OBJECTIVE: To examine the validity of the PainMatcher in chronic pain.
DESIGN: Comparison of parallel pain estimates from visual analogue scales with electrical stimulus magnitude matching.
Patients: Thirty-one patients with chronic musculoskeletal pain.
METHODS: Twice a day ongoing pain was rated on a standard 100-mm visual analogue scale, and thereafter magnitude matching was performed using a PainMatcher. The sensory threshold to electrical stimulation was tested twice on separate occasions.
RESULTS: In 438 observations visual analogue scale ranged from 3 to 95 (median 41) mm, and PainMatcher magnitudes from 2.67 to 27.67 (median 6.67; mean 7.78) steps. There was little correlation between visual analogue scale and magnitude data (r = 0.29; p < 0.0001). The mean sensory threshold was 3.67 steps, indicating that the PainMatcher, on average, stimulated at 2.1 times the perception threshold at matching point.
CONCLUSION: Electrical magnitude matching of chronic pain intensity elicited limited activation of nerve fibres at 2.0–2.2 times sensory threshold, indicating that the induced pain was evoked by coarse nociceptive Aδ fibres. While the visual analogue scale estimates covered the whole range of the instrument, the PainMatcher readings utilized only a small part of the instrument range and, importantly, had little or no relation to the visual analogue scale estimates. The validity of the PainMatcher procedure is doubtful.
Key words: electric stimulation; pain measurement; sensory threshold; visual analogue scale; reproducibility of results.
J Rehabil Med 2009; 41: 898–903
Correspondence address: Ann L. Persson, Rehabilitation and Research Centre for Torture Victims, Borgergade 13, PO 2107, DK-1014 Copenhagen K, Denmark. E-mail: ap@rct.dk
Submitted November 13, 2008; accepted May 27, 2009
INTRODUCTION
Self-assessment of pain intensity is commonly used to evaluate the therapeutic effects of clinical interventions and in clinical research (1). Techniques to assess pain intensity are either rating scales or matching methods, where patients are instructed to match their clinical pain to an induced sensation (2). However, a person’s reaction to pain is individual and highly dependent on the context (3).
The most common instrument for assessing clinical pain intensity is the visual analogue scale (VAS) (4–8). A difference of 9–13 mm on the scale has been found to correspond to a clinically significant change in acute pain conditions (9–12). The VAS is valid and reliable (13) in chronic pain as well as in experimentally induced pain (6, 7), and is more sensitive than verbal rating scales (14–17).
There is, however, renewed interest in magnitude matching of pain intensity, since many patients have difficulties in rating pain as a visual analogue amplitude measure (18). It has been argued that the use of direct matching to an induced experimental pain might be easier for the patient and might give a more objective assessment than comparing the pain intensity with a word or setting a mark on a line (19).
A recently developed instrument, the PainMatcher, is based on such perceptual matching by using electrical stimulation of sensory nerve fibres in the fingers (19). This stimulator works at a constant current amplitude and increases the pulse width, i.e. the electrical charge, in steps over time until the patient lets go of the instrument. This technique, to compare the magnitude of an electrically induced experimental pain sensation to a perceived clinical pain, has been reported to be as reliable and as sensitive to pain intensity changes as the VAS and the numerical rating scale (NRS) in patients with chronic nociceptive or neuropathic pain (19). It has also been reported to be valid for matching clinical and induced acute oral pain (20), and pre- and post-operative gynaecological pain (21). There have been greater difficulties, however, in using the instrument to assess pain from chronic whiplash injury, with a possible bias related to unpleasantness of the stimulation (22).
The aim of this study was to examine the validity of the PainMatcher technique in assessing pain intensity by comparing its data with parallel VAS estimates in patients with chronic pain. We chose to carry out the assessments in patients participating in a pain rehabilitation programme that did not have pain reduction as the main purpose, but increased activity. The assessments were carried out during a 2-week period, allowing spontaneous and activity-related variations of pain intensity. The research questions were: (i) Is there agreement between assessments with the 2 methods at the same point in time? (ii) Is the sensory threshold for electrical stimulation stable in individual subjects? (iii) Which cutaneous nerve fibres are activated at the stimulation intensities commonly produced by the PainMatcher? and (iv) Does the PainMatcher give valid measures of perceived chronic pain intensity?
METHODS
Patients
Patients disabled by chronic musculoskeletal pain were recruited during participation in a Commission on Accreditation of Rehabilitation Facilities (CARF)-accredited (23), 5-week cognitive-behaviourally oriented interdisciplinary pain rehabilitation programme at Umeå University Hospital in Sweden. They were referred to the rehabilitation centre with chronic pain present for a minimum of 6 months, the majority coming from primary care physicians in the area. Only those with whiplash-associated disorders present for less than 1.5 years were excluded, since they were managed in a separate programme.
Thirty-one adult patients of working age agreed to participate in the study (24 women; mean age 39.3 years, and 7 men; mean age 43.2 years). The subjects gave written consent to participate and the study was approved by the local ethics committee. The inclusion criteria for the pain rehabilitation programme described above were: (i) disabling chronic pain from the musculoskeletal system (on sick leave or with major interference in daily life causing treatment seeking behaviour due to pain of at least 6 months duration); (ii) age 18–65 years; (iii) further medical investigations not needed; (iv) written consent to participate and attend the activities in the rehabilitation programme; and (v) agreement not to participate in other parallel treatments. The exclusion criteria were: (i) ongoing major somatic (e.g. inflammatory), or psychiatric disease; (ii) a history of significant substance abuse; and (iii) obvious state of acute crisis (major recent adverse life event as found on interview). Furthermore, subjects with sensory or motor deficits in the left hand on clinical testing (see Procedure) or having a pacemaker (contraindication) were excluded.
PainMatcher
The PainMatcher® (Cefar-Compex Scandinavia Inc., Malmö, Sweden) is a commercially available magnitude matching equipment. The instrument is held in the thumb and index fingers of the left hand and, on compressing the grip, which consists of carbon rubber electrodes, it sends a constant current of 15 mA square wave pulses at 10 Hz through the tissue. It can adjust for differences in skin resistance up to 13 kOhm. The pulse duration slowly increases from 0 to 450 µsec in increments of 7.5 µsec in 60 steps, quantifying the electrical charge delivered (pulse current times pulse duration) until the patient lets go of the grip. The display shows the final pulse duration. The instrument is used to match present clinical pain with the evoked experimental pain generated by the electrical stimulation of the volar skin of the fingers.
Procedure
The electrical stimulation was started when the patient squeezed the electrodes. For the somatosensory perception threshold the subject was instructed to interrupt the current (by letting go of the instrument) when they felt the slightest sensation. For pain magnitude matching, they were told to interrupt the current when the experimental pain from the stimulus current was equal (matched) in intensity to their clinical pain level. A value between 1 and 60 was stored in the instrument solid state memory, and it was set not to display the data to the patients, thus blinding the patient to the test results. The patients were always guided by one of 3 assistants, who were trained and familiar with the technique.
Protocol
All patients were first introduced to and acquainted with the PainMatcher and a mechanical 100-mm (plasticized) VAS (15, 17) before the assessments. They were also given written information about the procedure. To standardize the procedure the magnitude matching was always carried out after the VAS estimation. However, it has been shown that the order of pain assessment using different scales does not affect the results (19).
The patients rated their present pain intensity on the 100-mm plasticized VAS with the end-points “no pain” and “worst possible pain”, after which the scale was moved out of sight. The PainMatcher instrument was then used to perform 3 consecutive magnitude matchings of the present pain intensity (mean used for analyses). When possible, the procedure was repeated twice a day for up to 2 weeks, giving a maximum of 20 paired observations. Before the magnitude assessments each patient’s sensory perception threshold was assessed 3 times with 1–3 min intervals (mean values used for analyses) at the 2 first occasions.
Statistical analyses
For the electrical stimulation charges, consisting of quantitative data on pulse durations for perception thresholds and pain magnitudes, we employed parametric statistics, using the Statistical Package for the Social Sciences (SPSS, version 15) for statistical analyses. The mean of the 3 PainMatcher estimates at each session was used for calculations of mean and 95% confidence interval (95% CI) at group levels. For additional clarity, median and range were also calculated. There is ongoing discussion as to whether VAS observations should be considered as categorical or numerical data (24, 25). We chose to analyse them as being categorical data in the present study. To study the agreement between the VAS and the PainMatcher assessments of pain intensity, Spearman’s rank correlation test was used for data from all subjects as a group, as well as in individual analyses for all subjects with ≥ 15 observations.
To analyse which cutaneous nerve fibres were activated by the PainMatcher stimulation (cf. (26)), the ratio between the stimulation charge from the PainMatcher estimates matching present clinical pain and the charge at the threshold for sensory perception at the first measurement was calculated for all observations.
RESULTS
Visual analogue scale estimates and PainMatcher readings
In the 438 observations (one outlier at PainMatcher duration 60 was excluded) from 31 patients, VAS ranged from 3 to 95 (median 41) mm, whereas the PainMatcher magnitudes ranged from 2.67 to 27.67 (median 6.67) steps (Fig. 1A). Some patients gave personal comments about the VAS and about the PainMatcher, for example: “It is difficult to relate to the VAS, not having been free from pain for many years, it is easier having something to compare with – like when using the PainMatcher”. One patient experienced the PainMatcher stimulation as very uncomfortable and commented: “It feels like holding an electrical cattle fence”. Two patients expressed that the stimulation intensity increased so quickly that they were not able to let go of the apparatus in time to mark the perceived pain intensity correctly. In fact, 16 out of 438 PainMatcher observations (3.6%) had pulse duration ratios below 1 (see below and Fig. 1B), indicating inadequate handling of the procedure by the patient (sensory threshold above level of matched pain).
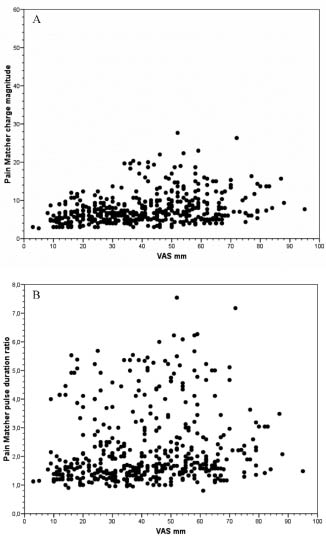
Fig. 1. (A) All observations of assessed ongoing pain intensity on standard visual analogue scale (VAS) (0–100 mm; x-axis) and PainMatcher readings of charge magnitude in arbitrary units (0–60 steps; y-axis). (B) As for A, but y-axis data represent PainMatcher pulse duration ratios (in relation to individual sensory thresholds, see Methods).
Correlations between visual analogue scale and PainMatcher assessments
The paired assessments of pain intensity with VAS estimates and PainMatcher pulse durations (n = 438) for the whole group of patients (n = 31), performed morning and evening for up to 10 consecutive weekdays showed little correlation (r = 0.29; p < 0.0001; Spearman’s test) (Fig. 1A). This plot also reveals that none of the patients employed the upper half of the stimulation range of the PainMatcher (1–60 steps), whereas the full VAS range was used.
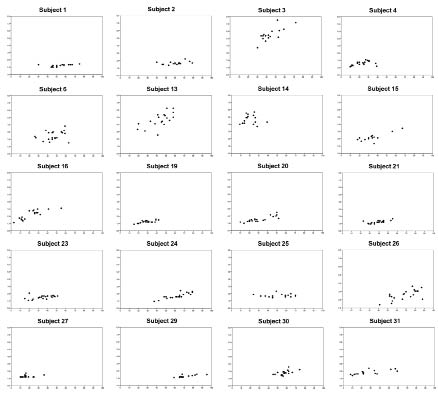
A plot of pulse duration ratios (cf. below) in relation to VAS estimates can be seen in Fig. 1B. It is evident that there is no correlation between the 2 types of pain intensity assessments (r = 0.17; p < 0.0001; Spearman’s test) when pooling data from all patients. The corresponding individual data-sets for the 20 patients with more than 15 observations are given in Figs. 2 and 3, which reveals that in less than one-third of the cases is there a visible correlation between VAS estimates and PainMatcher pulse duration ratios. For those subjects (in total 366 observations), the individual correlations between VAS and PainMatcher assessments using Spearman’s test varied dramatically with correlation coefficients from r = 0.16 to r = 0.86, with a median of r = 0.64.
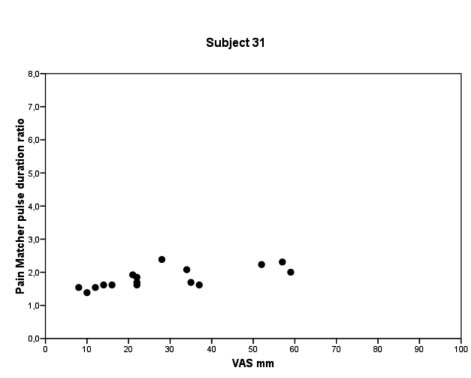
Fig. 2. Individual data-sets for patients with ≥ 15 measurements, showing the relation between visual analogue scale estimates and PainMatcher pulse duration ratios. For larger view in one patient see Fig. 3.
Fig. 3. Data from patient 31, enlarged to display scales and axis titles. VAS: visual analogue scale.
Nerve fibres activated by PainMatcher stimulus charges
The mean PainMatcher sensory perception threshold of the volar skin areas of digiti I–II was 3.67 (95% CI 3.46–3.88) steps (× 7.5 = 27.5 µsec pulse duration). There was no significant difference between the mean thresholds at the first and second measurement occasions (paired t-test). Such cutaneous nerve threshold determinations are usually stable over time (26).
The mean PainMatcher magnitude estimation of chronic pain intensity in our subjects from all observations (n = 438) was 7.78 (95% CI 7.40–8.16) steps (× 7.5 = 58.3 µsec pulse duration). This indicates that the PainMatcher, on average, stimulated at around 58.3/27.5 = 2.1 (95% CI 2.0–2.2) times the perception threshold when the patient let go of the instrument, i.e. when the patient found the intensity of his/her chronic pain to match the experimental pain in the digital skin areas, evoked by the electrical stimulus.
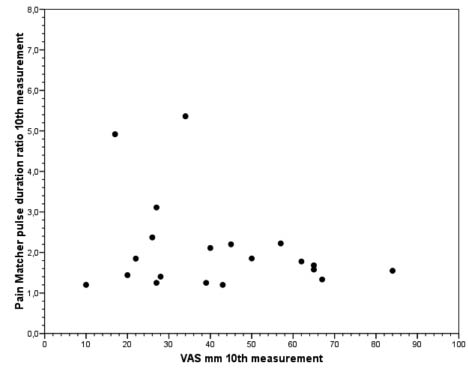
To further examine which cutaneous nerve fibres that may have been stimulated by the actual PainMatcher stimulus charges, we chose to plot, as an example, the ratios between the magnitudes of the tenth observed experimental pain evoked stimulus and those at the threshold, in each of the subjects contributing pain assessments 15 times or more (cf. above; Fig. 4). It can be seen that the stimulation intensities (pulse duration ratios) varied between 1.2 and 5.4 times the sensory perception threshold, more or less irrespective of the corresponding actual VAS estimates.
Fig. 4. Example of visual analogue scale (VAS) estimates and PainMatcher pulse duration ratios, from the tenth observed experimental pain evoked stimulus in each of the subjects (n = 20) contributing pain assessments 15 times or more.
DISCUSSION
Assessing pain intensity by way of the 100-mm VAS is by far the most commonly used technique in pain research and management (12, 13, 15, 17). Even if single researchers have recently argued that the VAS is less than adequate (24, 25), there is overwhelming evidence for the validity and the reliability of the VAS in acute pain assessment (12, 14, 17, 27–29). The VAS scale is also often used for chronic pain intensity even if chronic pain is associated with multiple dimensions, such as intensity, distress, affect, and impact on activity, which might confound the pain intensity ratings (30). On the other hand, a similar problem would hold true for the PainMatcher when used in chronic pain.
The approach of assessing pain intensity by the person matching his/her current pain with an experimentally induced sensation (2), such as that from an electrical stimulation, has recently been advocated through the PainMatcher technique (19). The present data show, however, that while the VAS estimates related to ongoing clinical pain in a group of patients with fluctuating musculoskeletal chronic pain covered the whole range of the VAS, the PainMatcher readings had little or no relation to the corresponding VAS estimates. As is amply demonstrated here, a certain PainMatcher stimulus charge may correspond to almost any reported VAS pain intensity, both at the group and at the individual levels.
The validity of matching methods has been questioned previously, since the affective components in clinical pain are not present when a person is stimulated with a pain that is controlled by the person themselves, both regarding intensity and duration. However, it has been argued that using direct pain matching might be easier for the patient to manage, as well as giving a more objective assessment than comparing the pain intensity to a word or to setting a mark on a line (31, 32).
Furthermore, the PainMatcher readings utilized only a small part of the instrument range. The same phenomenon appears in the studies published by Lundeberg and colleagues (19, 33), that the patients and subjects only use the lower third of the stimulation range of the PainMatcher instrument to match their full range of pain intensities. This observation is to be expected if the instrument stimulates a homogenous group of nerve fibres with a narrow range of electrical stimulation thresholds.
An important aspect is that the use of electrical stimuli for eliciting experimental pain in a matching technique, such as with the PainMatcher, introduces a confounder. When assessing sensory function, the sensory threshold (34) can be measured by increasing the stimulus continuously to a level where it is detectable, and thereafter decreasing it to a level where it is no longer detectable. When the electrical stimulation intensity has reached the sensory threshold, first the tactile Aβ nerve fibres are selectively activated (26, 35). When the current is increased, the threshold of Aδ nociceptive fibres is approached at 2–10 times the sensory threshold. Since the order of activation is connected to the nerve fibre diameter, the thin unmyelinated nociceptive C fibres are activated only at a much higher intensity (> 30 times) than that of Aβ fibres (35, 36).
The present results indicate that the PainMatcher unit was usually working at around 2.1 times the threshold of large Aβ fibres and merely straddling the threshold of Aδ fibres when the patient let go of the instrument (in our sample 1.2–5.4 times the sensory threshold, Fig. 4). In the study by Sang et al. (26) the mean of the ratio between pain threshold and tactile threshold was 10.9 (range 2.0–28.3). Since it is usually agreed that only tactile Aβ fibres occur in the range 1–2 times the sensory threshold (35), the present PainMatcher ranges indicate firstly, that precision was low (sometimes only tactile stimulation occurred (Fig. 1B), and secondly, that the magnitude matching of chronic pain intensity elicited a very limited activation of myelinated nociceptive fibres (mean 2.1; range 0.8–7.6 times threshold) (Fig. 1B). This means that the PainMatcher induced experimental pain was always evoked by the coarsest Aδ nerve fibres, and never by C fibres (26, 35). In fact, it can be questioned whether the PainMatcher procedure measures anything but nociceptive Aδ nerve fibre thresholds. Furthermore, it is tempting to speculate that other mechanisms were in operation, such as phenomena related to fear/avoidance and to sensitization or modulation via descending fibres, e.g. via diffuse noxious inhibitory controls that may influence the reaction (37).
It should be remembered that heat-sensitive nociceptive C fibres (38, 39) were most certainly activated by the old heat lamp technique (dolorimetry) by Hardy et al. (2), and further developed by Lipman et al. (40, 41). On the contrary, the experimental pain elicited by the “artificial” PainMatcher stimuli utilizes only a small part of the available nociceptor channels to the brain compared with heat stimulus matching. This qualitative difference may explain the lack of concordance between the VAS estimates and the PainMatcher readings, pointing to parallel, but independent, processes.
However, there may be other explanations. For example, in the study by Alstergren & Förström (20, Fig. 6 in their paper), almost half of the patients assessed their ongoing clinical pain intensity as being lower than their pain threshold, indicating that the PainMatcher measured something else than pain intensity, or was simply too difficult to master for the patients. It could even be that the patients experienced the stimulation so disagreeable that they let go of the PainMatcher prematurely (20). These authors were of the opinion that since a high level of discomfort during a pain assessment resembles chronic pain, the instrument would be suitable when assessing pain intensity in patients with chronic pain. Our results do not confirm this hypothesis. A recent study by Bunketorp Käll et al. (22) indicates that one bias may actually be that the PainMatcher assessments are partly associated with unpleasantness rather than with pain.
Taken together, our results do not support the use of the PainMatcher as a valid outcome measure for assessing pain intensity, at least not in patients with chronic musculoskeletal pain, which to a large extent is transmitted by C fibres (group IV fibres; (42)). Earlier studies have found the PainMatcher to be as reliable as the VAS and the NRS, more for acute pain situations (19–21, 43) than for chronic ones (19); but see Bunketorp et al. (22) who raise the concerns about the associations with unpleasantness.. Since the validity is now questioned, the earlier reported reliability and repeatability (19, 33, 43) can be considered to be of minor importance for the clinical use of this instrument. It is probable that the previously reported reliability of the PainMatcher technique is due to a reliable Aδ nerve threshold determination rather than to a matching phenomenon. Hence, our conclusion is that the validity of the PainMatcher procedure is doubtful.
ACKNOWLEDGEMENTS
The authors would like to thank all the patients for their participation, Maria Bylund, Section of Rehabilitation, Umeå University, Umeå, Sweden for skilfully performing some pain assessments, and Cefar Compex Scandinavia Inc. for lending us the PainMatcher units. The co-authors Sofia Westermark and Daniel Merrick contributed equally to the study. The study was supported by Umeå University.
REFERENCES