Jesús Devesa, MD, PhD1,2, Pedro Reimunde, BSc1,2, Ana Devesa, BSc1, Sonia Souto, PhD3, Manuel Lopez-Amado, MD, PhD4, Pablo Devesa, BSc1,2 and Víctor M. Arce, MD, PhD2
From the 1Medical Center Project Foltra, Teo, A Coruña, 2Department of Physiology, School of Medicine, Santiago de Compostela, 3School of Physiotherapy, University of A Coruña, 4Medical Center Modelo, A Coruña, Spain
OBJECTIVE: To report an unusual case of significant neurological recovery in a 26-year-old growth hormone-deficient female patient with significant neurological sequelae resulting from brain surgery at 11 years of age.
DESIGN: Case report.
RESULTS: Most of the neurological sequelae present at admission recovered after 8 months of combined growth hormone administration and kinesitherapy/speech therapy. These include an increase in tongue size and mobility and in the amount and quality of saliva, improvement in vocal cords function, recovery of oesophageal peristalsis and disappearance of sleep apnoea.
CONCLUSION: Since the patient had undergone intensive physical rehabilitation for a 15-year period with no significant improvement, it is tempting to speculate that the correction of growth hormone deficiency improved her rehabilitation. Therefore, we propose that growth hormone treatment, combined with the adequate kinesitherapy, may be a useful therapy for effective recovery from some neurological deficits in patients with growth hormone deficiency.
Key words: cranial nerve injuries, vocal cord paralysis, growth hormone.
J Rehabil Med 2009; 41: 775–777
Correspondence address: Jesús Devesa, Medical Center Project Foltra. Carretera Santiago-La Estrada, Chalet, s/n, ES-15886-Teo, Spain. E-mail: jesus.devesa@usc.es
Submitted March 20, 2009; accepted June 09, 2009
INTRODUCTION
Affectation of oropharyngeal structures are fairly common sequelae of oncological surgery of the head and the neck. This affectation frequently presents as disabling symptoms related to dysphagia, aspiration and dysphonia. The oropharyngeal defects cannot be neurologically restored (1, 2).
CASE REPORT
A 26-year-old woman presented with significant neurological sequelae (including paralysis of oropharyngeal structures, paralysis of vocal cords and lack of primary oesophageal peristalsis) secondary to iatrogenic palsy of cranial nerve pairs IX, X and XII resulting from brain surgery (bulbar astrocytoma) at age 11 years. Her growth velocity had also slowed down soon after surgery and lineal growth had stopped one year later. At that time, the patient was diagnosed with growth hormone (GH) deficiency, but GH therapy was discouraged due to the risk of tumour recurrence. The final height of the patient (1.51 m) was approximately 15 cm less than her predicted height.
At admission, the patient was unable to speak or swallow and was at high risk of aspiration. A tracheostomy cuff was maintained inflated 24 hours a day, and she required frequent clearance of secretions. Feeding was provided through a gastric tube. Sleep apnoea was also present and required a positive airway pressure system. Exploration showed the existence of an atrophic and hypotonic tongue and non-functioning oropharyngeal structures and vocal cords (despite intensive speech therapy and oropharyngeal electrotherapy/ kinesitherapy for 15 years). The presence of GH deficiency (GHD) was confirmed on the basis of the reduced GH peak response (< 9 ng/ml) to the growth hormone releasing hormone plus arginine test. The existence of cognitive affectation was ruled out as the patient had high academic scores (she had gained a first degree in biology after GH therapy was started). A positron emission tomography (PET) scan of the brain showed no residual tumoural activity. There were no other symptoms of note. The patient was scheduled for oropharyngeal kinesitherapy and speech therapy (45 min/day, 5 days/week, for each therapy) and, concurrently, GH treatment was started. GH doses (0.01 mg/kg) were administered subcutaneously at 10.00 h daily, prior to rehabilitation therapy. One month later, the GH dose was increased to 0.02 mg/kg.
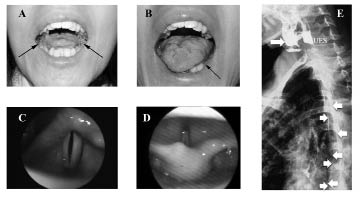
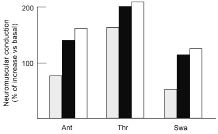
The time-line of the clinical evolution of the patient is shown in Table I. An obvious change in the type and amount of saliva produced was observed as soon as 15 days after the start of treatment, with the highly dense mucous saliva changing into a more fluid secretion (Fig. 1A and B) indicating a recovery of parasympathetic functionality. One month after the start of treatment, surface electromyography (EMG) patterns improved, indicating an enhancement in neuromuscular conduction to the suprahyoid group, tongue and pharynx (Fig. 2). Tongue size and mobility also increased significantly at this time (Fig. 1A and 1B). Furthermore, the patient’s speech improved progressively, and her vocal cords were fully functional 2 months after the start of treatment (Fig. 1C and 1D). Primary oesophageal peristalsis also recovered by this time. After 4 months of treatment, functional outcome assessment of swallowing (FOAMS) scale (3) score had improved from grade 1 on admission to grade 3–4, although the patient’s upper esophageal sphincter function did not completely recover, avoiding resumption of complete oral feeding (Fig. 1E). Sleep apnoea also disappeared 2.5 months after the start of treatment. Interestingly, the patient entered into an rapid eye movement (REM) phase as soon as she fell asleep, and this phase lasted for approximately 76% of the sleep cycle (data not shown). Four months after starting the treatment, the tracheal cannula was withdrawn, the tracheostomy incision was surgically closed, and nocturnal volumetric ventilation was discontinued. No aspirations or adverse clinical events were recorded secondary to this procedure. The overall condition of the patient improved significantly after treatment. However, it is difficult to know whether these findings depend directly on GH actions, since some improvements secondary to the treatment, such as the withdrawal of volumetric ventilation or the absence of sleep apnoea, may explain the existence of a better condition and alertness.
| Table I. Time-line of the clinical outcome of the patient |
| Time (months) | Clinical outcome |
| 0.5 | Reduced accumulation of saliva in the mouth Predominance of serous saliva |
| 1 | Increased tongue size and motility Increased oropharyngeal electromyography conduction |
| 2 | Recovery of vocal cord function (videolaryngoscope) Recovery of primary oesophageal peristalsis (oesophagoscopy) |
| 2.5 | Absence of episodes of sleep apnoea (polysomnography) |
| 4 | Removal of volumetric ventilation Surgical closure of the tracheostomy |
| 8 | Presence of deglution (barium transit) |
Fig. 1. (A, B) Changes in tongue size and mobility; (C, D) images from videolaryngoscopies showing vocal cords functionality; and (E) barium transit across the upper esophageal sphincter (arrows). Upper arrow shows the persistence of barium in the vallecula. Images were obtained before the start of treatment (A, C), and after 1 (B), 2 (D), or 4 (E) months of treatment. Also notice the difference in the amount of saliva present in the mouth (arrows) between (A) and (B).
Fig. 2. Neuromuscular conduction to the suprahyoid group, tongue and pharynx muscles. Surface electromyographic (EMG) activity was measured immediately before the beginning of the treatment and then every week for 3 months. Two pairs of bipolar skin electrodes (Ag-AgCl) were connected to an EMG device and the EMG signal was registered during the maximal muscle contraction sustained for 3 sec. The movements requested were: antepulsion of the tongue, thrust of the tongue on the palate, and swallowing. Results are shown as the percentage increase compared with basal, after 1 (grey), 2 (black) or 3 (white) months. Ant: antepulsion; Thr: thrust; Swa: swallowing.
DISCUSSION
We observed a dramatic improvement in several neurological functions in an adult patient with GHD following combined kinesitherapy/speech therapy and GH administration. Until now the relative impact of the aforementioned treatments on the clinical course of the disease had not been clear. However, the patient had been undergoing intense rehabilitation for a 15-year period with no significant improvement, while she began to recover shortly after starting GH treatment. Therefore, it is reasonable to assume that GH treatment is responsible for the clinical benefits reported here. Whilst GH roles in the nervous system are starting to emerge, it is presently clear that, apart from regulating somatic growth and metabolic processes, GH is involved in the regulation of brain growth and development. Thus, it has been shown that GH treatment stimulates both proliferation and differentiation of neural progenitor cells (4), and may promote axonal growth in developing nervous tissues (5). A role of GH in the regeneration of the adult central nervous system (CNS) has been also proposed, since GH treatment induces cell proliferation in the brain of adult hypophysectomized rats (6). This effect is probably due to the ability of GH to stimulate the proliferation and differentiation of adult neural stem cell pools present within the CNS (7). All these GH actions can be exerted in a direct fashion, through stimulation of the GH receptor, which is widely expressed through the CNS (8, 9). However, GH may also promote neuronal recovery via stimulation of Insulin-like growth factor-1 (IGF1), since IGF1 can be produced locally in the brain, and its expression is driven by GH (10). In this regard, it is also interesting to note that IGF1 gene transfer has been shown to stimulate laryngeal reinnervation in rats (11). Finally, GH may also stimulate the release of other neurotrophic factors that may have a positive impact of neurological recovery (12, 13). In summary, we report here for the first time, that correction of GH deficiency improved rehabilitation in a GH-deficient patient. In view of these findings, it can be envisaged that treatment combined with the adequate kinesitherapy may be a useful therapy for effective recovery from neurological deficits in adult patients with GHD. In addition, the existence of a GH deficit should be also taken into consideration in patients with low response to rehabilitation therapy.
ACKNOWLEDGEMENTS
This study was carried out with the financial support of Project Foltra and Fundación María Esperanza Romero Rubido.
REFERENCES