OBJECTIVE: To compare costs of function- and pain-centred inpatient treatment in patients with chronic low back pain over 3 years of follow-up.
DESIGN: Cost analysis of a randomized controlled trial.
Patients: A total of 174 patients with chronic low back pain were randomized to function- or pain-centred inpatient treatment.
METHODS: Data on direct and indirect costs were gathered by questionnaires sent to patients, health insurance providers, employers, and the Swiss Disability Insurance Company.
RESULTS: There was a non-significant difference in total medical costs after 3 years’ follow-up. Total costs were 77,305 Euros in the function-centred inpatient treatment group and 83,085 Euros in the pain-centred inpatient treatment group. Likewise, indirect costs after 3 years from lost work days were non-significantly lower in the function-centred inpatient treatment group (6354 Euros; 95% confidence interval –20,892, 8392) and direct medical costs were non-significantly higher in the function-centred inpatient treatment group (574 Euros; 95% confidence interval –862, 2011).
CONCLUSION: The total costs of function-centred and pain-centred inpatient treatment were similar over the whole 3-year follow-up.
Key words: low back pain, exercise therapy, vocational rehabilitation, rehabilitation outcome, costs, cost analysis.
J Rehabil Med 2009; 41: 919–923
Correspondence address: Stefan Bachmann, Klinik für Rheumatologie und Rehabilitation des Bewegungsapparates, Rehabilitationszentrum Klinik Valens, CH-7317 Valens, Switzerland. E-mail: s.bachmann@klinik-valens.ch
Submitted December 12, 2008; accepted June 18, 2009
INTRODUCTION
In Switzerland musculoskeletal disorders represent the third largest illness group, resulting in 9.4 million consultations per year (1). Of these, approximately 30% are for low back pain (LBP). In 2002 LBP had the highest prevalence of all medical conditions in the working age population in Switzerland, with 8% of women and 13% of men affected in a 4-week period (2). Total costs of LBP in Switzerland are estimated at 7.4 billion Euros, of which direct medical costs are 3.4 billion Euros (6.7% of total Swiss healthcare expenditure), and indirect costs to 4.0 billion Euros (3). Furthermore, chronic LBP, defined as LBP present for more than 12 weeks, is one of the most frequent reasons for persistent disability and lost work days. The expenses of the Swiss Disability Insurance Company increased by 215% from 1990 to 2005, and approximately 20% of disability pensions in 2008 were due to musculoskeletal diseases, among which chronic LBP plays a predominant role (4).
Similar figures are reported from other industrialized countries (5–8). In the USA chronic LBP causes 33–41% of all work-related pension costs and is responsible for 16–19% of disability pensions (9). The highly skewed distribution of costs due to LBP is well documented, with patients with chronic LBP accounting for up to 80% of total costs associated with LBP (10, 11). Meta-analyses have shown that exercises and multidisciplinary function-oriented rehabilitation programmes with the aims of changing illness behaviour and improving physical function can reduce pain and disability and increase the number of work days among patients with chronic LBP (12, 13). Regarding long-term costs, these programmes have not yet been well evaluated, but cost data are of great importance for health authorities.
Despite consensus in the literature promoting function-oriented treatment, pain- and symptom-oriented treatments for patients with chronic LBP are still widely used in Switzerland. Earlier, we performed a randomized controlled trial (RCT) in patients with chronic LBP and work-related disability, comparing a function-centred inpatient treatment (FCT) programme with a pain-centred inpatient treatment (PCT) programme in Switzerland (14). After 12 months follow-up post-treatment, the FCT programme significantly increased the number of days at work compared with the PCT programme (15).
The aim of the present study was to compare direct medical costs and indirect costs from productivity loss and disability pensions of this patient population after 1 and 3 years. Our a priori hypothesis was that the FCT programme would also be cost beneficial over the 3 years of follow-up.
METHODS
Patient selection, randomization and data collection
Cost analyses of treatment outcomes were performed in a RCT with 3 years’ follow-up. The study was registered at ClinicalTrials.gov (ID: NCT 00652236) and approved by the ethics committee of the Canton St Gallen, Switzerland (EKSG 03/035). Between January 2000 and May 2003 we recruited patients attending a rehabilitation centre for work-related health problems in Valens, Switzerland. Eligible patients were between 20 and 55 years with chronic non-specific LBP according the Quebec Task Force classification (16) who had taken at least 6 weeks of sick leave due to LBP in the previous 6 months. Patients with co-morbidities interfering with treatment or ability to work, and patients with two or more positive predictive tests for non-return-to-work according to Kool et al. (17) were excluded.
Patients who gave informed consent to participate were divided into 4 strata, defined by work status (employed, unemployed) and work-load (lifting loads < 10 kg or > 10 kg) based on the definitions of the US Department of Labour (18). An independent blinded research assistant performed concealed randomization within strata. Two separate teams of therapists treated patients 6 days per week for a 3-week period in the FCT and PCT programmes. Patients in one programme were not informed about the treatment of the other programme. To ensure that patients were unaware of the other treatment they stayed in different wards in order to reduce contact with patients from the other treatment group.
An independent blinded research assistant assessed costs and number of work days after 1 and 3 years of follow-up based on questionnaires sent to patients, employers, health insurance providers, and the Swiss Disability Insurance Company. Respondents were blinded to treatment assignment and non-respondents received up to 2 reminders within 2 months of the original request.
Treatment
Function-centred treatment. FCT was based on a functional restoration programme for 4 h per day for 3 weeks. The programme comprised work simulation, strength and endurance training through isokinetic exercise, cardiovascular training, sports therapy, and independent exercise. FCT used only group treatment offered by 2 therapists for groups of 8 patients. Therapist time per patient was approximately 1 h per day.
Pain-centred treatment. The PCT programme was 2.5 h per day including 1 h of individual treatment and 1 and 0.5 h of treatment in groups of 8 patients. The PCT programme included mobilization, stretching, strength training, aquatic exercises and a mini-back-school. Passive pain modulating treatments (hot packs, electrotherapy, and massage) were also used. Estimated therapist time per patient was approximately 1.25 h per day. The treatment duration was 3 weeks.
In both groups, a rheumatologist prescribed medications and applied local injections in the musculature and other soft tissue of the lumbar region when deemed medically necessary. If required, a psychologist also offered counselling. Treatment following rehabilitation was at the discretion of the patient’s primary physician, but patients in the PCT programme were told to continue with their independent exercises twice weekly if they were pain-free, while patients in the FCT programme were encouraged to continue their strength and endurance training twice weekly even if they had pain.
Outcome measurement
Costs were considered from a societal perspective (direct medical costs and productivity losses), a health insurance perspective (direct medical costs) and a disability pension fund perspective (disability pension payments).
Initial funding included 1 year follow-up. Additional funding was obtained for 3 year follow-up and assessed costs during the second and third year. All cost data were recorded in Swiss Francs and converted to Euros (exchange rate 1 January 2007: 1.604 CHF/1 Euro).
Direct costs. Direct costs of inpatient treatments were calculated based on daily rates. Cost per unit accounting was not used in the study centre, thus detailed per patient costs of personnel, material and overhead were not available. Data on direct medical costs during the 3 year follow-up period were collected by questionnaire sent to patients’ health insurance providers. We requested data on the total amount of costs related to the treatment of LBP. Unfortunately, health insurance providers were not able to report specifically on LBP-related healthcare consumption, although Swiss physicians are obliged to indicate the reasons and diagnoses for all healthcare reimbursement claims. Therefore, reported direct cost data are not LBP-specific, but represent the total costs of healthcare consumption. Direct cost data represent the lower bound of total direct medical costs because they do not include cost sharing and out-of-pocket payments by patients and public subsidies to hospitals.
Indirect costs. Indirect costs were assessed by questionnaire sent to patients and employers. As work absenteeism is the main cost factor regarding indirect costs, and no national database is available to assess work days, these data were assessed with questionnaires. Patient questionnaires were used to assess working status (currently working or not) and disability insurance status (whether the patient received a disability pension). The employer questionnaire assessed work absenteeism and disability-related reductions in daily working hours. In case of disagreement patients and employers were contacted by telephone to resolve the differences. Time-reduced work was taken into account. For example, a work day with 30% time reduction was counted as 0.7 work days. The costs for days on sick leave were then calculated according to a human capital approach by multiplying time on sick leave with the patients’ pre-tax income as a proxy for the value of lost productivity.
Detailed information on disability pensions was obtained from the Swiss Disability Insurance Company and included the date of adjudication of the disability pension, monthly payment amount, and the degree of the disability pension (i.e. 25%, 50%, 75%, or 100% according to their disability level). Partial disability pensions were analysed in the same manner as time-reduced work. Total transfers for disability payments were calculated by multiplying the number of months with a disability pension in the 3-year period with the monthly pension. The analysis of disability pensions was complicated by the long time period between the application and the decision by the Swiss Disability Pension Company. At the end of 2007 the applications of 12 patients were still unsettled. Furthermore, 10 patients withdrew their informed consent during the follow-up period and 18 patients could not be contacted because they moved back in their home countries and no forwarding addresses were provided.
For further analyses costs arising form work absenteeism and productivity losses and disability pension costs were summarized under the term “indirect costs”, although in modern health economics disability pensions are not part of indirect costs as they are income transfers and not productivity losses.
Statistics
Power calculations based on the primary outcome, work days during the first year after treatment, using a type I error of 0.05 and 80% power, indicated that 90 patients per group were needed to detect a difference of 40 work days between the 2 treatment groups during the first follow-up year. Because the RCT recruitment rate was lower than expected, only 174 patients instead of the planned 180 patient were included. Intention-to-treat analyses were conducted using Stata statistical software (Stata Corporation, College Station, TX, USA), version 9.
Tests for differences between the 2 treatment groups were performed with the 2-sample t-tests (Student’s t-test). Although the distribution of the cost-data variables is often skewed, only the arithmetic mean is of relevance for cost analysis (19). Boot-strapping was used to determine confidence intervals (CI).
Cost analyses were performed after 1 and 3 years of follow-up. While the relevant data for cost analysis were complete for the first year, a number of patients withdrew their informed consent, or data were missing for other reasons, in the subsequent years. Missing data for the number of work days between 1 and 3 years’ follow-up were imputed with a least-squares regression and a logistic hurdle model. These methods do not affect the magnitude of the differences between the treatment groups, but improve the precision of the estimates. Logistic hurdle regression is particularly useful for count data with an excess of zero counts. In a first step, logistic regression was used for analysing patients without work days or disability pension. In the second step, a least-squares regression was used to impute the number of work days or the disability allowance.
RESULTS
Participants
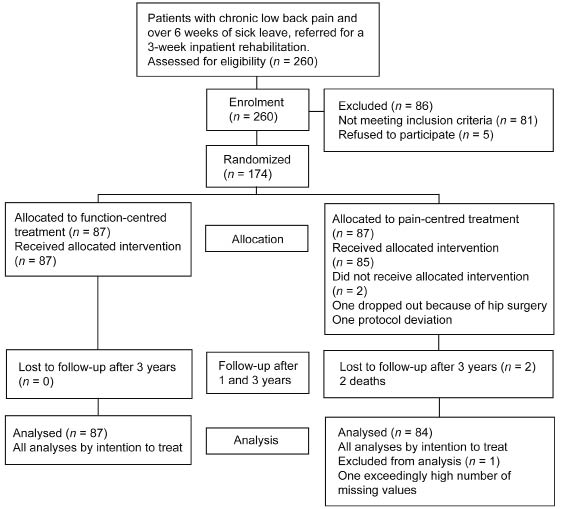
Fig. 1 shows the study flow of the patients. Cost analysis of the 3-year follow-up period was reduced to 171 patients, as 2 patients died after the first year and one patient was excluded due to an exceedingly high number of missing values. Baseline characteristics of the patients in the 2 groups are listed in Table I. Missing data were imputed for numbers of work days in 38 patients (FCT n = 14, PCT n = 24) and disability pension in 29 patients (FCT n = 11, PCT n = 18). After 3 years, 131 patients received a disability pension and were included in the examination of the effects of the treatments on disability pensions over the whole follow-up period.
Fig. 1. Patients in the study (according to the CONSORT statement).
| Table I. Baseline characteristics of the patients in the function- or pain-centred treatment (FCT or PCT) groups for chronic low back pain |
| Characteristics | FCT | PCT |
| Patients, n | 87 | 84 |
| Age, years, mean (SD) | 41 (8) | 43 (8) |
| Gender, men, % | 79 | 78 |
| Cultural background, n (%) | | |
| Switzerland | 36 (43) | 35 (42) |
| Southeast Europe* | 18 (21) | 13 (15) |
| Southwest Europe† | 30 (36) | 36 (43) |
| No professional education, n (%) | 32 (38) | 36 (43) |
| Heavy work (work load > 10 kg), n (%) | 64 (76) | 70 (83) |
| Days of sick leave in 2 years before treatment, mean (SD) | 186 (161) | 218 (154) |
| *Bosnia, Macedonia, Serbia, Turkey. †Italy, Portugal, Spain. SD: standard deviation. |
Costs
The results of cost calculations in the 2 follow-up periods are shown in Table II.
| Table II. Direct, indirect and total costs in Euros* of the initial rehabilitation treatment (n = 174) and after 1 (n = 174) and 3 years (n = 171) in the two treatment groups |
| | FCT | PCT | Difference FCT-PCT | p-value‡ |
| Mean | SE | Mean | SE | Mean | 95% CI† |
| Rehabilitation treatment | | | | | | | |
| Direct costs | 5,889 | 106 | 5,935 | 107 | –46 | –342–252 | 0.77 |
| 1 year |
| Direct costs | 2,658 | 282 | 1,926 | 226 | 732 | 33–1,431 | 0.02 |
| Indirect costs | 22,737 | 1,627 | 26,601 | 1,341 | –3,864 | –8,132–405 | 0.10 |
| Total costs | 25,395 | 1,714 | 28,527 | 1,379 | –3,132 | –7,307–1,044 | 0.08 |
| 3 years |
| Direct costs | 4,843 | 561 | 4,269 | 496 | 574 | –862–2,011 | 0.22 |
| Indirect costs | 72,462 | 4,619 | 78,816 | 4,072 | –6,354 | –20,892–8,392 | 0.15 |
| Total costs | 77,305 | 4,747 | 83,085 | 4,231 | –5,780 | –18,363–6,477 | 0.18 |
| *Original values in Swiss Francs. Exchange rate 1 January 2007: 1.604 CHF/1 Euro. †Estimated by boot-strapping. ‡Independent 2-sample t-test. CI: confidence interval; FCT: function-centred treatment; PCT: pain-centred treatment; SE: standard error. |
There was a non-statistically significant difference in total costs of the 2 treatment groups. Total costs were higher in the PCT group, with a difference of 3132 Euros (95% CI –7307 to 1044; p = 0.08) after 1 year, and to 5780 Euros (95% CI –18,363 to 6477; p = 0.18) after 3 years. Costs of inpatient treatment were the same in both groups (FCT 5889 Euros, PCT 5935 Euros). Direct medical costs after 1 year were significantly higher in the FCT group (732, 95% CI 33–1431; p = 0.02). No other differences in direct and indirect costs were found during the 3 year follow-up period.
DISCUSSION
To our knowledge this is the first study in Switzerland comparing the costs of function-centred and pain-centred inpatient rehabilitation programmes with 3 years follow-up. With regard to all costs we could not show a statistically significant difference between the 2 rehabilitation programmes over the 3 years of follow-up. Therefore, our a priori hypothesis, that the FCT programme would be cost beneficial over the 3-years of follow-up, has to be rejected.
Nevertheless, we found a small (but non-statistically significant) cost benefit from the FCT programme. The difference in total costs over 3 years amounted to 5780 Euros in favour of the FCT, corresponding to approximately 2 months’ salary for an average Swiss blue-collar employee. Direct medical costs were higher in the FCT programme in the first year, but this was offset by lower indirect costs over the 3-year follow-up period.
Some differences between our results and other research evaluating costs from LBP need to be taken into consideration. In an earlier observational study of a FCT programme we collected and calculated all programme costs over a 1-year period (20); direct medical costs were 7802 Euros per patient and indirect costs 26,000 Euros per patient. Compared with the present study, direct costs of our observational study were higher at 1-year follow-up, while the indirect costs are comparable. This difference may be due to the different methods used to determine direct medical costs, while the indirect costs were calculated similarly in both studies. Specifically, in our earlier study, direct costs were assessed using data from primary care physicians (e.g. number of visits, number of medications, and number of radiographs during the 1-year follow-up) and then valued, whereas in the present study cost data from the health insurance providers was used. Therefore, it could be that, in the present trial, not all consultations or all medications were reported to the health insurance provider, or that some were paid by the patients, leading to lower direct costs. Compared with the cross-sectional survey by Ekman et al. (21) of patients with chronic LBP in a primary care setting in Sweden, the direct medical costs in our RCT were slightly lower (2300 vs 3100 Euros) and indirect costs slightly higher (24,600 vs 17,600 Euros). These discrepancies might be due to the differences in recruitment, inclusion criteria, rates and salaries. Ekman et al. (21) included patients with chronic LBP in a primary care setting, whereas we studied patients who participated in an inpatient rehabilitation programme. In the primary care setting, the duration of the complaints is shorter and the probability of patients returning to work is therefore higher, leading to lower indirect costs. Patients participating in an inpatient rehabilitation programme are examined and documented thoroughly so that no further expensive investigations are necessary after discharge, and subsequent direct costs are expected to be lower. Steenstra et al. (22) published an economic evaluation of a multi-stage return-to-work programme for workers on sick leave due to LBP. Similar to our FCT programme, the return-to-work programme resulted in a statistically significant increase in work days after 52 weeks at slightly higher treatment costs compared with usual care. From a societal perspective, the higher direct medical costs of work-related interventions over the first year were offset by lower indirect costs from sick leave and disability pensions during the first year of follow-up.
Several limitations and strengths should be mentioned. Insufficient power is a limitation of this study that probably contributes to the imprecision of between-group cost differences. The a priori power calculation was based on the number of work days during the first follow-up year; therefore, this study was underpowered for cost analyses over 3 years of follow-up. Insufficient power is a common problem in cost-effectiveness studies, which are often performed alongside RCTs (23). Another limitation of our study is the low treatment contrast, as both groups received 3 weeks of inpatient rehabilitation. Because we recruited patients from a single rehabilitation centre with an established care protocol it would have been unethical and medically impossible to use a “usual care” or “no treatment” control group. An earlier meta-analysis demonstrated larger effects in experimental studies comparing exercise with usual care than in studies comparing 2 treatments that both used exercise (13). Therefore, the effect on costs of FCT, if compared with “usual care”, is expected to be larger.
In this pragmatic trial the intensity of treatment (treatment hours per day) was different. The number of treatment hours was higher in the FCT group. This is due to the fact that FCT was based on the concept of work-hardening programmes using behavioural principles and exercise aiming at facilitating return to work and increasing activity despite pain. The PCT group passed a more traditional “usual care” programme with a biomedical approach to reduce pain as a primary goal before increasing activity. Furthermore, patients in the FCT were told to continue their training despite pain. All these facts may have influenced the outcomes in favour of the small, but non-significant, effects of the FCT, since recent trials reported better outcomes in patients with chronic LBP using a work-hardening approach (13) or an intensive training programme (12). Although we made all attempts to minimize confounding by keeping patients unaware of the other treatment, it cannot be ruled out that discussions between patients regarding therapy components and goals affected the results. Another limitation of our study is that health insurance providers reported total costs without distinguishing between costs attributable to LBP and other diseases. This may have resulted in an overestimation of LBP-associated direct medical costs in both groups, but would not be a systematic bias affecting the main results or conclusions, because between-group comparisons were performed. Additionally, direct costs data from health insurance providers are considered to be more accurate than information from patients, physicians or healthcare professionals. In Switzerland, patients are free to visit specialists and other healthcare professionals without contacting their primary care physician, thus tracing all healthcare professionals involved in care is difficult.
Finally, there was a non-statistically significant difference at baseline in days of sick leave in the 2 years before treatment. In the analysis and presentation of the work days results (15) we took into account potential pre-treatment covariates as sick leave before treatment, age, sex, education, job qualification and cultural background. Including these covariates in the analysis did not change the results. Therefore we believe that the differences in the current results after the first year can be attributed to the effect of FCT and not to baseline differences between groups.
Taking into account the 3-year follow-up duration, a major strength of this study is the relatively high completeness of data (75%). Another strength is that this study is based on actual and reliable data from health insurance providers and the Swiss Disability Insurance Company. In contrast, other cost-effectiveness analyses often use modelling techniques based on best estimates of expected costs and benefits. Data from the Swiss Disability Insurance Company are the best available in Switzerland, as every person that is out of work due to an illness or injury is reported to this insurance for the purpose of vocational measures or pension payments. However, our reported data may underestimate total indirect costs because they do not consider lower productivity in the workplace (presenteeism) and possible replacement costs for the employer.
In conclusion, this cost analysis of a RCT with 3-year follow-up showed similar costs of function- and pain-centred treatment programmes. The cost benefit for the FCT programme, of 5780 Euros, was not significant. Because costs are similar, and the current literature promotes active function-oriented therapies in the treatment of patients with chronic LBP, we believe that FCT programmes should be the treatment of choice for patients with chronic non-specific LBP. Nevertheless, further cost analyses in larger studies are urgently needed.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Swiss Federal Office of Health (grant-number 00.00473) and the Swiss National Science Foundation (grant NFP 53 405340-111500). Dr Bachmann received additional funding from the Robert Bosch Foundation, Stuttgart (grant-number 32.5.1141.0021.0).
We thank Cornelia Hotz for her help in collecting the data and Ariane Knüsel and Kerri Clough-Gorr, DSc, MPH, for their assistance in preparing the manuscript.
REFERENCES