OBJECTIVE: To evaluate the effectiveness of repetitive locomotor training using a newly developed electromechanical gait device compared with treadmill training/gait training with respect to patient’s ambulatory motor outcome, necessary personnel resources, and discomfort experienced by therapists and patients.
METHODS: Randomized, controlled, cross-over trial. Sixteen non-ambulatory patients after stroke, severe brain or spinal cord injury sequentially received 2 kinds of gait training. Study intervention A: 20 treatments of locomotor training with an electromechanical gait device; control intervention B: 20 treatments of locomotor training with treadmill or task-oriented gait training. The primary variable was walking ability (Functional Ambulation Category). Secondary variables included gait velocity, Motricity-Index, Rivermead-Mobility-Index, number of therapists needed, and discomfort and effort of patients and therapists during training.
RESULTS: Gait ability and the other motor outcome related parameters improved for all patients, but without significant difference between intervention types. However, during intervention A, significantly fewer therapists were needed, and they reported less discomfort and a lower level of effort during training sessions.
CONCLUSION: Locomotor training with or without an electromechanical gait trainer leads to improved gait ability; however, using the electromechanical gait trainer requires less therapeutic assistance, and therapist discomfort is reduced.
Key words: brain injuries, walking, rehabilitation, therapy.
J Rehabil Med 2009; 41: 734–739
Correspondence address: Jan Mehrholz, Institute of Rehabilitation Science, Klinik Bavaria in Kreischa, An der Wolfsschlucht 1-2, DE-1731 Kreischa, Germany. E-mail: jan.mehrholz@klinik-bavaria.de
Submitted January 6, 2009; accepted May 27, 2009
INTRODUCTION
Restoration of walking ability is a major goal in the rehabilitation of patients with acquired brain damage and after spinal cord injury. Accordingly, the primary goal of therapeutic efforts should be to restore independent walking ability.
Insights into the mechanisms of brain plasticity, recovery, and learning have led to the development of new and (at least for a subgroup of conditions) potentially more effective training concepts that rely largely on the principles of repetition and massed practice (1). Such training-based interventions have been reported to achieve good clinical results (2–5).
The therapeutic intervention is goal-directed, meaning that training content is the task itself. Task-oriented training regimes are based on fundamental principles of motor learning (6) and are thought to involve mechanisms of central neuroplasticity (7) leading to cortical reorganization (8, 9).
During the last 2 decades, the promising treatment option of treadmill training with or without partial body weight support has been introduced in rehabilitation approaches for neurologically impaired patients. Treadmill training allows repetitive practice of complex gait cycles. As such, it encompasses the 2 crucial aspects of task-specificity and repetition (10, 11). However, a disadvantage of treadmill training concerns the number of physiotherapists needed to set the patient’s paretic limbs and control weight shift. In addition, therapists must expend high levels of physical effort in assisting severely handicapped patients and often complain about exhaustion or physical strain. The duration of therapy sessions is therefore limited. Systematic correction of a patient’s weight shift and stride length is often not possible. Hence, as Kosak & Reding (12) have pointed out, therapists prefer to use task-oriented walking on the floor (with ankle-knee bracing) instead of treadmill training. To overcome these shortcomings, electromechanical-assisted and robotic-assisted gait training devices, such as the gait trainer (GT1) (13) or Lokomat (14), have been developed recently and used in neurological rehabilitation.
The LokoHelp (LokoHelp Group Germany) is another newly developed electromechanical device for improving gait after brain injury. This device is placed on a treadmill and can easily be installed and removed. Although the application of this new gait training device seems promising and has been shown to be feasible (15), its efficacy has not been evaluated in a randomized, controlled trial.
The aim of our study was therefore to compare the effects of 2 forms of task-oriented gait training with respect to patient’s walking ability as well as the number of therapists and the level of therapeutic effort needed to conduct the respective types of training.
METHODS
This prospective randomized controlled trial was approved by the hospital’s ethics committee, and all participants or their legal representatives gave their written informed consent.
Subjects
Participating patients demonstrated a hemi- or tetra-paresis as a result of brain injury, stroke, or spinal cord injury. All patients met the following inclusion criteria: (i) unable to walk independently (Functional Ambulation Category (FAC) score 2 or less (16), i.e. the patient could not walk at all or required the help of 1 or 2 therapists, irrespective of the use of a walking aid); (ii) able to sit; (iii) able to participate in 1 h of physiotherapy, and (iv) able to understand and follow instructions. Due to the study protocol, the expected length of inpatient rehabilitation had to be longer than 10 weeks. Exclusion criteria were a restricted passive range of motion in the hip or knee joints > 20° and/or instabilities (instable fractures, joint injuries) of the lower extremities.
Eligible patients were recruited between October 2006 and March 2007. The interval between onset of the impairment and the onset of the study treatment protocol was between 1 and 180 months. Participating patients’ ages ranged from 11 to 38 years. All participants were inpatients for the entire duration of the clinical trial. All recruited patients finished the study programme and completed interventions A and B.
Randomization
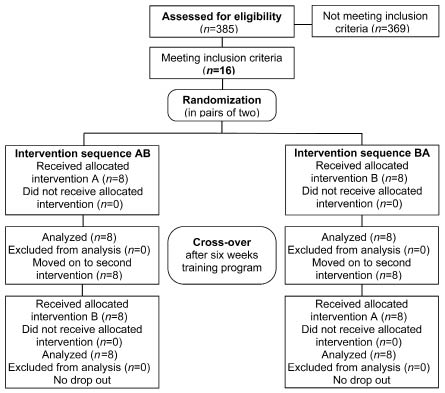
Corresponding to the cross-over design of the study, the type of the first intervention to be administered to each patient was determined by a paired randomization process (Fig. 1). For every 2 patients sequentially recruited for the study, a lot was drawn (by a person not involved in screening, testing, or treatment of the participants) indicating whether the patient should begin with intervention A or B.
Fig. 1. CONSORT flow diagram.
Study protocol
In addition to the normal comprehensive rehabilitation programme, 2 kinds of locomotor training interventions (A and B) were applied. During intervention A, patients received a total of 20 treatments of repetitive locomotor training with the electromechanical gait device (duration 30 min, excluding 15 min preparation time). Treatment sessions took place 3–5 times a week and had to be completed within 6 weeks. During intervention B, patients received a total of 20 treatments on the treadmill or practiced walking on the floor (duration 30 min, excluding 15 min preparation time). Treatment sessions took place 3–5 times a week and had to be completed within 6 weeks. After finishing intervention A, patients receive treatments according to intervention B and vice versa (cross-over design, Fig. 1). Participants continued to participate in the standard rehabilitation therapy sessions during intervention A and B. For each patient, measurements of the study variables took place before any interventions were initiated, and after the first and second interventions, respectively.
Description of intervention A
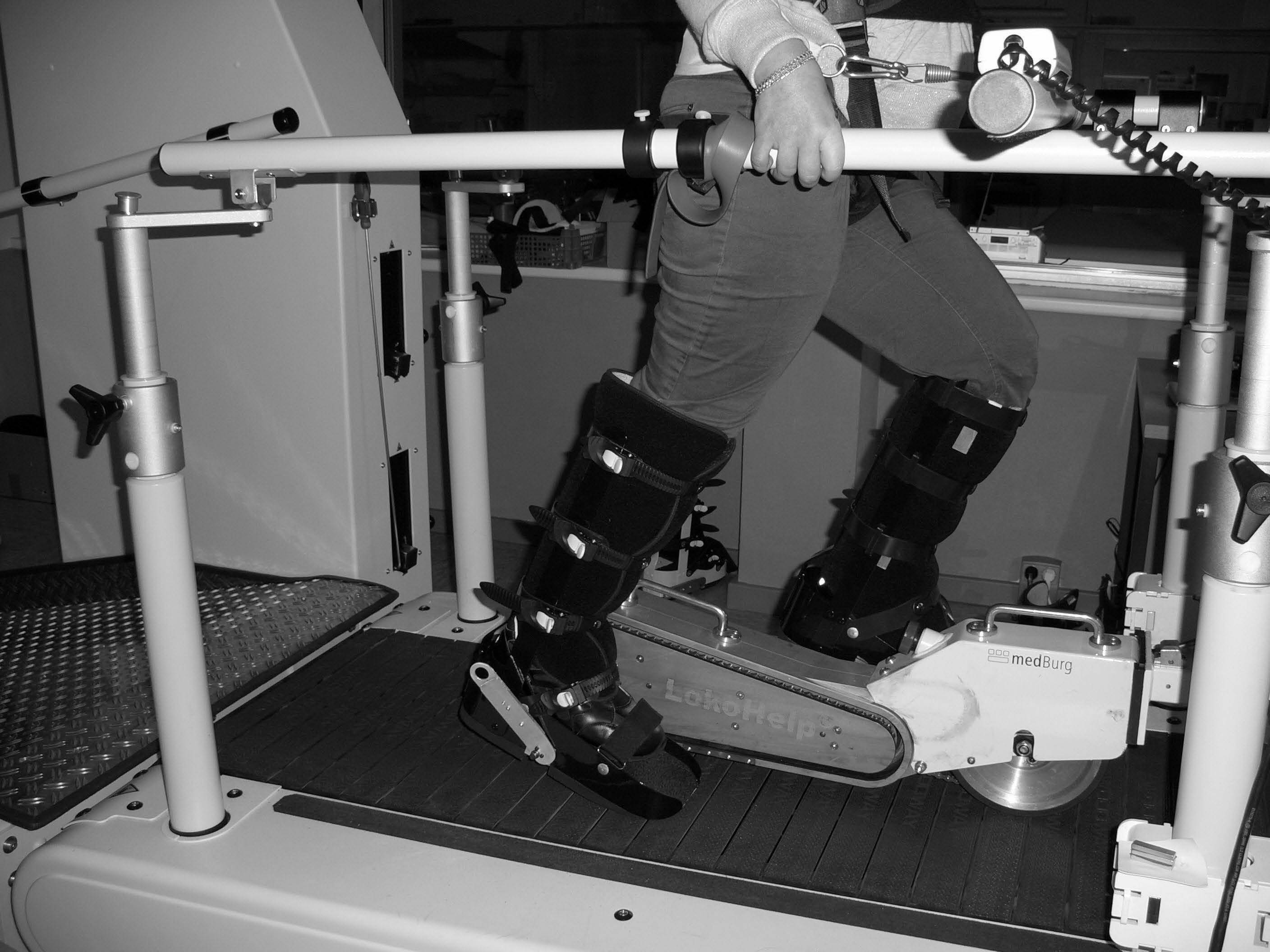
The therapy for intervention A consisted of walking training with the electromechanical gait device (LokoHelp). The LokoHelp device is fixed onto the band of a motor-driven treadmill and transmits the treadmill movement to levers positioned on both sides of the device. Simulation of gait is achieved by the track of the levers, which imitate the stance and swing phases in a sequentially accurate manner (Fig. 2). Velocity can be set individually from 0 to 2.5 km/h. Step length is fixed at 400 mm. The patient is secured with a harness that supports body weight. Each lower leg is set into an orthosis, which maintains the ankle joint at a 90° angle. The orthoses are then attached to the side levers (Fig. 2). The movements of the centre of mass are controlled by ropes attached to the side and front bars, which the patient may grasp. Physical assistance (e.g. for the control of the knee or hip extension in the stance phase) was provided according to individual needs.
Fig. 2. Patient engaged in treadmill training with the Lokohelp device.
Description of intervention B
Therapy consisted of either motor-driven treadmill training or walking practice on the floor. During treadmill training, the patient was secured with a harness that provides body weight support. The movements of the centre of mass were controlled by ropes attached to the side and front bars, which the patient could grasp. Treadmill speed could be varied from 0 to 5 km/h. Physical assistance (e.g. to set the limbs or to help the patient to extend the trunk or shift weight) was administered according to individual needs. If necessary, a second therapist was available to provide assistance.
The training sessions for interventions A and B were conducted at a demanding level. The velocity of the treadmill was set to a maximum speed tolerated by the patients. Therapists motivated patients to actively move the legs and to bear weight. The initial body weight support ranged from 10% to 30%, which was reduced as soon as possible.
Therapists administering the treatment had to complete a form after each therapy session, thereby providing information about the total distance walked, body-weight support, number of therapists needed for the treatment, and strain and complaints of patients and therapists.
Measurements
Primary outcome variable.
• Gait ability was assessed according to the FAC, a reliable and valid instrument for assessing gait ability (16). Six categories (0–5) distinguish the level of physical support needed while walking, irrespective of technical aids used. Level 0 describes a patient who is unable to walk or who requires the assistance of 2 or more people. At level 1, a patient needs continuous support from one person in order to carry weight or control balance. At level 2, a patient needs intermittent physical support, and at level 3, a patient needs only verbal support. Level 4 indicates that a patient is able to walk on even surfaces without help, and level 5 means that a patient can walk independently everywhere, including stairs.
Secondary outcome variables
• The number of therapy sessions requiring a second therapist was documented for interventions A and B, respectively, according to treatment protocol.
• Walking velocity was assessed by measuring the time a patient took to walk a distance of 10 m (if the patient was able to perform the task) (17).
• Lower limb motor power was assessed by the Motricity Index (MI) leg score. The MI (score values range from 1 to 100) assesses the motor power of the affected lower limb. Ankle dorsiflexion, knee extension, and hip flexion of the affected limb or limbs are rated. Because some subjects were diplegic, we added the MI for both legs to achieve a sum score of 2–200 (18).
• The patient’s activity level was assessed by the Rivermead Mobility Index (RMI, score range from 0 to 15). This instrument includes 15 mobility-related items, from turning over in bed to running. Items are assigned a value of 0 (unable to perform activity) or 1 (able to perform activity) (19).
• Posture control and balance were assessed using the Berg Balance Scale (BBS), which consists of 14 items. Items test an individual’s ability to maintain positions or perform movements of increasing difficulty by diminishing the base of support from sitting, standing on 2 legs, and standing on 1 leg. The total score ranges from 0 to 56 points (20).
• The Modified Ashworth Scale (MAS) was used to document resistance to passive movement and hence, muscle tone. Resistance is rated from 0 = no resistance to 5 = maximal resistance to passive movement. The MAS was used to assess resistance to passive movements in the ankle, knee, and hip joints of both lower limbs. The results for each limb were added (i.e. maximal score for both limbs = 30) (21).
• Distance walked during training sessions was also documented. The mean walking distances achieved during interventions A and B were calculated according to treatment protocols.
• Discomfort of patients and therapists was assessed. The total number of therapy sessions in which complaints were made by either the patient or therapist during interventions A and B were documented according to treatment protocols.
• Physical stress of patients and therapists was assessed according to treatment protocols. Physical stress during interventions A and B was categorized. For statistical evaluation, dichotomous sum scores (exhausting/high vs moderate/low) were calculated.
The measured parameters were evaluated by trained therapists not involved in the study before and after each treatment phase. The therapists were blind to the intervention phase.
Statistical analysis
The patients’ characteristics were first summarized with descriptive statistics. Differences between groups and interventions A and B were analysed with Fisher’s exact tests for frequencies and Wilcoxon signed-rank test, which is a non-parametric alternative to the dependent t-test for continuous variables (22). We applied non-parametric statistics because a normal distribution was not achieved, as checked visually (Q-Q plots and histograms) and statistically with Kolmogorow-Smirnov Tests a priori. Fisher’s exact tests were used for frequencies because of their known independence on large-sample distribution assumptions and their appropriateness for sparse tables (22). The alpha level was set as 0.05 for all comparisons. SAS/STAT® software package 9.1.3 (SAS Institute Inc., Cary, NC, USA, 2006) was used for all calculations.
RESULTS
A total of 16 patients were included in our study. All of them completed both interventions, and no patient dropped out of the study. Patient characteristics are shown in Table I.
| Table I. Patient characteristics |
| | Group AB n = 8 | Group BA n = 8 | p-value* |
| Age, years, mean (SD) | 22.4 (6.0) | 25.8 (6.1) | 0.41 |
| Female, n | 3 | 2 | 1.000 |
| Duration of illness, months, mean (SD) | 16 (15) | 56 (69) | 0.225 |
| Diagnosis, TBI/stroke/SCI | 6/1/1 | 6/1/1 | 1.000 |
| FAC at baseline | | | |
| Mean (SD) | 0.5 (0.8) | 1.0 (0.9) | 0.296 |
| Median (IQR) | 0.0 (1.0) | 1.0 (0.9) | |
| RMI at baseline, median (IQR) | 3.3 (1.9) | 4.1 (2.7) | 0.624 |
| BBS at baseline, median (IQR) | 9.5 (12.7) | 12.8 (12.5) | 0.308 |
| *p-values determined by Wilcoxon rank-sum test for continuous variables and by the Fisher’s exact tests for frequencies. SD: standard deviation; TBI: traumatic brain injury; SCI: spinal cord injury; FAC: Functional Ambulation Classification; IQR: interquartile range; RMI; Rivermead Mobility Index; BBS: Berg Balance Scale. |
Gait ability improved for all patients, but did not differ significantly between groups (as shown in Table II, FAC improvement after intervention A: mean 0.9 (SD 1.4), median 0 (IQR 2.0); after intervention B: mean 0.5 (SD 1.0), median 0 (IQR 1.0); p = 0.155). Walking velocity, RMI, MI, BBS and MAS scores also did not differ significantly between interventions A and B (Table II). However, as shown in Table II, the distance walked during training sessions was significantly higher during intervention A, mean 553 m (SD 116), than during intervention B, mean: 400 m (SD 245), p = 0.009. We were unable to find statistical evidence for any period or carry-over effects.
| Table II. Results by intervention (A or B) |
| | Intervention | |
| Variable | A | B | p-value* |
| FAC | | | |
| Mean (SD) | 0.9 (1.4) | 0.5 (1.0) | 0.155 |
| Median (IQR) | 0.0 (2.0) | 0.0 (1.0) | |
| Walking velocity, m/sec, mean (SD) | 0.2 (0.2) | 0.2 (0.4) | 0.624 |
| MI, sum score, mean (SD) | 9.1 (15) | 10.8 (17.9) | 0.167 |
| RMI, points, mean (SD) | 0.9 (1.5) | 1.4 (1.8) | 0.677 |
| BBS, points, mean (SD) | 3.9 (6.6) | 4.6 (7.8) | 0.717 |
| MAS, sum score | | | |
| Mean (SD) | 0.5 (2.4) | –0.3 (1.0) | 0.011 |
| Median (IQR) | 0.0 (0.0) | 0.0 (1.0) | |
| Distance (m) walked during training, mean (SD) | 553 (116) | 400 (245) | 0.009 |
| Second therapists needed for training session, times, n | 0 | 80 | < 0.001 |
| *p-values determined by Wilcoxon signed-rank test for continuous variables and by the Fisher’s exact tests for frequencies. IQR: interquartile range; SD: standard deviation; FAC: Functional Ambulation Classification; MI: Motricity Index; RMI: Rivermead Mobility Index; BBS: Berg Balance Scale; MAS: Modified Ashworth Scale. |
| Table III. Patients’ and therapists’ efforts and complaints |
| | Patients’ effort | Patients’ complaints |
| Exhausting/high | Moderate/low | Fisher’s exact test | Complaints | No complaints | Fisher’s exact test |
| Intervention A | 98 | 222 | p = 0.15 | 34 | 286 | p = 0.064 |
| Intervention B | 116 | 204 | 20 | 300 |
| First phase of treatment | 115 | 205 | p = 0.21 | 36 | 284 | p = 0.015 |
| Second phase of treatment | 99 | 221 | 18 | 302 |
| Sequence AB | 117 | 203 | p = 0.11 | 34 | 286 | p = 0.064 |
| Sequence BA | 97 | 223 | 20 | 300 |
| | Therapists’ effort | Therapists’ complaints |
| Exhausting/high | Moderate/low | Fisher’s exact test | Complaints | No complaints | Fisher’s exact test |
| Intervention A | 0 | 320 | p < 0.001 | 2 | 318 | p < 0.001 |
| Intervention B | 56 | 264 | 60 | 260 |
| First phase of treatment | 54 | 266 | p < 0.001 | 53 | 267 | p < 0.001 |
| Second phase of treatment | 2 | 318 | 9 | 311 |
| Sequence AB | 2 | 318 | p < 0.001 | 9 | 311 | p < 0.001 |
| Sequence BA | 54 | 266 | 53 | 267 |
The number of therapists needed for therapy sessions was significantly lower during intervention A. As shown in Table II, a second therapist was never needed for the 320 treatment sessions conducted during intervention A. In contrast, a second therapist was needed in 80 of the 320 treatment sessions conducted during intervention B (p < 0.001).
Patient discomfort was slightly, but not significantly, higher during intervention A, and patient effort was slightly but not significantly lower during intervention A. Therapist discomfort and physical effort during the training sessions were significantly lower during intervention A (as shown in Table III). Therapists’ efforts were significantly higher, and they complained significantly more often in the first phase of treatment than in the second (phase effect). Efforts were greater and complaints more frequent when the sequence of interventions was BA vs AB (sequence/carry-over effect).
DISCUSSION
Our results support and confirm the findings of previous reports that gait rehabilitation following repetitive and task-oriented treatment guidelines is beneficial to non-ambulatory patients with severe hemi- and tetra-paresis after acquired brain damage and incomplete spinal cord injury (11, 12, 23–26). Patients’ gait ability, muscle strength, and other motor functions improved during both treatment phases. Since most of the patients were in a chronic state, spontaneous recovery can be excluded and the observed beneficial effects attributed to the treatment.
Comparison of the effects of the electromechanical device (LokoHelp) training and treadmill training alone revealed that the improvement in walking ability (FAC) after intervention A was greater than after intervention B. However, this difference was not statistically significant. These results are in line with studies concerning 2 different types of mechanically and robot-assisted gait training (27–29). In addition, although approximately 5 min more preparation time was needed in intervention A compared with intervention B, the distance walked during training sessions was significantly higher in intervention A.
During LokoHelp training session, the mean distance walked was 553 m compared with 400 m during the treadmill training. Bearing in mind that the number of repetitions seems to be crucial for relearning a task (1, 6), it is reasonable to assume that in a larger patient sample, training using devices would lead to significant results, as has been demonstrated in studies on the gait trainer (30, 31). In contrast to a recently published paper from Peurala et al. (32), we set the treatment duration at 45 min per training session. In our study, this treatment duration included the time used to get the patient into the device, and no additional time for preparation was provided as described in another study using the GT1 (30). Although set-up procedures in intervention A takes slightly longer, the mean achieved walking distances were higher in intervention A compared with intervention B.
Recently Hidler et al. (33) have reported better walking ability in a population of ambulatory patients after therapist-assisted training compared with robot-assisted gait training using the Lokomat. However, in our study we included only patients who could not walk at study onset; therefore it is difficult to compare the results of both studies.
Finally, our results are in contrast with the results of a Cochrane review including GT1 and Lokomat trials, which found that patients who receive electromechanical-assisted gait training are more likely to achieve independent walking than patients receiving gait training without these devices (34). However, an update of the evidence using mechanical and/or robot devices is expected in due course.
The MAS scores increased slightly after intervention A and decreased after intervention B. This may be due to the fact that for one patient intervention A was conducted between one and 3 months after spinal cord injury. Thus, the recorded increase might be rather the result of the known phenomenon of increasing spasticity that occurs after spinal cord injury and not of the intervention studied.
The MI of the lower limbs improved during both treatment phases, indicating that repetitive, task-oriented gait training can lead to amelioration in voluntary muscle activity. Although this finding is supported by studies of patients with supratentorial lesions (29), it differs from those obtained in samples of patients with spinal cord lesions (35, 36). The relationship between strength and walking function is still not fully clear, albeit a recent review has shown a positive correlation between these factors (37, 38).
A main finding of our study was that the number of therapists needed to accomplish the treatment during treadmill training was significantly higher than during the electromechanically assisted training intervention. Whereas a second therapist was never needed for treatments in intervention A, additional therapists were needed in 80 of the 320 treadmill intervention sessions, especially for setting the limbs. This meant a 25% increase in staff requirements. Given that personnel resources in the public health system are becoming increasingly limited, the number of therapists needed to achieve a patient’s improvement is not a trivial consideration. Previous studies have reported the generally high number of personnel needed for gait-training therapies involving similar patients (29–31). Werner et al. (27) assessed the number of personnel required and found that, overall, 2 therapists per patient were needed to conduct treadmill training.
The number of therapists’ complaints (mostly regarding wrist and lower back pain) was greater and the level of physical stress experienced by the therapists was significantly higher during intervention B (see Table III). The therapy protocols revealed that therapists described the treatment as being exhausting/high in 56 of the total 320 therapy sessions (= 17.5% of the therapy sessions) during treadmill training, but never during LokoHelp training. Werner et al. (27) maintain that the overexertion of therapists during the treadmill therapy sessions may lead to non-optimal gait patterns and, in the long run, to worse walking ability. At least it is reasonable to assume that when the therapists are overexerted, they seem to initiate fewer repetitions of gait cycles.
Interestingly, there was also a phase and sequence effect for the discomfort and physical stress of therapists. Complaints and high levels of effort were reported more often in the second phase of treatment, and at the same time more often when the sequence of the 2 interventions were B and then A compared with A and then B (Table III). Obviously, complaints were registered less often during treadmill training when the patients had already completed intervention A. Therapists reported that the setting of the limbs and maintaining trunk extension were easier in this training condition. It is possible that these patients had learned how to set their limbs and to maintain trunk extension during intervention A (gait training with LokoHelp). This improvement, which is also reflected by the increase in mean FAC scores, could lead to easier training conditions for therapists (i.e. less assistance needed to be provided) and might explain the phase and sequence effect on the complaints and efforts of therapists.
Patients’ complaints during gait training using the treadmill and the LokoHelp were comparable (Table III). Patients’ complaints (mostly related to the body weight support system and once to knee pain) decreased over the course of the treatment period. During the total of 640 treatment sessions (320 sessions of each intervention A and B), no other unwanted side-effects or complications occurred.
Both interventions were well accepted by the patients. Overall, the patients rated the physical effort needed during the treadmill and LokoHelp training phases as being equal (Table III). Therefore, we conclude that the muscle activity during mechanically assisted gait training is not lower than during treadmill training or gait training on the floor.
Based on our findings, we conclude that task-oriented gait training with treadmill training or with a mechanical gait-training device leads to an improvement in walking ability and related scores. The training paradigm involving the latter, newly developed device reduces the physical strain for therapists and the amount of personnel needed to conduct gait-training therapies.
Certain limitations of our study should be noted. First, our conclusions were based on patients’ reports of their efforts and not on measurements of muscle activity made during LokoHelp- or therapist-assisted treadmill training sessions.
One could also argue that another limitation of our study is the large variation in the studied group. However, this is due to the fact that the population for this study was drawn from our inpatient rehabilitation centre, which is specialized for severely handicapped patients after brain injury, stroke and spinal cord injury.
Another limitation is that we cannot provide follow-up results and exact data pertaining to the gait patterns of the 2 kinds of gait training protocols.
ACKNOWLEDGEMENTS
The authors thank the physiotherapists who conducted the testing procedures and the patients who agreed to participate in the study. We also thank Dr Willi Nagel, Department of Psychology, University of Konstanz, for his expert advice on statistical questions.
The authors report no conflicts of interest. The company Medburg (Basel, Suisse) provided the LokoHelp gait device for the period of the study and funded one physiotherapist for additional treatments. The company had no influence on the interpretation of data and the final conclusions drawn.
REFERENCES