OBJECTIVE: To explore the associations amongst the on-and-off kinetics time constants, ambulatory activity outcomes, and physical functional performance in stroke survivors.
SUBJECTS: Ten stroke survivors (time since stroke: mean 7.5 years (standard deviation 8.3) ; gender: 4 males, 6 females) and 10 control subjects matched for age and physical activity level.
METHODS: Oxygen uptake kinetics (on-and-off kinetics) were measured using a submaximal exercise test with a recumbent cycle. The continuous-scale physical functional performance test was used to measure functional ability. Ambulatory activity outcomes including steps per day, number of activity bouts and length of activity bouts were measured using a Step Activity Monitor.
RESULTS AND CONCLUSION: Shorter activity bouts were significantly associated with longer off-kinetics time constants in stroke survivors (or longer time to recover from an exercise bout). Future research may test the effect of activity interventions designed to increase the length of activity bouts on-kinetics outcomes and functional ability.
Key words: oxygen uptake kinetics; ambulatory activity; stroke survivors.
J Rehabil Med 2010; 42: 259–264
Correspondence address: Trish Manns, 2-50 Corbett Hall, Department of Physical Therapy, University of Alberta, Edmonton, Alberta, T6G 2G4, Canada. E-mail: trish.manns@ualberta.ca
Submitted November 26, 2008; accepted October 22, 2009
INTRODUCTION
Oxygen uptake (VO2) kinetics quantifies the time required to adapt from one metabolic load to another, for example from rest to a new steady state submaximal exercise load, or from that submaximal exercise bout to passive recovery. It describes the rate of change in VO2 in response to a submaximal exercise bout (1), and is a surrogate measure of cardiopulmonary fitness (2). Less time taken to adapt to a new exercise load reflects increased efficiency in the ability of the cardiopulmonary and skeletal muscle systems to deliver and/or utilize oxygen (1). In clinical populations, cross-sectional studies have shown that VO2 kinetics are slowed in people with heart failure (1, 3) and those with pacemakers (4). Similarly, studies with young adults with cystic fibrosis demonstrate slowed recovery kinetics (5, 6).
Limited studies with individuals with neurological conditions have been conducted. An early investigation trained 9 individuals with spinal cord injury (SCI) using functional electrical stimulation (FES) and showed that 24 bi-weekly sessions of FES exercise resulted in significant improvements in VO2 kinetics (7). More recently, a cross-sectional study with trained individuals with SCI found that people with SCI adapted and recovered faster from a submaximal exercise load compared with an untrained control group (8). These authors speculated that the peripheral muscle adaptations in the arms of the trained SCI subjects may have led to less lactate production, accounting for their findings (8). A study of people after stroke reported improvements in VO2 on-kinetics following an 8-week program of low-intensity aerobic exercise (9). Shorter on-kinetics time constants were also associated with ambulatory activity (10). However, the methods used to measure on-kinetics (1 repetition) and ambulatory activity (pedometer) limit the conclusions we can draw from these studies (9, 10). Finally, a study with older adults explored the associations between on-and-off kinetics time constants and functional ability (2). Alexander and associates reported significant positive associations with on-and-off kinetics time constants and selected functional tests including the get-up-and-go and the 6-minute walk (2). The authors suggest that kinetics testing may predict functional ability because energy expenditure during kinetics testing approximates that of functional abilities.
The associations between on-and-off kinetics time constants and functional ability have not been explored in stroke survivors. Ambulatory activity parameters measured with a more accurate measurement tool than pedometry, and their associations with kinetics outcomes have also not been explored in stroke survivors. This study will explore the associations amongst the on-and-off kinetics time constants, ambulatory activity outcomes, and physical functional performance in stroke survivors. Descriptive data related to submaximal on-kinetics, total daily ambulatory activity, and functional ability (CS-PFP10) has been published previously (11, 12). This manuscript includes data regarding submaximal recovery kinetics (off-kinetics), as well as more detailed information related to activity profiles (activity bouts, and length of activity bout). We also document and explain in detail the measurement of oxygen uptake kinetics and explore possible clinical application of this outcome in rehabilitation medicine.
METHODS
Participants were tested on 2 separate days, at least 2 days apart. Testing on day 1 consisted of questionnaires (health history, physical activity), and measurement of participant characteristics (height, weight, gait speed, use of walking aids). A graded exercise test (for measurement of peak VO2) and set up of the step activity monitors were also completed on day 1. On the second day of testing, participants completed a submaximal exercise test (for VO2 kinetics) and the continous-scale physical functional performance (CS-PFP10) test battery. The study was approved by the Health Research Ethics Board at the University of Alberta and all participants gave informed consent prior to participation.
Measurement of on-and-off kinetics time constants
A recumbent cycle ergometer was used for all testing (Ergo-metrics 800S, Roxon Inc., Montreal, QC, Canada). Two tests were completed, a graded exercise test to determine peak VO2 and a steady state submaximal exercise test to determine VO2 kinetics. The methods used for the measurement of peak VO2 and VO2 kinetics have been described previously (12). Briefly, the peak VO2 test results, and subsequent determination of ventilatory threshold (13) were used to establish workload for the submaximal test (i.e. VO2 kinetics test). A steady-state submaximal exercise test below the ventilatory threshold was completed to measure VO2 kinetics during exercise. The exercise protocol followed 5 min of quiet rest to determine baseline VO2, and involved an abrupt pedalling start to an intensity corresponding to the VO2 ranging from 75% to 90% of the ventilatory threshold. The exercise period lasted 5 min, and measurement on the metabolic cart continued for 7 min following exercise cessation. The protocol was repeated 3 times, with a 20-min rest between each repetition.
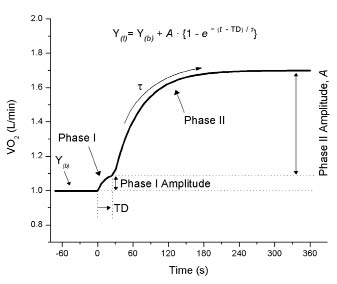
Outcomes measured from VO2 kinetics include phase I, phase II, time delay, and the time constant or tau (τ) (Fig. 1). The phase I response is referred to as the cardiodynamic phase (14). This phase does not reflect an exercise-induced increase of VO2 but a wash out of the metabolites in the resting muscle as a result of exercise-induced increases in pulmonary blood flow returning to the lungs (14). The time delay outcome reflects the time from the start of exercise to the end of phase I. The phase II response, by contrast, is a reflection of the system’s ability to deliver oxygen to the muscles and/or utilize the available oxygen to adapt to energy demands associated with the new exercise load. The time constant (τ) reflects the time to reach a 63% change in respective variables, with longer (greater) time constants indicating a slower period of adaptation to an exercise perturbation. At exercise onset, the rate of increase in VO2 reflects an acquired oxygen deficit, or the period at the beginning of exercise when the demand of the muscles exceeds what the pulmonary system is able to deliver (2). By contrast, the time constant following exercise is reflective of the oxygen debt to be repaid, and is closely related to the inorganic phosphate/phosphocreatine ratio (15). The primary kinetics outcomes reported in this study are the on-and-off kinetics time constants (τ).
Fig. 1. Profile of a bi-phasic response in oxygen uptake (VO2) to square wave exercise at a constant work rate approximating 80% of the ventilatory threshold. Time 0 indicates exercise onset. The corresponding mathematical equation distinguishing the phase II response is shown. Y(t): VO2 at any given time; Y(b): VO2 at baseline; TD: time delay to phase II onset; τ (tau): time constant or time for VO2 to reach 63% of the response; Phase, A: amplitude response of respective phases.
Physical functional performance
Physical functional performance in 5 domains (upper-body strength, lower-body strength, upper-body flexibility, balance and coordination, and endurance) was measured using the CS-PFP10 (16). The CS-PFP10 uses a 10-task test battery that includes everyday tasks, such as donning and doffing a jacket, getting up and down off the floor, and carrying a pot. The CS-PFP10 takes approximately 30–40 min to complete and is a valid and reliable performance measure that is responsive to changes with an intervention in healthy older adults (16). The procedures and methods of scoring have been described previously (11).
Ambulatory activity
Ambulatory physical activity was measured for 4 days using the step activity monitor (SAM) (17). Step activity monitors (Cyma Corporation, Seattle, WA, USA) use an accelerometer to determine number of strides per day, and can also measure pattern and intensity of activity. These monitors are accurate and reliable in the measurement of ambulatory ability in people after stroke in different environmental conditions including stairs, level and uneven ground, and outdoors (18). Prior to providing the participant with the SAM, an accuracy check took place. Using the SAM software, the monitor was set to the participant’s height, typical walking speed (i.e. slow, normal, fast) and leg motion (i.e. dynamic/fidgety, normal, gentle/geriatric). The participants walked for 30 s at a comfortable gait speed and an observer used a manual step counter to count strides taken during that time. The manual count was compared to the count from SAM (red light flashing when step counted) and 95% accuracy or greater was achieved on the first calibration trial with all participants.
Participants wore the monitor on their non-paretic leg and were instructed to wear it at all times except when sleeping or bathing. After retrieval, SAM information was downloaded via a USB port and visually inspected with the participant for anomalies (e.g. seeking confirmation of periods of activity or inactivity, “what were you doing at about 6 pm?”). An algorithm was written using MatLab to allow calculation of absolute daily activity (summation of number of strides, in all non-zero or non-one cells), activity bouts, and length of activity bouts. Activity bouts were defined events when the participant switched from inactivity (stride count ≤ 1/min) to activity (stride count > 1/min), and ended when they returned to inactivity. Length of activity bouts were the number of consecutive minutes with stride counts > 1 in each defined activity bout. SAM data is presented as strides and was multiplied by 2 to represent steps. Results are presented as the daily mean over a 4-day period.
Data analysis
Descriptive statistics using means, standard deviations, and percenta–ges were used to describe participant characteristics. Pearson correlation coefficients were used to assess the relationship amongst the primary variables of physical functional performance, ambulatory activity outcomes (absolute ambulatory activity, number and length of activity bouts), and on-and-off kinetics time constants. Data analysis specific to oxygen uptake kinetics has been reported previously (12).
RESULTS
Ten stroke survivors (4 males, 6 females) and their matched controls participated in the study. Detailed participant characteristics have been previously reported (12). Briefly, 6 stroke survivors had right hemiplegia, and mean age and time since stroke was 54.3 years (SD 3) and 7.5 years (SD 8.3) (median 4.2 years), respectively. Nine of 10 stroke survivors used a walking aid (6 straight cane, 3 quad cane), 6 wore an ankle foot orthoses, and gait speed ranged from 21–72 m/min (mean 43 m/min (SD 16)). Controls were not different from stroke survivors for height, weight, or physical activity (p > 0.221).
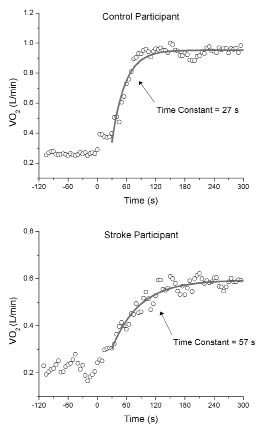
Fig. 2 provides an illustration of the difference in the time to adapt to a submaximal exercise bout between a stroke survivor and their matched control. The shallower slope of the curve for the stroke survivor demonstrates slower adaptation to submaximal exercise. Similar to findings for on-kinetics (12), time to recover from submaximal exercise (off-kinetics) was significantly longer for stroke survivors compared with controls (mean 63.8 s (SD 18.5) vs mean 48.0 s (SD 6.9); p = 0.022).
Fig. 2. Oxygen uptake (VO2) responses to exercise onset for a healthy control and a patient after stroke. Data points represent 5-second averages. Monoexponential curve fits for phase II VO2 and their respective time constant are also shown.
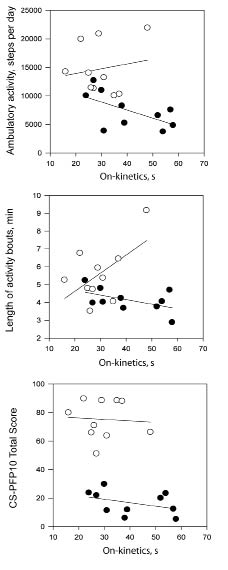
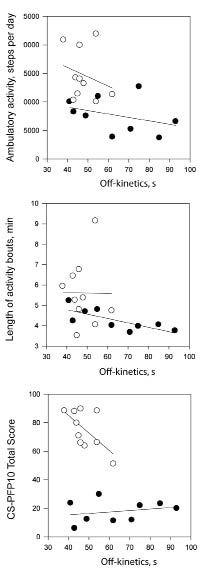
The number of daily activity bouts were not different between stroke survivors and controls (mean 64.0 (SD 19.0) vs 74.2 bouts per day (SD 10.4); p = 0.154), but mean length of activity bouts was shorter in stroke survivors (mean 4.1 (SD 0.7) vs 5.6 min (SD 1.6); p = 0.015). There were no significant correlations between the absolute number of activity bouts and the CS-PFP10 or kinetics outcomes. The associations amongst the on-and off-kinetic time constants, absolute ambulatory activity (steps per day), length of activity bouts, and physical functional performance are displayed in Figs 3 and 4. In stroke survivors, the association between absolute ambulatory activity and the on-kinetics time constant approached significance in (r = –0.598, p = 0.068). Longer times to recover from submaximal exercise were associated with shorter activity bouts in stroke survivors (Fig. 4; r=0.760, p=0.017). In control participants, the relationships between slower on-kinetics and longer activity bouts (r=0.563, p= 0.090) and slower off-kinetics and lower CS-PFP10 scores (r= 0.626, p=0.053) approached significance.
Fig. 3. Scatter-plots between on kinetics time constant, absolute ambulatory activity, length of activity bouts, and physical functional performance and for stroke survivors (•) and matched controls (○).
Fig. 4. Scatter-plots between off-kinetics time constant, absolute ambulatory activity, length of activity bouts, and physical functional performance for stroke survivors (•) and matched controls (○).
DISCUSSION
This investigation reports and describes the protocols for measurement of 2 outcomes; kinetics and pattern of activity that have been minimally reported in research with stroke survivors. The measurement of on-and-off kinetics utilizes a submaximal exercise test to measure fitness, and may be better tolerated by stroke survivors than maximal exercise tests (19). The associations between the on-kinetics time constant, absolute ambulatory activity and length of activity bouts in stroke survivors approached significance (Fig. 3). Two earlier studies reported significant associations between ambulatory activity (as measured by pedometer) and the on-kinetics time constant (9, 10) in stroke survivors. Our findings show similar, though non-significant, results and enhance our knowledge in this area as superior measurement techniques were used. We utilized an accelerometer that provides valid measurement of ambulatory activity in individuals with stroke (18), compared with a pedometer, whose accuracy is decreased at slower gait speeds (20, 21). In addition, we used 3 repetitions vs 1 repetition for VO2 kinetics testing, which provides superior accuracy in modelling the exercise onset and recovery time constants (22).
In terms of off-kinetics (recovery), longer times to recover from a submaximal exercise bout were associated with shorter activity bouts. Recently, we reported that length of activity bouts increased significantly from rehabilitation discharge to 6 weeks post-discharge in sub-acute stroke survivors (23). Taken together these findings suggest that length of activity bouts may be an appropriate target for interventions. A recent study with diabetic participants used accelerometry to measure breaks in sedentary time (i.e. activity bouts), and found that more interruptions in sedentary time were associated with better metabolic outcomes (24). It is possible that interventions that focus on increasing the average length of activity bouts (increasing breaks from sedentary time) may lead to faster recovery times and ultimately the ability to perform a greater number of functional activities throughout the day (i.e. because recovery time is faster). Future research could explore that question with stroke survivors or other neurological populations who may have limitations in functional abilities.
The scatter-plots suggest that there may be a relationship between longer activity bouts and longer time to adapt to submaximal exercise in control participants (see Fig. 3). This unexpected finding may be explained solely by the small sample size. When the 1 control participant with exceptionally long average activity bouts (9.16 min) was removed from the analysis the correlation was r = –0.011(p = 0.978). Off-kinetics and CS-PFP10 scores also showed correlations approaching significance in control participants. This relationship is in the expected direction. In spite of the differences between stroke survivors and control participants in terms of muscle metabolism (25), we have no data to suggest that relationships amongst outcomes in this study would be in disparate directions for the 2 groups.
There were no significant associations between the on-and-off kinetics time constants and physical functional performance in stroke survivors or controls (Figs 3 and 4). A study with older adults (age range 76–82 years) tested the relationship between the on-and-off kinetics time constants, functional performance, and peak VO2 (2). These authors reported that in impaired older individuals (peak VO2 ≤ 18 ml/kg/min), the off-kinetics time constant (time to recover from a submaximal exercise bout) was highly associated with functional tests such as the 6-minute walk and a bag carry, than peak VO2. In our sample of stroke survivors, whose mean peak VO2 was 16 ml/kg/min, the association between physical functional performance and the VO2 on-kinetics time constant was not significant. In theory, because VO2 kinetics testing utilizes workloads that mimic metabolic equivalent (MET) values for functional activities (26), we would expect to observe relationships between on-and-off kinetics time constants and functional abilities. We may not have observed the same relationships between VO2 kinetics and functional measures as Alexander et al. (2) because our functional test measured serial performance of functional tasks (as opposed to one functional task, i.e. bag carry) and we used the recumbent cycle for VO2 kinetics testing as opposed to the treadmill. The CS-PFP10 requires primarily walking tasks and we may have observed stronger relationships between VO2 kinetics time constants and the CS-PFP10 if we had used a more task-specific mode of exercise for VO2 kinetics testing.
The use of oxygen uptake kinetics testing in clinical settings has been questioned because of the testing time required if multiple repetitions are to be completed and because of difficulties with reproducibility (27). A reduction in the number of repetitions required, if possible, is clearly desirable for use in more clinical settings. We calculated intra-class correlation coefficients (ICC) from our data and found that the mean ICC for stroke survivors using all 3 repetitions was 0.964, and for 2 repetitions 0.952. Our results suggest that 2 repetitions are adequate in terms of reproducibility. Our ICC values are higher than reported for patients with chronic heart failure; however, we started the pedalling from rest, and this protocol, as opposed to starting from unweighted pedalling (27), may be superior in terms of reproducibility. Reproducibility may also be affected by the amplitude of the change in VO2 (22), and we observed lower amplitudes of increases in VO2 with stroke survivors. However, we maximized amplitude, and arguably reproducibility, by using a workload during testing that was specific to the abilities of the participant, one relative to ventilatory threshold. Our ICC values support this statement. In addition, we practiced and emphasized the importance of a quick transition to the optimal pedalling speed.
Other possible clinical applications of the concept of adap–tation and recovery from exercise is the use of heart rate measurement. Heart rate recovery is an important predictor of mortality, and measurement of heart rate during recovery from peak (28) or submaximal exercise (29, 30) may be an important, simple, and widely available method to learn more about a participants’ ability to recover from an exercise bout. This method does not require a metabolic cart, and heart rate monitors are widely accessible in clinical settings. Future research could examine relationships between magnitude of heart rate reduction following exercise, and recovery kinetics for oxygen uptake.
There are limitations to our study that require discussion. Because of the cross-sectional design used in this investigation, it is not possible to examine causal relationships between outcomes. Moreover, our participants were more active than other groups of stroke survivors (31–33), with ambulatory activity levels similar to a sample of older adults with pre-clinical disability and reduced physical functional performance (34). This difference may reflect the longer time since stroke in our sample, but it also simply characterizes our small sample of individuals with stroke, 6 of 10 of whom exercised 2–3 times weekly at an exercise centre for people with disabilities. Our stroke survivors were not atypical from an impairment perspective with gait speeds comparable with what is reported in the literature. It is likely that less active groups of stroke survivors would report greater limitations in VO2 kinetics and functional abilities.
In summary, this study provides information about the associations amongst kinetics outcomes, ambulatory activity, and physical functional performance in stroke survivors. The ambulatory outcomes related to pattern of activity, and the kinetics outcomes have been minimally described in the stroke literature (9, 10, 23). The submaximal nature of kinetics testing, and the challenges of maximal testing in people with stroke (19), make kinetics testing an exercise testing protocol that should be explored to a greater extent as a fitness measure in people with stroke. Our data suggest there may be a relationship between the time it takes to adapt and recover from a submaximal exercise bout and ambulatory outcomes such as steps per day, and length of activity bouts. The associations with physical functional performance were not significant. Future studies with larger samples of stroke survivors will help to elucidate these relationships further. Intervention studies may explore the effect of changes in ambulatory activity or kinetics outcomes on physical functional performance.
ACKNOWLEDGEMENTS
We acknowledge the funding support of the University of Alberta Endowment Fund for the Future (EFF): Support for Advancement of Scholarship.
REFERENCES