OBJECTIVE: To evaluate the effectiveness of ultrasonographic guided botulinum toxin type A injections into the plantar fascia to reduce pain and improve gait in patients with unilateral plantar fasciitis.
DESIGN: A randomized double-blind control study.
SUBJECTS: Fifty patients with chronic unilateral plantar fasciitis were recruited, and divided into experimental and control groups.
METHODS: Subjects in the experimental group were injected with 50 units botulinum toxin type A, reconstituted with normal saline, into the plantar fascia under ultrasonographic guidance. Follow-up evaluations were made 3 weeks and 3 months after injection. The control group subjects were injected with normal saline under ultrasonographic guidance. Outcome measures included comparing scores from the
visual analogue pain scale, changes in thickness of the plantar fascia and fat pad, and gait assessment including the maximal centre of pressure velocity during first step loading response.
RESULTS: Visual analogue pain scale and plantar fascia thickness in the symptomatic foot decreased significantly, as noted at follow-up 3 weeks and 3 months after botulinum toxin type A injections (p < 0.001). However, the fat pad thickness remained unchanged. The centre of pressure velocity during loading response increased 3 months after injection (p < 0.05). Outcome measures of the control group remained unchanged.
CONCLUSION: Botulinum toxin type A is effective in the treatment of foot pain associated with plantar fasciitis and increases the centre of pressure velocity during loading response without inducing fat pad atrophy.
Key words: plantar fasciitis; botulinum toxin; centre of pressure; ultrasonography.
J Rehabil Med 2010; 42: 136–140
Correspondence address: Yung-Cheng Huang, Department of Physical Medicine and Rehabilitation, Cheng Hsin General Hospital, No.45, Cheng Hsin St. Pai-Tou, 112 Taipei, Taiwan. E-mail: jeremy0681@gmail.com
Submitted November 24, 2008; accepted October 9, 2009
INTRODUCTION
Plantar fasciitis is a common condition of the foot that causes heel pain, especially in people who are obese or athletic, or who have restricted ankle flexion (1). Long-term repetitive overstretching of the plantar fascia has been observed to cause micro-tears in the insertion site near the medial calcaneal tuberosity, which may lead to inflammatory reactions and heel pain, localized to an area 1–2 cm distal to the medial calcaneal tuberosity. When the plantar fascia is stretched from a contracted equinus position after prolonged inactivity, such as sleeping or sitting for a long time, the pain is usually most severe during the first few steps, which causes gait difficulties (2).
Current treatments for plantar fasciitis include shoe inserts, night splints, ultrasound, laser treatment, stretching exercises, local corticosteroid injections, extracorporeal shockwave therapy and surgery (3–5). Effectiveness of splint and orthosis in the treatment of this lesion is inconclusive. Patients often feel discomfort when wearing shoe inserts and heel cups. Corticosteroid injection is a common treatment for plantar fasciitis and can reduce pain in the short term, but can also cause a range of unwanted effects, such as subcutaneous fat atrophy, plantar fascial rupture, peripheral nerve injury and muscle damage (6).
Botulinum toxin type A (BoNT-A) has been widely used for treating spasticity in patients with hemiplegia and cerebral palsy. In recent years, BoNT-A has been used for treating chronic muscular and neuropathic pain (7), such as migraine, myofascial pain syndrome and piriformis syndrome (8–10). Botulinum toxin has been found to have anti-nociceptive and anti-allodynia effects (11) and acts by modulating pain neurotransmitters including substance P, glutamate and anti-inflammatory reactions (12, 13).
Injection under ultrasonographic guidance may increase precision and therapeutic effects (14). We therefore hypothesized that BoNT-A injection under ultrasonographic guidance could reduce pain and improve the gait pattern without adverse effects. The effects were evaluated using a visual analogue pain scale (VAS), plantar fascia thickness by ultrasonography, and by changes in gait. If the plantar fascia thickness decreased, as in the case of fat pad atrophy, then the fat pad thickness was also measured by ultrasonography.
METHODS
Subjects
A randomized double-blinded control method was designed for this study and the study protocol was approved by Cheng Hsin General Hospital IRB, Taipei, Taiwan. Informed consent was obtained before patients entered the study. Subjects matched the inclusion criteria if they were at least 18 years old, had had unilateral heel pain for at least 3 months, and were diagnosed with plantar fasciitis by physical examination and ultrasonography. The acceptable criteria for diagnosing plantar fasciitis by ultrasonography included plantar fascia rupture or a plantar fascia thicker than 4 mm, as well as a decrease in echogenicity (15). Subjects were excluded on the basis of any of the following: if they had a foot deformity (congenital or acquired), pes cavus (the angle formed between the distal medial malleolus, the navicular tuberosity, and the 1st metatarsal head =180°), any systemic disease that could have induced foot pain or a sensory disorder (e.g. diabetes, rheumatoid arthritis, seronegative arthritis), or previous surgical treatment of the affected foot. Subjects were also excluded if there was a known allergy to BoNT-A, neuromuscular junction disorder (e.g. myasthenia gravis, Lambert-Eaton disease), a current treatment with anticoagulation medication, or if they were pregnant or breastfeeding.
Fifty subjects with unilateral heel pain were recruited from the outpatient rehabilitation medicine department of Cheng Hsin General Hospital IRB, Taipei, Taiwan. Subjects were randomly assigned into 2 groups: 25 subjects in the experimental group received a BoNT-A injection, while 25 subjects in the control group received normal saline. The patients, the person doing the injections and the observer for outcome measures were all blinded to solutes of the injections. There were no significant differences in physical or demographic characteristics between the experimental and the control group among age (mean 54.4 (standard deviation (SD) 9.6) vs 51.5 (5.5) years), weight (mean 63.0 (SD 11.2) vs 64.9 (9.3) kg), height (mean 160.6 (SD 8.7) vs 161.0 (8.3) cm) and male-female ratio (6:19 vs 6:19). Subjects in the experimental group received 50 units of BoNT-A (Botox, Allergan, Irvine, CA, USA), reconstituted in 1 ml normal saline, by injection into the plantar fascia under ultrasonographic guidance using a 25-gauge, 1.5 inch needle (Fig. 1). Subjects in the control group were injected with 1 ml normal saline into the plantar fascia under ultrasonographic guidance.
Fig. 1. Posterior botulinum toxin injection into the plantar fascia under ultrasonographic guidance.
Subjects lay prone with their feet hanging over the edge of the bed. An ultrasonographic probe (8L-RS, GE Medical Systems, Milwaukee, WI, USA) was placed against the painful heel and the plantar fascia was visualized. The needle was inserted into the fascia via a posterior approach below the calcaneus. Ultrasonographic images of the needle showed a linear object with high echogenicity, and the needle was further inserted until the plantar fascia was reached, at which point the injection was administered.
Outcome evaluation: Pain intensity and plantar fascia thickness
Before injection, pain intensities were recorded using the 100-mm VAS, where 0 represents no pain and 10 the worst pain ever experienced by the subject. For ultrasonography (Logiq Book, GE Medical Systems), subjects were examined in a prone position with their feet hanging over the edge of the table. A 10 MHz linear probe was placed against the heel parallel to the plantar fascia. Only slight pressure was applied on the heel while the plantar fascia was examined. The fascia thickness was measured (in mm) near the insertion point of the calcaneal tendon (Fig. 2). The heel fat pad thickness (in mm) was measured above the plantar fascia without applying any pressure, in order to avoid compressing the fat pad.
Fig. 2. Plantar fascia and thickness measurements by ultrasonography. (Image modified for best contrast using Photoshop CS3 (Adobe, San Jose, CA, USA)).
Centre of pressure velocity measurement
The velocity of the centre of pressure (COP) in gait and the foot pressure were measured using a pressure mat (FSA, Manitoba, Canada). The first step method for evaluating the COP velocity was used in this study (16).
Prior to the test, subjects were asked to sit for at least 20 min, and then to walk in a straight line for 3 m. The first step was imposed on the pressure mat without targeting the mat. The bilateral foot COP was recorded in real time using FAS 4 software (FSA, Manitoba, Canada). A set of COP coordinates were represented as COP(X,Y), and consecutive COP coordinates as (X1,Y1), (X2,Y2), (X3,Y3)….(Xn,Yn) (17).
A custom software was written with LabVIEW 8.5 (National Instruments, Austin, TX, USA) to calculate COP displacement, velocity and acceleration. The displacement between consecutive points of the COP was calculated using the following formula:
√(X2–X1)2 + (Y2–Y1)2
COP velocity (cm/s) values were derived from the COP displacement data by differentiation.
The COP velocity represents movements of the foot centre during the stance phase of the gait. The maximal velocity during the loading response was recorded, where the loading response phase was defined as the period from the initial contact of the foot with the floor until shortly before the foot became flat (18). The COP velocity during the loading response is used to predict the velocity of the foot when first making contact with the ground. Compared with the gait cadence, the COP velocity represents the foot velocity during the stance phase.
The subjects were examined 3 weeks and 3 months after the BoNT-A injection, respectively. At each examination, VAS, plantar fascia thickness, fat pad thickness, COP velocity and foot pressure were measured. BoNT-A has been proved to be effective in pain reduction and anti-inflammation (19). In the current study, the authors studied changes in VAS and plantar fascia thickness, respectively, to assess the effects of the injection. Ensemble gait function changes were measured by the COP velocity.
Statistics
Normal distribution was confirmed in both groups. Repeated analysis of variance (ANOVA) measures combined with the post hoc test were used to determine differences in VAS, plantar fascia thickness and fat pad thickness in the experimental and control group before injection and 3 weeks and 3 months post-injection, respectively. Bilateral feet differences before and changes in the symptomatic foot before and after injection were compared using the paired t-test comparing COP velocities before and 3 months after injection. The Pearson bi-variant test was used to analyse the correlation between VAS and plantar fascia thickness. All data were analysed using SPSS 13.0 statistic software (SPSS, Chicago, IL, USA).
RESULTS
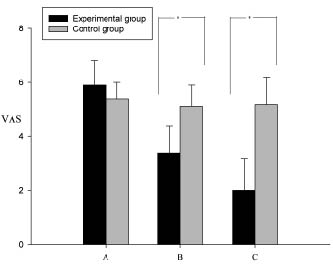
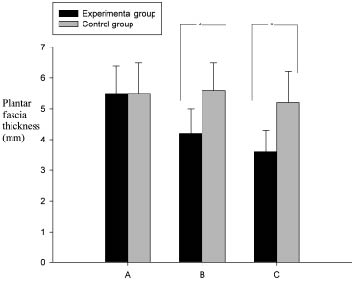
Compared with the control group subjects, the VAS of the BoNT-A treated subjects decreased significantly (p < 0.001) in the experimental group 3 weeks and 3 months after injection, respectively (Table I). The VAS and plantar fascia thickness were reduced 3 weeks post-injection, and were further reduced after 3 months (Table I). The fat pad thickness remained unchanged between pre-injection and 3 weeks and 3 months post-injection (p = 0.652), respectively. In the control group, the pain scale, plantar fascia thickness and fat pad thickness showed no difference between pre-injection, and 3 weeks and 3 months post-injection (p > 0.05), respectively. Compared with the control group, the VAS and plantar fascia thickness in the BoNT-A-treated subjects decreased significantly (p < 0.001) (Figs 3 and 4). Patients in the control group revealed no change in pain scores. In the Pearson bi-variant test, the results showed a high correlation (p < 0.001, r = 0.754) between change in VAS and change in fascia thickness.
| Table I. Pain scale, plantar fascia and fat pad thickness in the Experimental and the Control group Data are presented as mean (standard deviation) |
| | Prior to injection | After 3 weeks | After 3 months | p-value of ANOVA |
| VAS Experimental group* Control group | 5.9 (0.9) 5.4 (0.6) | 3.4 (1.0) 5.1 (0.8) | 2.0 (1.2) 5.2 (1.0) | < 0.001 0.575 |
| Plantar fascia thickness, mm Experimental group† Control group | 5.5 (0.9) 5.5 (1.0) | 4.2 (0.8) 5.6 (0.9) | 3.6 (0.7) 5.6 (1.0) | < 0.001 0.960 |
| Fat pad thickness, mm Experimental group Control group | 9.7 (1.9) 10.1 (1.2) | 10.1 (1.7) 10.1 (1.1) | 10.1 (1.6) 10.0 (1.1) | 0.652 0.960 |
| *Post hoc p-values all < 0.001 prior to injection, after 3 weeks and after 3 months. †Post hoc p-values < 0.001 prior to injection, after 3 weeks and after 3 months. Post hoc p-value = 0.043 after 3 weeks and after 3 months. ANOVA: analysis of variance; VAS: visual analogue scale. |
Fig. 3. Visual analogue pain scale (VAS) changes including mean (standard deviation) in the experimental and the control group. A: VAS prior to injection; B: VAS 3 weeks after injection; C: VAS 3 months after injection. (*p < 0.001).
Fig. 4. Thickness changes including mean (standard deviation) of the plantar fascia in the experimental and control groups. A: thickness prior to injection; B: thickness 3 weeks after injection; C: thickness 3 months after injection. (*p < 0.001).
Centre of pressure velocity and analysis
Before injection, the maximum COP velocity during the loading response in the symptomatic foot was slower than in the asymptomatic foot (Table II). The painful heel had a lower velocity during the loading response (p = 0.018). The COP velocity in the symptomatic foot increased significantly after BoNT-A injection (p = 0.012), matching the COP velocity of the asymptomatic foot.
| Table II. Centre of pressure velocity during loading response in the Experimental group (n = 25). Data are presented as mean (standard deviation) |
| | Prior to injection | After 3 weeks | p-value |
| Velocity of the symptomatic
foot (cm/s) | 31.5 (6.9) | 39.7 (6.2) | 0.018 |
| Velocity of the asymptomatic
foot (cm/s) | 43.4 (14.9) | 46.4 (11.7) | 0.208 |
| p-value | 0.012 | 0.028 | |
DISCUSSION
In this study, the pain scale (VAS) and plantar fascia thickness decreased incrementally and significantly (p < 0.001) 3 months post-injection. The pain scale and plantar fascia thickness also have a strong correlation in our study based on the Pearson bi-variant test. Although heel pain was reduced by BoNT-A, not every subject was free of painful sensations. Hence in our study, BoNT-A decreased the pain but did not completely cure the condition.
The plantar fascia thickness is regarded as an indicator of the extent of inflammation, in which increased thickness is consistent with more severe inflammation (20). In a previous study of plantar fasciitis injection, the BoNT-A was directly injected from the sole to the fascia by palpated guidance (21). In this procedure, the physician palpated the most tender point prior to injection. The needle penetrated the fat pad directly, and hence the medication may not have been injected directly into the plantar fascia. An ultrasonographic guide injection method can provide dynamic images that allow more accurate injections. Compared with palpation guidance, sonographic guidance has the advantage of lower recurrence rates (14) and a reduced risk of fat pad injury.
We chose a posterior approach for injecting the BoNT-A due to safety considerations. Other approaches, such as medial approach injection method, would have resulted in lateral plantar nerve injury with numbness of the 3rd, 4th and 5th toes (22).
Increased heel pressure is considered a cause of plantar fasciitis. In this study, the fat pad remained unchanged post-injection in both the experimental and the control group. In a previous case report, steroid injections against heel pain may have caused fat pad atrophy (23), yet recurrent heel pain is caused by fat pad atrophy. Hence steroid treatment can amplify fat pad atrophy in a vicious circle. Compared with steroid injection, BoNT-A treatment does not appear to promote fat pad atrophy. Patients with plantar fasciitis usually complain of heel pain when taking their first step after waking up, or after a prolonged rest, which then lessens after further walking. We chose the measure of first step COP velocity during the loading response to analyse gait changes between bilateral feet post-injection. The COP velocity represents the foot velocity during the stance phase of the gait. In normal subjects, the COP velocity between bilateral feet is the same. In hemiplegic and knee joint osteoarthritis patients, the COP velocity can predict the difference in gait velocity between the symptomatic and asymptomatic limbs (24). The heel pain may not cause a change in the average step length and cadence. It indicates that both step length and cadence may not be sensitive parameters in measuring changes after the injection. Therefore foot COP velocity was used in this study.
Prior to injection, the COP velocity of the symptomatic foot was lower compared with the asymptomatic foot, but increased significantly after injection. The delayed COP progression may be caused by patients having a hesitant tendency because of the pain at heel strike. Another possible cause of the delayed COP progression could be the impact force from the ground. Walking more quickly leads to a greater impact force, which may induce more pain.
Recalcitrant plantar fasciitis is a difficult condition to cure, and has a tendency to recur after therapy (25). The plantar fascia is a deep tissue without a rich vascular supply. Physical therapy modalities, such as ultrasound, and oral medication, such as non-steroidal anti-inflammatory drugs (NSAID), are usually not effective in treating plantar fasciitis because the vascular supply is poor, and because symptoms readily recur after treatment.
An injection for treatment of plantar fasciitis is considered when the medication has to be delivered directly into the inflammatory plantar fascia. Steroid injection is effective in treating plantar fasciitis in the short term, but the condition has a high recurrence rate, and fat pad atrophy is sometimes noted after injection (23).
Plantar fasciotomy is an invasive method for treating recalcitrant plantar fasciitis. However, post-operative strains in the ligaments and bone were found to be excessive (26). The operation is irreversible, and future outcomes require more scrutiny to evaluate the usefulness of this procedure.
Injecting BoNT-A against plantar fasciitis was previously reported as having a long-term benefit in pain relief (27); however, we have further developed this procedure by using ultrasonographic guidance. The earlier study differed from ours by using palpation guidance injection, which has the disadvantage of less accurate drug-delivery, causing unwanted side-effects, such as fat pad atrophy and lateral plantar nerve injury. In the previous study, injections were made into the arch of the foot, and the needle effects may have been confused with myofascial pain syndrome. In contrast, we used ultrasonographic guidance to inject the BoNT-A directly into the inflammatory plantar fascia, avoiding needle penetration through the fat pad.
Previous studies have demonstrated that inflammation in plantar fascia tissue is associated with increased thickness of the fascia due to oedema and perifascial fluid collections (15, 20). Measuring the fascia thickness is an objective method for evaluating the inflammatory degree. However, the normal fascia thickness in this region is 2–4 mm. Hence, using the plantar fascia thickness as a guide for the extent of inflammation is only reliable when the thickness exceeds 4 mm.
The clinical use of BoNT-A has expanded beyond the original use of its effects on cholinergic neurons. The increasing interest in its potential role in treating chronic pain conditions is partly based on the effects of BoNT-A on modulating the release of substance P, calcitonin gene-related peptide (28), and glutamate (29). Otherwise, BoNT-A was found to show effects such as inhibiting inflammatory pain and releasing neurotransmitters from primary sensory neurons in a rat formalin model. It inhibits peripheral sensitization, which leads to an indirect reduction in central sensitization (11).
In conclusion, treatment of unilateral plantar fasciitis with BoNT-A led to significant pain relief and a reduction in the plantar fascia thickness 3 weeks and 3 months post-injection, respectively. The ultrasonographic guidance injection method provided an accurate and safe method for treating chronic plantar fasciitis, and thus may be preferable to other methods, such as palpation guidance.
The subjects treated with BoNT-A demonstrated an increase in COP velocity during loading response. It is thought that delayed COP progression during the loading response in the symptomatic foot may result from hesitancy in stepping forward in an attempt to lessen heel pain.
The thickness of the plantar fascia is closely correlated with the VAS pain scale. Hence, plantar fascia thickness can be a predictive and objective tool for evaluating the severity of plantar fasciitis.
REFERENCES