OBJECTIVE: To explore the occurrence of, and risk factors for, spasticity until 6 months after first-ever stroke.
METHODS: Forty-nine patients were examined at day 2–10, at 1 month, and at 6 months. The modified Ashworth Scale was used to assess resistance to passive movements. A comprehensive clinical examination was performed to identify other positive signs of upper motor neurone syndrome, in accordance with a broader definition of spasticity, and to evaluate whether spasticity was disabling. Neurological impairments were determined by use of the National Institutes of Health Stroke Scale and global disability by use of the modified Rankin Scale.
RESULTS: Spasticity was present in 2 patients (4%) at day 2–10, in 13 patients (27%) at 1 month, and in 11 patients (23%) at 6 months. Severe paresis of the arm at day 2–10 was associated with a higher risk for spasticity at 1 month (odds ratio = 10, 95% confidence interval 2.1–48.4). Disabling spasticity was present in one patient at 1 month and in 6 patients (13%) at 6 months.
CONCLUSION: Spasticity according to the modified Ashworth Scale usually occurs within 1 month and disabling spasticity later in a subgroup. Severe paresis of the arm is a risk factor for spasticity.
Key words: stroke; spasticity; incidence; prevalence; prediction.
J Rehabil Med 2010; 42: 296-301
Correspondence address: Erik Lundström, Department of Neuroscience, Neurology and Rehabilitation Medicine, Uppsala University Hospital, SE-751 85 Uppsala, Sweden. E-mail: erik.lundstrom@gmail.com
Submitted June 1, 2009; accepted November 17, 2009
INTRODUCTION
Stroke often affects sensory-motor networks and descending tracts, as reflected by the negative and positive signs of upper motor neurone (UMN) syndrome (1). Spasticity is one of the positive signs, most often defined according to Lance (2) and clinically characterized by a velocity-dependent increase in the resistance to passive movement. A broader definition of spasticity that incorporates other positive signs of UMN syndrome, such as co-activation of antagonist muscles during voluntary activity and dystonic posturing of limbs, as well as flexor spasms, has been suggested (1). Spasticity in this broader sense may interfere with motor function, and is a common reason for clinical interventions such as by physiotherapy, use of orthoses or other technical devices or drugs. The clinical significance of such disabling spasticity is reflected by the increasing number of intervention studies during recent years, in which intramuscular injections of botulinum toxin or intrathecal baclofen are used to target one or more of the positive signs of UMN syndrome (3, 4).
Data on the prevalence as well as on the significance of the positive signs of the UMN syndrome after stroke with regard to disability, as conceptualized by the International Classification of Functioning, Disability and Health (ICF), are scarce (5). This probably reflects both conceptual and methodological problems related to the definition and assessment of spasticity (1, 6). Recent studies yield some information on the prevalence of spasticity as assessed by the Modified Ashworth Scale (MAS). This scale permits grading of the resistance to passive movements at rest but does not differentiate resistance due to increased reflex activity from resistance due to biomechanical factors (7). The reported prevalence of spasticity according to MAS at 3 months or later after first-ever stroke in studies of unselected, consecutive patients after stroke is around 20% or higher (8–11). Neurophysiological studies indicate that reflex-mediated increase in muscle tone reaches its maximum already within 3 months after stroke (12, 13), but conclusive data on the occurrence early after first-ever stroke are lacking and data on the prediction of spasticity are scarce. In a modelling study by Leathley et al. (14), early arm or leg weakness, as well as lower day 7 Barthel Index score, were significant predictors of abnormal muscle tone at 12 months. A prospective study by van Kuijk et al. (15), including only patients with severe stroke and presenting with upper extremity paralysis, demonstrated a higher prevalence of muscle hypertonia according to MAS (63%) than in unselected study samples. No association was found between other, initial neurological impairments, or Barthel Index at week 1, and spasticity at 26 weeks after stroke.
There is no single measure that captures all relevant aspects of spasticity according to the broader definition (1), and data on the prevalence of such spasticity are scarce. In a recent study, we used a comprehensive clinical evaluation to identify positive signs of the UMN syndrome and to evaluate their impact on motor function, activity performance and participation (8). The estimated prevalence of disabling spasticity with a need for intervention in a representative sample of patients at 1one year after first-ever stroke was 4% (8).
Early identification of potentially disabling spasticity is important to enable preventive intervention. Therefore, the present study was performed to explore the occurrence of, and risk factors for, spasticity until first 6 months after first-ever stroke among those with any initial central paresis.
Materials and METHODS
Patients
Patients were recruited consecutively from Uppsala University Hospital between February 2005 and March 2008, with the last follow-up in September 2008. Uppsala University Hospital is responsible for the stroke population in 5 municipalities with a total of 265,000 inhabitants. Inclusion criteria were: (i) a first-ever stroke (cerebral infarction or intracerebral haemorrhage) defined according to the World Health Organization (WHO) criteria (16); (ii) any central paresis in the face, arm, hand or leg, at stroke onset; (iii) age between 18 and 84 years; (iv) resident in the catchment area; and (v) ability to give informed consent. Exclusion criteria were: (i) any other neurological disorder that might affect muscle tone, (ii) transient ischaemic attack, and (iii) subarachnoid haemorrhage. Before entering the study, the patients received written and oral information. In case of language problem, proxy approval by next-of-kin was used. All participating patients, or their next-of-kin, gave informed consent. The study was approved by the regional human ethics committee (Ups 2004: M-444).
Study design
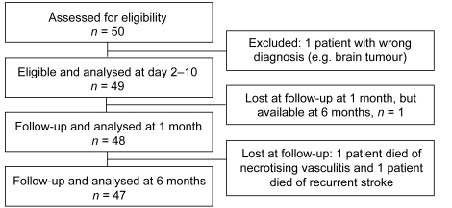
Details of the study design and inclusion procedures are shown in Fig. 1. Fifty patients were recruited, all performed a computerized tomography scan of the brain in the acute stage to distinguish between haemorrhagic and ischaemic stroke. One patient was excluded during the course of study because of a revised diagnosis of brain tumour instead of ischaemic infarction, leaving 49 eligible patients. The patients were examined at 3 different time-points: At inclusion (2–10 days after the stroke onset), and at 2 follow-ups; 1 and 6 months after the stroke. Stroke severity, paresis, sensory disturbance, spasticity and disability were assessed at all 3 time-points. All acute assessments and most of the follow-ups were carried out at the Uppsala University Hospital. Baseline characteristics are summarized in Table I.
Fig. 1. Consort flow diagram for the patient sample.
| Table I. Baseline characteristics of the cohort (n = 49) at inclusion, day 2–10 after the stroke onset |
| Age, median (min–max), years | 74 (35–84) |
| Women, n (%) | 21 (43) |
| Stroke type*, n (%) | |
| Ischaemic stroke | 41 (84) |
| Haemorrhagic stroke | 8 (16) |
| NIHSS points, median (min–max) | 5 (0–19) |
| Disability, n (%) | |
| Modified Rankin Scale 0–1 | 11 (22) |
| Modified Rankin Scale 2–5 | 38 (78) |
| Paresis†, n (%) | 45 (92) |
| Paresis in the face‡ n (%) | 26 (53%) |
| Paresis in the arm or hand§ n (%) | 32 (65) |
| Paresis in the leg¶ n (%) | 29 (59) |
| Sensory disturbance for painº, n (%) | 20 (41) |
| *Stroke was classified into ischaemic or haemorrhagic. †Definition of paresis is ≥ 1 on any of item 4 (face), 5 (arm), 6 (leg) or 12 (hand) on the NIHSS. ‡Item 4 on the NIHSS, §Item 5 and/or 12 on the NIHSS, ¶Item 6 on the NIHSS, ºItem 8 on the NIHSS. NIHSS: National Institutes of Health Stroke Scale. |
Stroke severity including severity of paresis and sensory disturbance
The National Institutes of Health Stroke Scale (NIHSS) is a 46-point ordinal scale that assesses neurological deficit and is frequently used in stroke studies (17, 18). The NIHSS is considered reliable and valid, as well as quick and easy to use (19–21). We used the NIHSS for the following 3 purposes:
• To assess the severity of the stroke (total NIHSS score).
• To assess the severity of paresis. Severe paresis was defined as a score of ≥ 2 on item 5.
• To assess the presence of sensory disturbance, defined as a score of ≥ 1 on item 8.
Spasticity
Spasticity was assessed by use of the MAS. The MAS is a 6-point ordinal scale with documented reliability (22, 23). All patients were assessed by the first author (EL), and those exhibiting any signs or symptoms of spasticity were also assessed by the last author (JB), as well as by a physiotherapist and/or occupational therapist who were members of a neurorehabilitation team specialized in motor disorders. Spasticity according to the MAS was assessed with the patient in a resting position in an outpatient ward, except for those few who required examination at another institution or at home. Assessment of spasticity included flexion and extension movements around upper (shoulder, elbow, wrist, and fingers) and lower extremity (hip, knee, and ankle) joints, with the patient in resting position. Spasticity was defined as a MAS score ≥ 1 for any of the passive movements performed, in accordance with most previous studies on spasticity after stroke (8–11).
The definition of disabling spasticity has been described in detail previously (8). Briefly, a comprehensive clinical evaluation, based on a semi-structured interview and a detailed neurological examination, was performed by a doctor and a team therapist to identify any positive signs of the UMN syndrome and to evaluate if these had a clinically significant impact on motor function, activity performance or social life.
Global disability
The modified Rankin Scale (mRS) was used to assess global disability. The mRS is a 7-point ordinal scale commonly used in stroke studies (available at http://www.strokecenter.org/trials/scales/rankin.html). The inter-rater reliability and validity for stroke outcome is well-documented (24). The mRS outcome was dichotomized into mRS ≤ 1 vs mRS ≥ 2 (17, 18).
Statistics
Descriptive statistics were used for baseline characteristics (Table I) and for some of the follow-up data (Table II).
| Table II. Temporal course and distribution of spasticity and disabling spasticity |
| | At inclusion (day 2–10)
n = 49 | At 1
month
n = 48 | At 6
months
n = 47 |
| Spasticity, n (%) | 2 (4) | 13 (27) | 11 (23) |
| Upper extremity, n (%) | 2 (4) | 12 (25) | 10 (22) |
| Lower extremity, n (%) | 2 (4) | 6 (13) | 6 (13) |
| Severity of spasticity | | | |
| MAS maximum 1, n (%) | 0 | 9 (19) | 3 (6) |
| MAS maximum 1+, n (%) | 2 (4) | 2 (4) | 1 (2) |
| MAS maximum 2, n (%) | 0 | 0 | 3 (6) |
| MAS maximum 3, n (%) | 0 | 1 (1) | 3 (6) |
| MAS maximum 4, n (%) | 0 | 1 (1) | 1 (2) |
| Disabling spasticity, n (%) | 0 (0) | 1 (2) | 6 (12) |
| Severe paresis in the arm*, n (%) | 18 (37) | 8 (17) | 4 (9) |
| Severe paresis in the leg†, n (%) | 7 (14) | 2 (4) | 3 (6) |
| Sensory disturbance, pain‡, n (%) | 20 (41) | 18 (38) | 15 (32) |
| NIHSS, median (min–max) | 5 (0–19) | 2.5 (0–15) | 1 (0–20) |
| *Defined as > 2 on item 5 (arm) on the National Institutes of Health Stroke Scale (NIHSS). †Defined as > 2 on item 6 (arm) on the NIHSS ‡Defined as > 1 on item 8 on the NIHSS. MAS: Modified Ashworth Scale. |
As previous studies showed a frequency around 20% of spasticity according to MAS in non-selected stroke populations, we estimated that spasticity according to MAS in the current sample, including only patients with initial motor impairments, would be around 30% or higher. In the current sample, this would allow testing of the independent effect on spasticity of variables, which we previously found to be associated with spasticity at one year after first-ever stroke (8). Univariate analyses compared the clinical characteristics of patients with no spasticity vs patients with spasticity at 1 month and 6 months after stroke. Since the parameters age and NIHSS did not show Gaussian distributions, we used the Mann-Whitney U test to compare the two groups. The univariate analysis was performed with χ2 for categorical data. When expected values were less than 5, Fisher’s exact test was used. A p-value (2-tailed) < 0.05 was set as significant.
At first, univariate analysis was performed for identifying variables associated with spasticity at 1 month. Stroke type, severe paresis of the arm and sensory disturbance were associated with spasticity at 1 month. The time-point of 1 month was chosen because we found that spasticity was evident as early as 1 month after stroke. At 1 month, 13 patients with spasticity were found, allowing us to test for 2 variables in the multiple logistic regression model. Thus, the variables stroke type (ischaemic or haemorrhagic) and severe paresis of the arm were entered in the model. The reason for choosing severe arm paresis was based on previous studies (8, 14) in which paresis and spasticity were found to be associated with spasticity, whereas sensory disturbance lost its significance in our own study (8). Odds ratios are given with 95% confidence intervals.
Cochrane’s Q test for repeated measurements was used for analysing whether the frequency of spasticity changed during the study period. Statistics were performed by use of SPSS version 16.0 for Macintosh.
RESULTS
Demographic and clinical data are summarized in Table I. Median age of the study sample (n = 49) was 74 years (range 35–84), for women 75 (range 45–83) and for men 74 (range 35–84) years. The proportion of women was 43%. The stroke was ischaemic in 84% and haemorrhagic in 16% of the patients.
Spasticity according to Modified Ashworth Scale
The incidence of spasticity at day 2–10, at 1 month and at 6 months after stroke is shown is Table II. Of all 49 patients, 2 patients (4%) had MAS ≥ 1 at inclusion. In one of these, spasticity was present also at 1 month and at 6 months. During the whole follow-up, 17 out of 49 (35%) patients showed spasticity at one or more of the 3 time-points. At 1 month, 13 out of 48 patients (27%) showed spasticity. At 6 months, 11 out of 47 patients (23%) showed spasticity. Seven of these patients showed spasticity at both 1 and 6 months, whereas 3 only at 6 months. Five patients had spasticity at 1 month, but not at 6 months. The Cochrane’s Q test showed significantly different frequency of spasticity (p = 0.002) by time.
Spasticity was most often observed in the upper extremity. At inclusion, 2 patients had spasticity in both the upper and lower extremity. At 1 month, 12 out of 48 patients (25%) had spasticity in the upper extremity, 6 (13%) had spasticity in the lower extremity and 1 (2%) had spasticity solely in the lower extremity. At 6 months, 10 out of 47 patients (22%) had spasticity in the upper extremity, 6 had spasticity in the lower extremity and 1 had spasticity solely in the lower extremity. MAS scores tended to increase during the observation time (Table II).
Univariate analyses demonstrated correlations between spasticity at 1 month and age, stroke type, severe paresis in the arm and sensory disturbance at inclusion (Table III). By entering these variables in a multiple regression analysis we found an independent association between severe arm paresis at inclusion and spasticity at 1 month (odds ratio (OR) = 10, 95% confidence interval (CI) 2.1–48.4).
| Table III. Comparisons between patients with no spasticity vs patients with spasticity at 1 month |
| | No spasticity (n = 35) | Spasticity
at 1 month (n = 13) | p-value |
| Age, years, median (min–max) | 75 (45–83) | 68 (35–84) | 0.28 |
| Women, n (%) | 16 (46) | 4 (31) | 0.35 |
| Stroke type, n (%) | | | 0.014 |
| Ischaemic stroke | 32 (91) | 8 (62) | |
| Haemorrhagic stroke | 3 (9) | 5 (38) | |
| NIHSS points at inclusion, median (min–max) | 3 (0–19) | 12 (2–17) | 0.002 |
| Severe paresis in the arm at inclusion*, n (%) | 8 (23) | 10 (77) | < 0.001 |
| Sensory disturbance at inclusion†, n (%) | 11 (31) | 9 (69) | 0.018 |
| Disability at inclusion, n (%) | | | 0.030 |
| Modified Rankin Scale 0–1 | 10 (29) | 0 | |
| Modified Rankin Scale 2–5 | 25 (71) | 13 (100) | |
| *Severe paresis in the arm, defined as > 2 points at item 5 in the NIHSS. †Defined as > 1 on item 8 on the NIHSS. NIHSS: National Institutes of Health Stroke Scale. |
Disabling spasticity
Of all 49 patients, 6 (12%) developed disabling spasticity during the 6-month follow-up period and all scored in the severe mRS category. No patient had disabling spasticity at inclusion, 1 (2%) had disabling spasticity at 1 month, and 6 (13%) had disabling spasticity at 6 months (Table IV). The Cochrane’s Q test showed significantly different frequency of spasticity (p = 0.002) by time. All 6 patients with disabling spasticity at 6 months had an initial severe paresis in the arm.
| Table IV. Comparisons between patients with no spasticity and disabling spasticity at 6 months |
| | No spasticity (n = 41) | Disabling spasticity at 6 months (n = 6) | p-value |
| Age, years, median (min–max) | 74 (35–83) | 54 (43–84) | 0.062 |
| Women, n (%) | 19 (46) | 2 (33) | 0.68 |
| Stroke type, n (%) | | | 0.27 |
| Ischaemic stroke | 35 (85) | 4 (67%) | |
| Haemorrhagic stroke | 6 (15) | 2 (33%) | |
| NIHSS points at inclusion, median (min–max) | 4 (0–19) | 14 (12–17) | < 0.001 |
| Severe paresis in the arm at inclusion*, n (%) | 12 (29) | 6 (100) | < 0.002 |
| Sensory disturbance at inclusion†, n (%) | 20 (49) | 4 (67%) | 0.67 |
| Disability at inclusion, n (%) | | | 0.31 |
| Modified Rankin Scale 0–1 | 11 (27) | 0 (0) | |
| Modified Rankin Scale 2–5 | 30 (73) | 6 (100) | |
| *Severe paresis in the arm, e.g. >2 points at item 5 in the NIHSS. †Item 8 on the NIHSS. NIHSS: National Institutes of Health Stroke Scale. |
DISCUSSION
The present study explored the occurrence of spasticity in a cohort of patients followed until 6 months after first-ever stroke. One main finding is that spasticity according to MAS most often appeared already within the first month and then persisted in the majority of these patients. Thus, 27% of the patients exhibited spasticity at 1 month and 23% of the patients at 6 months. These prevalence rates are somewhat higher than those reported in previous studies of Swedish cohorts, but no data have been available so far for the time-points of 1 month and 6 months after disease onset. Sommerfeld et al. (9) reported 19% spasticity after 3 months, Lundström et al. (8) 17% after 12 months, whereas Welmer et al. (11) found spasticity in 20% of a patient population 18 months after stroke. In a UK study by Watkins et al. (10) of 270 consecutive, hospitalized patients with stroke, 106 were followed up at 12 months by use of both the MAS and the Tone Assessment Scale (TAS) (10). The reported prevalence according to MAS was 27%. It may be speculated that this somewhat higher prevalence reflects differences with regard to admission criteria, rehabilitation programmes or assessment procedures.
Most previous prevalence studies have included patients with stroke regardless of initial paresis. For inclusion in our current study, an initial paresis was required in order to capture patients specifically at risk for spasticity and thus to increase the proportion of patients with the outcome of interest. Basically, for spasticity to occur, damage to both the corticospinal and other corticofugal descending tracts has to occur (25, 26) and a strong, inverse correlation between primary motor function according to, for example, the Fugl Meyer Scale and spasticity after stroke has been demonstrated (27, 28). Furthermore, a recent study by van Kuijk et al. (15), which included only patients with severe stroke and presenting with upper extremity paralysis, showed a much higher prevalence (63%) of muscle hypertonia according to the Ashworth Scale at 26 weeks after the stroke, compared with studies of unselected samples.
The design of this study allowed us to monitor the occurrence of spasticity over time during a 6-month period following first-ever stroke. We found that for the majority of patients, who exhibited spasticity according to MAS at 1 month, this was also present at the 6 months examination. However, as might be expected during this early phase post stroke, there was variability within the patient population, probably reflecting that recovery and maladaptative processes are running in parallel. Thus, 5 patients with spasticity at 1 month showed no spasticity at 6 months, while 3 patients with no spasticity at 1 month exhibited spasticity at 6 months. Interestingly, MAS scores tended to increase by time. This might indicate ongoing nervous system reorganization as well as increasing muscle stiffness, and points to the need for clinical methods to differentiate neural and biomechanical components of resistance to passive movements, as pointed out previously (29, 30). Thus, further studies utilizing such methods should be of interest in order to identify the time course of different components of the increased resistance to passive muscle stretch, to identify risk factors for these and to allow the design of improved early interventions.
Stroke type, severe paresis in the arm and sensory disturbance were associated with the occurrence of spasticity at 1 month after stroke. However, only severity of upper extremity paresis in the arm came forward as an independent variable in the multivariate analysis, which might be due to the small sample size in the present study. Overall, this finding is in agreement with the observations in the prediction model described by Leathly et al. (14), demonstrating that early weakness of the arm or the leg were significant predictors of abnormal muscle tone at 12 months after stroke. The finding is also in agreement with findings in the study by van Kuijk et al. (15), demonstrating a high prevalence of spasticity in patients presenting with a severe upper extremity paresis but no association between sensory disturbances, or other acute neurological impairments, and spasticity at 26 weeks after the stroke.
Another finding in the present study is that a subgroup of patients with spasticity according to MAS is at risk for developing disabling spasticity and that this mainly develops later than 1 month post-stroke. Thus, disabling spasticity was observed in only 1 patient at 1 month and in another 5 patients at 6 months, yielding a prevalence rate of 12% at that time-point. This rate is higher than the prevalence rate of 4% that we observed in a previous study of patients at 1 year after first-ever stroke (8). As discussed above, one reason for the higher prevalence rate in the present study is probably that paresis was required for inclusion. Although the small number of patients with disabling spasticity at 6 months does not allow us to draw any conclusions, the data suggest that an initial severe paresis is a risk factor for disabling spasticity.
The concept, definition and assessment of spasticity are matters of debate (6, 31). In the present study, we used the MAS to quantify spasticity in order to enable comparison with prior studies. We also used a clinical approach, as described in detail in a previous study (8), to capture other positive signs of the UMN syndrome and their impact on motor and activity performance. This is accordance with the wider definition of spasticity that has been proposed by Pandyan et al. (1), i.e. disordered sensory-motor control, resulting from an UMN lesion, presenting as intermittent or sustained involuntary activation of muscles. There is no established single assessment instrument that captures all relevant aspects of spasticity according to this broader definition. The TAS (22, 32), which covers not only resistance to passive movements, but also posturing at rest and reactions associated with voluntary activation, indeed has merits. In the study by Watkins et al. (10), the prevalence of spasticity according to MAS only was 27%, but according to MAS and TAS combined was 38%, indicating a much higher prevalence rate of spasticity after stroke according to the broader definition. However, the TAS has not been used much and even if a correlation between spasticity according to TAS and Barthel scores was demonstrated, the TAS does not “describe or measure the resulting disability” (10). Thus, there is no single measure that addresses spasticity-related disability. Therefore, in this explorative study, we used a comprehensive clinical evaluation to achieve a clinically relevant estimate of the prevalence of disabling spasticity after first-ever stroke in addition to conventional measures of disability.
Interpretation of our data must be cautious and consider both the sample size, the inherent limitations of the MAS with regard to validity and possible confounders of even a comprehensive clinical evaluation. However, this study clearly indicates that spasticity according to the MAS mainly develops within 1 month after first-ever stroke while disabling spasticity develops later in a subgroup and that patients with an initially severe arm paresis are at higher risk for developing spasticity. The findings should be useful for further studies with more elaborate design aiming at the early identification of patients at risk for disabling spasticity.
ACKNOWLEDGEMENTS
This study was supported by grants from Selanders foundation and Stroke-riksförbundet. We thank all the patients and the staff at the Stroke Unit at Uppsala University Hospital.
REFERENCES