OBJECTIVE: The aim of this study was to investigate the influence of different volumes of saline vehicle on the effects of botulinum toxin type A in reducing ankle plantarflexor spasticity and improving gait pattern in children with cerebral palsy.
DESIGN: Children with cerebral palsy having ankle plantarflexor spasticity were recruited. They were divided randomly into 2 groups. Botulinum toxin type A mixed with 2 ml or 8 ml saline was injected into the gastrocnemius in each group. Passive range of movement of ankle joint, Modified Ashworth Scale, and results of 3-dimensional motion analysis obtained at pre-treatment, 4, 12, and 24 weeks after treatment were compared.
RESULTS: Ankle dorsiflexion was increased and ankle plantarflexor spasticity was decreased significantly after botulinum toxin type A treatment. Linear parameters were generally improved, and these improvements persisted until 12–24 weeks. The ankle dorsiflexion angle in the stance phase was also increased, and this increase was maintained until 24 weeks, as revealed by 3-dimensional gait analysis. However, no significantly different effect of varying the amount of saline vehicle was detected.
CONCLUSION: Botulinum toxin type A improved physical findings and gait pattern in patients with cerebral palsy. The volume of saline mixed with botulinum toxin type A did not result in significant differences in physical evaluation or gait analysis. However, the large-volume group revealed side-effects more frequently and showed no clinical benefits compared with the small-volume group. We conclude that 2 ml of dilution is preferable for botulinum toxin type A treatment in children.
Key words: botulinum toxin, cerebral palsy, gait, muscle spasticity, gastrocnemius muscle.
J Rehabil Med 2009; 41: 740–745
Correspondence address: In Young Sung, Department of Physical Medicine and Rehabilitation, Asan Medical Center, Ulsan University College of Medicine, 388-1 Pungnap-2dong, Songpa-gu, Seoul, 138-736, Korea. E-mail: iysung@amc.seoul.kr
Submitted November 6, 2008; accepted May 7, 2009
*Part of this paper was presented at oral presentation session in Annual Assembly of APMR in 2006, Honolulu, Hawaii.
INTRODUCTION
Cerebral palsy (CP) refers to a group of disabilities caused by damage to the immature brain before, at, or shortly after, birth. Spasticity of the ankle plantarflexors, including the gastrocnemius muscle has frequently been found to lead to an abnormal gait pattern in children with CP, including inappropriate heel touch or insufficient dorsiflexion during ambulation. Botulinum toxin type A (BoNT-A) has been used widely to reduce spasticity resulting from upper motor neurone diseases, such as CP, traumatic brain injury, stroke, or spinal cord injury (1–5). It also aids in reducing the over-activity of muscles and it improves efficiency in movement patterns in patients with CP.
In addition to physical or neurological examination, some studies have used a 3-dimensional motion analyser to assess improvements in ambulation more objectively and quantitatively after BoNT-A injection (6–9). Sutherland et al. (6, 7) described the improvement in the peak angle of ankle dorsiflexion in the stance and swing phases among kinematic parameters of 3-dimensional gait analysis in children with CP.
Generally, a dosage of BoNT-A ranging from 3 to 12 U/kg per child was used to accomplish a remarkable reduction in limb spasticity, while avoiding the side-effects of the toxin (10, 11). As with any toxin, the amount of saline used for dilution is very important. As a vehicle, saline delivers and distributes the injected toxin over the muscle. It was expected that if more saline was used, in spite of having a lower concentration, a larger area would be covered. However, there is no standard amount of saline used to dilute the toxin. Only a few articles have reported that a more dilute preparation of BoNT-A resulted in better muscle tone reduction in animal studies (12, 13). Human studies have been conducted, but these have mainly targeted the upper limbs of adult patients with a traumatic brain injury or stroke, and the effects were evaluated using a physical scale or electrodiagnostic study rather than by functional parameters (14, 15). Therefore, more quantitative and objective methods should be used to perform further studies examining the effects of BoNT-A with different volumes of saline vehicle in terms of functional parameters. These studies would be helpful to define the dilution effects and to establish the appropriate saline volume for treatment of patients. In addition, children with CP are one of the primary groups receiving BoNT-A treatment and they have relatively small muscles, thus the saline volume might influence the effects of BoNT-A, and this should therefore be established more precisely.
The aim of this study was to analyse the effects of using different amounts of saline vehicle with BoNT-A on the reduction in spasticity of the gastrocnemius muscle in children with CP. Increased range of motion of the ankle joint and improvement of the gait pattern were evaluated by 3-dimensional gait analysis.
PATIENTS AND METHODS
The sample size was determined by a previous study showing the changes in ankle dorsiflexion in the stance phase after BoNT-A treatment (16). At least 12 children were required to be recruited to each group in order to provide an 80% probability of detecting change at the 5% significance level.
Children were included who showed insufficient ankle dorsiflexion during ambulation with CP-related ankle plantarflexor spasticity; they were followed up in the outpatient department of physical medicine and rehabilitation. Subjects who were able to ambulate for a short distance, and thus could undergo 3-dimensional gait analysis without devices or support, were selected. Inclusion factors were: (i) they did not have any other disease, except CP, that might influence gait pattern; (ii) they had no fixed contracture of the lower limbs; (iii) they had not had any surgery related to the treatment of lower limb spasticity; and (iv) they had not received botulinum toxin, phenol injections, or other anti-spastic medicines for one year prior to the study. The parents of each child provided written consent after being informed of the purpose and methods of the study. Ethics approval was obtained from the Institutes of Review Board. A final total of 38 children (19 boys, 19 girls) participated in the study. Twenty-eight had diplegic and 10 had hemiplegic CP. Patients’ age at the time of injection ranged from 2 to 8 years. Mean age was 3.80 (standard deviation (SD) 1.61) years.
The 38 children were divided into 2 groups using a table of random numbers (Fig. 1). They did not know to which group they were assigned; however, the injector knew this information because they mixed the saline with the toxin. The dosage of BoNT-A (Botox, Allergan Inc., Irvine, CA, USA) used in this study was 3.0 U/kg body weight to one gastrocnemius muscle, diluted with 0.09% saline, 100 U/2 ml in one group (small-volume group; n = 19) and 100 U/8 ml in the other group (large-volume group; n = 19). The number of boys and girls were 11 and 8 in the small-volume group, and 8 and 11 in the large-volume group. The mean age of the small- and large-volume groups was 3.74 (SD 1.88) and 3.84 (SD 1.84) years, respectively. In the small-volume group 15 children were diplegic and 4 hemiplegic. In the large-volume group 13 were diplegic and 6 hemiplegic. No significant difference was noted between groups in gender proportion, mean age, or type of cerebral palsy. All patients had grade 1 or 1+ of ankle plantarflexor spasticity.
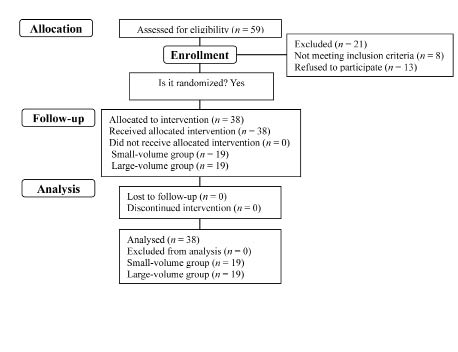
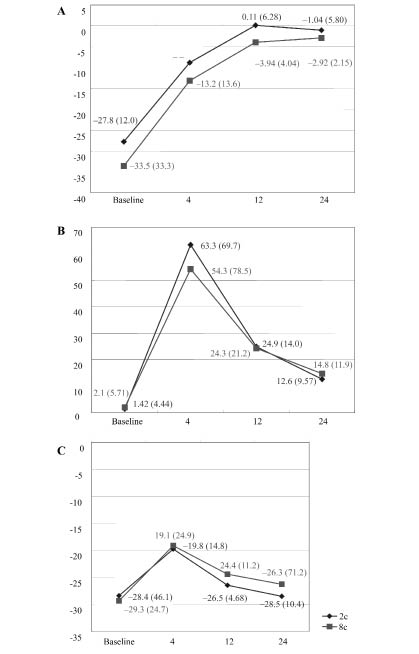
Fig. 2. Maximal ankle dorsiflexion angle was significantly increased (A) between 0–20% gait cycle and (B) between 20–60% gait cycle, and this effect persisted at 24 weeks. (C) The significant decrease in maximal ankle plantarflexion between 60–100% GC was shown at 4 weeks post-injection, but this change was not persistent at 12 weeks post-injection. No significant difference in ankle dorsiflexion was found through the gait cycle between the 2 groups. Values are means (standard deviations). The x-axis indicates the weeks after injection and the y-axis indicates the maximal ankle dorsiflexion angle. *p < 0.05 significant change compared with pre-treatment status.
All of the hemiplegic and diplegic subjects received the same dose per body weight in each affected gastrocnemius muscle. The total dose was divided equally and injected into 2 sites, the medial and lateral mid-belly of the muscle bulk, which were near the assumed motor endplate zones (17). The location of the injections was determined according to anatomical landmarks and palpation. No electromyography or motor point stimulation was used. The needle position was checked by flexing-extending both the knee and ankle joints.
All subjects underwent physical examination before injection, including passive range of motion (PROM) of the ankle joint and the degree of ankle plantarflexor spasticity. PROM of the ankle joint was assessed by goniometer with the knee extended. Ankle plantarflexor spasticity was graded through the Modified Ashworth Scale (MAS) (10).
Three-dimensional gait analysis was performed in order to assess gait characteristics (6, 7, 9). The Vicon 370 motion analysis system (Oxford Metrics Ltd, Oxford, UK) was used to obtain linear, kinetic, and kinematic variables of each individual. Passive markers were attached with adhesive tape on the following locations on both lower limbs: the anterior superior iliac spines, the sacrum, the anterior aspect of the mid-thigh, the knee and the mid-calf, the lateral malleolus, the base of the heel, and the dorsum of the foot between the second and third metatarsals. The child was asked to walk independently for approximately 10 m with bare feet, while the infrared camera determined the location of each marker, representing joint motion. These data were analysed by the VCM Software program (VCM Software Ltd.Jerusalem, Israel).
These physical and gait analysing tests were all performed within 3 days prior to injection, and 4, 12, and 24 weeks post-injection. Physical examinations were conducted by 1 physiatrist; 3-dimensional gait analysis was performed and its results were analysed by the other physiatrist. Neither examiner knew to which group each child was assigned.
Each affected limb was regarded as a single limb for the purpose of physical examination for statistical analysis. However, in diplegia only one limb was selected for statistical analysis of 3-dimensional gait analysis because side-to-side interaction might have influenced the results (6). Mann-Whitney U test of SPSS version 12.0 was used to compare the mean age between the small- and large-volume groups. For assessing the difference of gender and distribution of type of cerebral palsy at pre-treatment, χ2 with Fisher’s exact test was used. A repeated measures statistical design (Friedman analysis) was used to analyse both the changes in physical status (range of ankle dorsiflexion and spasticity of ankle plantarflexor) and the motion analysing test (linear parameter, maximal ankle dorsiflexion angle, ankle power and moment) before and after botulinum toxin injection, and to compare the ankle results between the 2 groups. For calculation of mean MAS, grade 1+ was regarded as 2. Statistical significance was set at a value of p < 0.05.
RESULTS
Physical examination
Ankle plantarflexor spasticity, estimated by MAS, decreased significantly after injection and this reduction was maintained until 12 weeks post-injection. The PROM of ankle dorsiflexion was significantly improved and was maintained until 24 weeks post-injection. There was no significant difference between the small- and large-volume groups (Table I).
|
Table I. Values of physical examination before and 4, 12 and 24 weeks after injection
|
|
|
Saline volume (ml)
|
Baseline
Mean (SD)
|
4 weeks
Mean (SD)
|
12 weeks
Mean (SD)
|
24 weeks
Mean (SD)
|
|
MAS of APF
|
2
|
1.67 (0.50)
|
1.00 (0.00)*
|
1.33 (0.50)*
|
1.78 (0.97)
|
|
8
|
1.63 (0.52)
|
1.50 (0.76)*
|
1.50 (0.53)*
|
1.59 (0.80)
|
|
PROM of ADF (degree)
|
2
|
–24.3 (11.4)
|
1.00 (11.9)*
|
2.25 (9.48)*
|
–2.08 (10.1)*
|
|
8
|
–24.8 (11.9)
|
1.00 (8.38)*
|
4.58 (10.3)*
|
–2.25 (9.57)*
|
|
*p < 0.05, significant change compared with baseline value. There is no significant difference in all parameters between the 2 ml and 8 ml saline groups.
Sample size of 2 ml and 8 ml group was 19, respectively.
SD: standard deviation; MAS of APF: Modified Ashworth Scale of ankle plantarflexor; PROM of ADF: passive range of motion of ankle dorsiflexion.
|
Three-dimensional gait analysis
Linear variables. Cadence and speed were increased, benefits that were maintained until 24 weeks post-injection. The stride length of the affected limb was also significantly increased, and this effect persisted until 12 weeks post-injection. Single limb support of the affected limb was significantly increased, and this persisted until 4, 12, and 24 weeks after injection in both groups. The large-volume group showed increased cadence compared with the small-volume group, although this was not statistically significant. There were no significant differences between the 2 groups in other variables (Table II).
|
Table II. Values of linear variables in 3-dimensional gait analysis
|
|
|
Baseline
Mean (SD)
|
4 weeks
Mean (SD)
|
12 weeks
Mean (SD)
|
24 weeks
Mean (SD)
|
|
Cadence, steps
|
|
2 ml
|
89.0 (42.0)
|
104.3 (37.5)*
|
120.2 (55.3)*
|
119.0 (34.6)*
|
|
8 ml
|
101.7 (14.0)
|
151.8 (16.5)*
|
148.8 (13.7)*
|
147.7 (23.1)*
|
|
Speed, m/sec
|
|
2 ml
|
0.44 (0.30)
|
0.61 (0.35)*
|
0.74 (0.42)*
|
0.53 (0.34)*
|
|
8 ml
|
0.45 (0.20)
|
0.98 (0.33)*
|
1.01 (0.27)*
|
0.88 (0.22)*
|
|
Stride length, m
|
|
2 ml
|
0.51 (0.23)
|
0.64 (0.23)*
|
0.69 (0.15)*
|
0.65 (0.24)
|
|
8 ml
|
0.52 (0.23)
|
0.77 (0.24)*
|
0.81 (0.21)*
|
0.71 (0.12)
|
|
Single limb support, % of gait cycle
|
|
2 ml
|
28.0 (12.5)
|
33.8 (9.78)*
|
35.4 (8.73)*
|
35.5 (6.98)*
|
|
8 ml
|
33.8 (3.90)
|
40.4 (4.61)*
|
40.0 (10.9)*
|
36.3 (4.83)*
|
|
*p < 0.05, significant change compared with baseline value.
There is no significant difference in all parameters between 2 ml and 8 ml saline group.
Sample size of 2 ml and 8 ml group was 19, respectively.
SD: standard deviation.
|
Kinematics variables. The maximal angle of ankle plantarflexion was significantly decreased for between 0–20% and 20–60% of the gait cycle (GC), and this effect persisted until 24 weeks post-injection. A significant decrease in maximal ankle plantarflexion between 60–100% GC was shown at 4 weeks post-injection, but this change did not persist to 12 weeks post-injection. No significant difference was found between the 2 groups (Fig. 2).
Kinetics variables. There was no significant change after the injection and no significant difference between the 2 groups in any kinetics variable.
Adverse effects
Six children reported post-injection calf pain, 4 in the large-volume group and 2 in the small-volume group. We observed no evidence of excessive muscle weakness following the BoNT-A injection. All adverse events were self-limited and did not require any treatment.
DISCUSSION
Many studies have shown improvement in gait characteristics, including linear variables, and kinematic and kinetic variables of the ankle joint, by utilizing 3-dimensional gait analysis after a BoNT-A injection to the gastrocnemius (6, 7, 9, 16). Our study indicated that linear variables were generally improved and ankle dorsiflexion increased mainly in the stance phase, but that there was no significant change in ankle power during the gait cycle. These findings mean that BoNT-A injected into the gastrocnemius leads to improved ankle dorsiflexion range without significant ankle plantarflexor weakness. Improvement in linear and kinematic variables could be obtained without improvements in kinetic parameters.
The duration of the BoNT-A effects on the various variables was interesting. It is well-known that the effects of BoNT-A on the reduction in muscle tone do not persist longer than 3 months because nerve terminals blocked by BoNT-A are recovered by collateral sprouting or regeneration (18–20). This study showed that although reduction in ankle plantarflexor spasticity induced by BoNT-A did not persist until 24 weeks after injection, increased ankle dorsiflexion in the physical examination and improvements in various variables of gait analysis were generally maintained until 24 weeks. One study documented that although muscle tone had increased again at 12 weeks after injection, the effect of decreased equinus during the GC was shown to be prolonged (21). Slawek & Kilmont (22) reported that patients with CP treated with BoNT-A demonstrated overall functional benefits, such as gross motor function measure (GMFM), despite the increased muscle tone 3 months after BoNT-A injection. These results suggest that clinical and functional improvements can persist in spite of the disappearance of the pharmacological effects of BoNT-A. This occurrence could be explained by children’s abundant nervous system plasticity, which accommodates their muscles learning new functions, biomechanical transformation and the lengthening of muscles acquired by muscle training after BoNT-A, and the antagonist strengthening during the weakened period of the injected muscle (5, 23, 24).
This study did not succeed in finding significant differences between small- and large-volume saline groups based on the variables of physical examination and gait analysis. Although some animal models have demonstrated dilution effects (12, 13), other human studies also failed to show these expected results. According to the single-blinded trial of Lee et al. (10), comparing different dilution volumes in patients with CP, a large volume (100 IU/4 ml) did not yield better results than a small volume (100 IU/1 ml) in compound muscle action potential, range of motion, and MAS. In addition, Francisco et al. (14) compared the effects of small- (100 U/1 ml) and large-volume (100 U/2 ml) preparations of BoNT-A on upper limb spasticity of adult patients with acquired brain injury and found no significant difference between the 2 preparations. Perhaps this can be attributed to the fact that most examiners used a high enough dosage of toxin to sufficiently reduce the spasticity, which may have masked the effects of differences in saline volume. In contrast to animal trials, the examiners were very reluctant to use a lower dose of toxin because they were afraid that it might not result in patient improvement. Francisco et al. (14) reported the high dose (240 U) injected as the main factor eliminating the possibility of difference. Lee et al. (10) also attributed no difference to the fact that a dose of 3–6 U/kg might be so large that it yielded significant responses no matter how much saline was used. We used the dose of 3 U/kg, which was a relatively lower dose, because children taking part in this study were younger and had smaller muscles.
The degree of volume difference could also be an important factor affecting the effects of saline volume. A 1:4 ratio of dilution was thought to be appropriate to investigate different volume effects of saline. According to other studies (10, 14, 15) authors usually used the ratios of 1:2 through 1:5. Francisco et al. (14) compared 100 U/1 ml and 100 U/2 ml, which did not show any differences according to dilution effects. Lee et al. (10) also demonstrated no difference by comparing 100 U/1 ml and 100 U/4 ml. They explained that this result was partly because the ratio 4:1 was too low to produce differences; in addition to that, a dose of 3–6 U/kg might be too large, as previously described. However, the study performed by Gracies et al. (15) revealed a more significant reduction in spasticity in the large-volume preparation group (20 U/ml) compared with the small-volume group (100 U/ml). Also, another study demonstrated that high-volume (500 U/5 ml) saline preparation of Dysport was more effective in reducing spasticity than low-volume (500 U/1 ml) preparations (25). It was assumed that at least the ratio of 5:1 in dilution was high enough to cause the difference. We used 2 ml and 8 ml of saline per 100 U. As in Lee et al. (10), the ratio of 4:1 might not have been high enough to show differences according to saline volume. The absolute volume that was used in dilution might also be an important factor. Two millilitres of saline were used in this study because this volume was the one most commonly used in a clinical setting for the purpose of reducing spasticity of the gastrocnemius in children with CP. Other researchers used 1 ml of saline in the small-volume group, even though in 2 studies (14, 15) the subjects were adult patients. Even 2 ml of saline used in the low volume group of our study could be large enough to distribute the BoNT-A along the muscle belly as extensively as 100 U/8 ml in the young children ranging from 2 to 8 years old who took part in our study. It was assumed that small-volume dilution (2 ml) was preferred because this revealed less side-effects than large-volume dilution and comparable clinical benefits to large-volume dilution. Future studies should include groups with lower volumes of saline with the same dose of toxin or lower doses of toxin diluted with various amounts of saline.
This study had limitations. We did not evaluate the functional aspect of the patients using functional assessment tools, such as the Gross Motor Function Classification System (GMFCS) or the GMFM, which might assess the children with respect to functionality, which is of interest to many physiatrists involved in paediatric rehabilitation. We did not receive reports from the children or parents that were helpful in determining the patients’ satisfaction and quality of life.
In conclusion, BoNT-A injected into the gastrocnemius had positive effects on: (i) reduction in ankle plantarflexor spasticity; (ii) increase in ankle dorsiflexion; and (iii) improvement of gait pattern, such as the linear parameters and ankle dorsiflexion in the stance phase. However, differences in the saline volumes used to dilute BoNT-A did not significantly affect the degree of improvement in the physical assessment or gait pattern.
Conflict of interest
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
REFERENCES