw from the study at this stage. The clinic receptionist, blinded to subjects, then assigned the qualifying subjects to either test or control groups by drawing slips of paper from a bin containing 40 slips, 20 labeled “test” and 20 “control”. This process continued until both groups had the requisite 20 subjects.
The specific aim of this study was to assess whether needle stimulation of a trigger point (sensitive locus) evokes anti-nociceptive effects in segmentally related (neurologically linked) trigger points. To quantify this, we calculated the segmental component of the PPT (PPTseg) based on the following rationale. Stimulation of the supraspinatus trigger point will potentially evoke both segmental and non-segmental anti-nociceptive effects at the infraspinatus site. Segmental anti-nociceptive mechanisms would impact the pressure sensitivity selectively at the infraspinatus trigger point, owing to the common neurologic link between supraspinatus and infraspinatus at C5, which is not shared by the supraspinatus and gluteus medius (L4,5S1) (20). Due to their generalized non-specific action, the impact of non-segmental (systemic, supraspinal) anti-nociceptive effects (e.g. diffuse noxious inhibitory control (DNIC)) would be comparable at both infraspinatus and gluteus medius trigger points. Accordingly, the difference in PPT values between the infraspinatus and gluteus medius trigger points (PPTseg) is a direct quantitative measure of the segmental anti-nociceptive influences acting at the infraspinatus point. Based on this rationale, increased values of PPTseg represent increased segmental anti-nociceptive effects (decreased sensitivity) at the infraspinatus SHL.
Data collection
The primary outcome measure used to quantify SHL sensitivity was the PPT, measured in units of Newtons (N). All force readings were performed using a Chatillon DFE Series Digital Force Gauge with a gauge tip contact area of 285 mm2 (19 mm × 15 mm). Prior to data collection, all qualifying subjects who were on a short-term course of medication (anti-inflammatory, muscle relaxants) refrained from taking their medication for 48 h prior to data collection. All PPT readings were performed on the right side of the body. Prior to actual data collection, subjects were trained in PPT identification on the contralateral side. Patients were placed comfortably in the prone position for all phases of data collection. One SHL was located in each of the right supraspinatus, infraspinatus and gluteus medius muscles and marked with a non-toxic marker for easy follow-up identification. PPT readings were taken with the force gauge by applying a consistently increasing force at the rate of 5 N/s (21) over the identified SHL; subjects were instructed to identify the onset of a deep dull achy or sharp stabbing sensation at the SHL site, at which point the instantaneous pressure reading was recorded as the raw PPT value.
Baseline PPT readings (time zero) were recorded from the right infraspinatus and right gluteus medius SHL sites prior to the treatment intervention (test, control). Subjects then received the needling intervention according to their group allocation. Sterile Carbo needles (0.25 × 40 mm) were used for all needling protocols. Test subjects received intramuscular dry needle injection into the identified supraspinatus SHL. The depth of penetration varied according to the subject; however, site specificity was confirmed by the presence of local and referred pain upon insertion. The presence of a jump sign and/or local twitch response was confirmatory, but not mandatory, for the identification of the SHL. Control subjects received a sham procedure involving intramuscular dry needle injection into the normal tissue surrounding the identified supraspinatus SHL (22). This sham procedure was validated by the absence of pain or discomfort (local, referred) as well as absence of a local twitch response. After the needle procedure, PPT readings were recorded by the second clinician from both the infraspinatus and gluteus medius sites at 1, 3, 5, 10 and 15 min intervals, post-needling. The needle remained in situ for the full duration of the experiment. Three PPT readings were recorded at each trigger point at each time interval, the mean of the closest 2 readings was reported as the raw PPT score. All raw PPT values were then normalized to the baseline (pre-needling) scores to standardize for variations between subjects. Subjects and the recording clinician were both blinded to intervention grouping.
Statistical analysis
Repeated measures analysis of variance (ANOVA) was performed, with time and treatment as independent variables and PPTseg as dependent variable. Time was treated as a factor, since time intervals were not evenly spaced. In order to detect significant differences in PPTseg between treatment conditions a univariate ANOVA was performed at each time point individually, using treatment as independent variable and PPTseg as dependent variable. Repeated measures analysis was performed with CRAN R statistical software (Version 2.51), Development Core Team, Vienna, Austria and univariate ANOVA within time points was performed with SPSS Statistical Software (Version 16.0) SPSS Inc., Chicago, USA. The level of significance in both analyses was set at 5%.
RESULTS
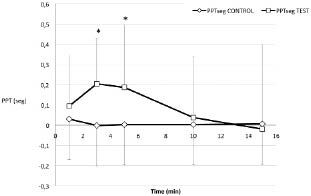
We observed significant short-term decreases in pressure sensitivity at the infraspinatus trigger point, compared with the gluteus medius point, in test subjects post-needling (Fig. 2). Significant overall interactions were observed for treatment group (p = 0.008) but not time (p = 0.214) or time*group (0.168). Comparisons of groups at individual time points revealed significant differences in PPTseg between test and controls at 3 (p = 0.002) and 5 (0.015) min post-needling, suggestive of a short-term segmental anti-nociceptive effect at the infraspinatus trigger point site. Raw PPTseg values are listed in Table II.
Fig. 2. Raw pain pressure threshold (PPT) readings were taken from the infraspinatus and gluteus medius trigger points at 1, 3, 5, 10 and 15 min post-needling, and were normalized to baseline (pre-needling) values. Test subjects receive dry needle puncture to the supraspinatus trigger point, while controls received sham intramuscular dry needle puncture. PPT(seg), the difference between the infraspinatus and gluteus medius PPT readings, represents the direct segmental effect acting on the infraspinatus trigger point. The PPT(seg) was significantly greater (i.e. decreased sensitivity at infraspinatus vs gluteus medius) in test subjects vs controls at 3 and 5 min (denoted by *). Bars represent 1 standard deviation.
| Table II. Raw pain pressure threshold (PPT)seg values with 95% confidence intervals (CI) recorded at each time interval by group. |
| Time (min) | Group | Mean | 95% CI |
| 1 | Control | 0.029 | –0.199–0.256 |
| 3 | Control | –0.002 | –0.291–0.288 |
| 5 | Control | 0.003 | –0.187–0.193 |
| 10 | Control | 0.003 | –0.354–0.360 |
| 15 | Control | 0.006 | –0.496–0.508 |
| 1 | Test | 0.095 | –0.398–0.587 |
| 3 | Test | 0.205 | –0.235–0.644 |
| 5 | Test | 0.187 | –0.419–0.792 |
| 10 | Test | 0.038 | –0.549–0.624 |
| 15 | Test | –0.020 | –0.834–0.795 |
DISCUSSION
The results of this study illustrate that site-specific stimulation of a SHL via intramuscular dry needle technique reduces short-term pain sensitivity in segmentally linked SHL. This response was not observed with needle stimulation of normal (non-SHL) tissues, confirming that these effects were direct manifestations of site-specific stimulation of the SHL. The clinical significance of this anti-nociceptive effect is unknown, as the minimal clinically important difference (MCID) of PPT values in trigger points or acupoints has not been studied previously. Analogous segmental anti-nociceptive effects have been previously reported using ultrasound to stimulate trigger points (23). The similar anti-nociceptive effect using different stimulating modalities validates the importance of the sensitive locus in mediating these effects.
The primary diagnostic criterion for the identification of the SHL in our study was hypersensitivity; we only included SHL with baseline values of 35 N or less. In addition, the presence of a tender nodule, patient pain and pain referral were confirmatory for the presence of a SHL. Lucas et al. (14) suggests that “worthwhile agreement might be achieved” if we emphasize tenderness and pain reproduction in the diagnostic protocol for trigger points. These criteria are also consistent with the neurogenic hypothesis for trigger point pathophysiology.
The effects of needling were not studied beyond 15 min, as we were more interested in first evaluating whether needling a SHL evoked a short-term segmental effect; the longer term effects (> 15 minutes) of needling need to be studied to investigate clinical applications of these results. The observed anti-nociceptive effect was short-lived (< 10 min); however, these effects were evoked by a single stimulation to only one SHL. It stands to reason, based on these results, that the simultaneous stimulation of multiple segmentally-linked SHL over repeated therapeutic sessions may evoke enhanced segmental responses. This mechanism may be the basis for the systematic effects observed with traditional acupuncture therapy and is the focus of follow-up studies.
Much of our understanding of the systematic physiologic effects of SHL stimulation is derived from the acupuncture literature. Evoked potential recordings demonstrate that signals induced from acupoints follow different neural pathways through the nervous system compared with those elicited from non-acupoints (24). In addition, changes in somatosensory evoked potentials (increased latency and decreased amplitude) (25) and visceral effects have been observed in both animals (9, 26) and humans (8).
Analgesia is one the most profound physiologic responses to acupoint stimulation, although the mechanism has yet to be elucidated. Site-specific needle stimulation of acupoints decreases pain sensitivity in a variety of clinical conditions, including headache (27), dental pain (28) and back pain (29). Moreover, these systematic effects have also been induced by stimulation of acupoints with other therapeutic modalities, such as pressure (30), magnets (31) and ultrasound (16), further emphasizing the physiologic importance, and modality-independence, of the SHL.
These collective observations demonstrate the profound physiologic and therapeutic impact of stimulating SHL. In contrast to traditional meridian acupuncture theory, the results of our study suggest that these systematic effects may be mediated via segmental neuromodulatory mechanisms. Moreover, since trigger point sensitivity has been linked to central sensitization (32), it is plausible that the segmental effects observed in this study are subsequent to modulation of central sensitization within the common neuromeric field of the SHL. This is the focus of a follow-up study.
The increased density of large, myelinated fibers in SHL regions may provide physiologic rationale for the segmental effects observed in this study. The inhibitory action of selective large fiber stimulation has been shown to significantly influence the central processing of pain (33). Repetitive electrical stimulation of large low-threshold cutaneous fibers inhibits the excitatory discharges of dorsal horn neurons in humans (34) while animal studies demonstrate that selective stimulation of large myelinated fibers induces “long-term depression” of primary synapses in the dorsal horn neurons (35). Accordingly, we hypothesize that the observed anti-nociceptive effects of site-specific SHL stimulation in this study may be mediated by segmental inhibitory effects evoked by selective stimulation of large myelinated fibers in the SHL. This may be an important mechanism responsible for the documented systematic effects (visceral and somatic) of acupuncture.
In this study, trigger point sensitivity was quantified using the PPT measure. Pressure algometry is a convenient and reliable method of assessing trigger point sensitivity and has demonstrated high inter- and intra-examiner reliability (36, 37). A strong correlation exists between PPT readings and pain perception, making the PPT a reliable outcome measure for the activation state of the trigger point. A change in the PPT value implies an underlying change in the subject’s pain sensitivity, including the collective influence(s) from spinal (segmental), supraspinal or other physiologic (biochemical, electrochemical, hormonal) mechanisms.
Age and sex are important confounding variables in the interpretation of pain. Studies suggest that pressure sensitivity is enhanced in elderly people, while sensitivity to non-noxious somatosensory stimuli is decreased (38). In contrast to the documented impact of age, more than 50% of existing studies do not report a sex bias (39), and the sex differences that are reported are small, inconsistent and subject to variations based upon experimental protocols (40). In this study, we are not concerned with the absolute values of PPT, but are specifically interested in the relationship between the PPT measures at the infraspinatus and gluteus medius muscles. In addition, by normalizing PPT scores to baseline our experiment focuses on the changes within individuals; for these 2 reasons, the effects of age and sex are relevent to the conclusions of our study. Another possible limitation of this study includes the potential modulating effect of repeated pressure testing on PPT values over time. Studies have shown, however, that repeated pressure algometry (7 trains of 2 repeated measurements over a 1-h test period) does not significantly impact PPT values over time (21).
In conclusion, we have observed significant short-term segmental anti-nociceptive effects after dry needling a secondary hyperalgesic locus (trigger point). Similar segmental anti-nociceptive effects have previously been reported with ultrasound stimulation of trigger points (23), suggesting that the SHL (trigger point) may be an important anatomic landmark for segmental neuromodulation. The pathophysiology of trigger points has previously been linked to central sensitization (41); in addition, the systematic effects of trigger point stimulation have been linked to the activation of networked spinal circuits (neuromeric fields) (42). Consequently, it is possible that trigger point stimulation directly modulates the magnitude of sensitization within the common neuromeric field. This mechanism may provide the physiologic basis to explain the broad profile of systematic (somato-visceral) physiologic effects documented in the acupuncture literature and make trigger points an important consideration in the management of pain.
Conflict of interest: The authors declare no conflicts of interest.
REFERENCES