OBJECTIVE: To determine whether pulmonary function at discharge from inpatient rehabilitation can predict respiratory infection in spinal cord injury in the first year after discharge, and to determine which pulmonary function parameter predicts best.
DESIGN: Multicentre prospective cohort study.
SUBJECTS: A total of 140 persons with spinal cord injury.
METHODS: Pulmonary function was tested at discharge from inpatient rehabilitation. Pulmonary function parameters (expressed in absolute and percentage predicted values) were: forced vital capacity, forced expiratory volume in 1 sec, and peak expiratory flow. Respiratory infection was determined one year after discharge by a physician. Differences between the respiratory infection and non-respiratory infection groups were tested; and receiver operating characteristic curves were used to determine how accurately pulmonary function parameters could predict respiratory infection.
RESULTS: Of the 140 participants, 14 (10%) experienced respiratory infection in the first year after discharge. All pulmonary function parameters were significantly lower in persons who experienced respiratory infection than in those who did not. All pulmonary function parameters were almost equally accurate in predicting respiratory infection; only percentage predicted forced vital capacity was less accurate.
CONCLUSION: Pulmonary function at discharge from inpatient rehabilitation can be used as a predictor of respiratory infection in the first year after discharge in spinal cord injury. No single pulmonary function parameter was a clearly superior predictor of respiratory infection.
Key words: spinal cord injury, complications, respiratory infections, pulmonary function test, rehabilitation, sensitivity and specificity.
J Rehabil Med 2009; 41: 729–733
Correspondence address: Karin Postma, Rijndam Rehabilitation Center, PO Box 23181, 3000 KD Rotterdam, The Netherlands. E-mail: k.postma@rijndam.nl
Submitted December 12, 2008; accepted May 7, 2009
*This article was presented in part at the 46th ISCoS Annual Scientific Meeting and 10th NoSCoS Congress, 27–30 June 2007, Reykjavik, Iceland.
INTRODUCTION
Although the life expectancy of persons with spinal cord injury (SCI) has improved in recent decades, this group is still prone to long-term secondary medical complications (1–4). Respiratory complications, such as respiratory infection (RI) and atelectasis, are some of the more serious long-term secondary medical complications in persons with SCI (2, 5). Long-term respiratory complications are a common cause of hospitalization (6) and a major cause of mortality (3, 4). Therefore, minimizing the risk of long-term respiratory complications should be an important goal of primary SCI rehabilitation and aftercare (7).
To minimize the risk of long-term respiratory complications it is important to identify persons at risk during primary rehabilitation. Persons with SCI who are neurologically the most impaired (i.e. those with complete high tetraplegia) are at greatest risk of respiratory complications (2). A logical explanation for this finding is that this latter group is also the most restricted in pulmonary function (vital capacity, cough, and respiratory muscle strength) (8–11). However, the relationship between lesion characteristics and pulmonary function is not straightforward: respiratory complications are not only restricted to complete tetraplegia, and pulmonary function varies strongly within different lesion groups. For example, Linn et al. (9) suggested that, within any given level of injury, it is likely that smaller vital capacity means a greater risk of respiratory complications and early death. From this point of view, pulmonary function has the potential to be a better predictor than lesion characteristics. Compared with lesion characteristics, another advantage of pulmonary function is that it is a modifiable factor that, clinically, can make it a more useful predictor. If pulmonary function is predictive of respiratory complications, it can be used not only to identify persons at risk in an early stage and as an alarm for preventive strategies, but also as the central topic in a specific training programme focusing on decreasing the risk of long-term respiratory complications.
The aim of the present study was to determine whether pulmonary function at discharge from inpatient rehabilitation can predict RI in SCI within the first year after discharge. Additionally, to determine which pulmonary function parameter can best predict RI.
MATERIALS AND METHODS
For this study, data of a Dutch multicentre prospective cohort study on the restoration of mobility during SCI rehabilitation were used (12, 13).
Subjects
In the Dutch multicentre study, persons with SCI admitted for primary rehabilitation to one of the 8 participating rehabilitation centres from August 2000 to July 2003 were included if they were between 18 and 65 years of age and were expected to remain (at least in part) wheelchair-dependent. Subjects were excluded if they had a progressive disease, a psychiatric condition interfering with constructive participation, or if they did not sufficiently comprehend the Dutch language. The study was approved by the medical ethics committee, and prior to participation all subjects gave their written informed consent. For the present study we included persons who were still enrolled in the Dutch multicentre study at discharge from inpatient rehabilitation.
Pulmonary function
Pulmonary function (PF) was determined at discharge from inpatient rehabilitation by forced spirometry measurements with the Oxycon Delta® (Jaeger, Hoechberg, Germany). Persons were tested seated in a wheelchair and wearing a nose clip. Three repeated flow volume curves were made; in case of a non-characteristic curve, an extra measurement was performed (14). The best trial, determined as the trial with the highest sum of forced vital capacity (FVC) and forced expiratory volume in 1 sec (FEV1), was used in further analysis. The FVC, FEV1 and peak expiratory flow (PEF) were expressed in absolute values and percentage of the predicted values (based on able-bodied persons of the same age, gender, and height).
Respiratory infection
A physician determined one year after discharge from inpatient rehabilitation, whether the subject had had RI since discharge and how many days they stayed in bed due to RI. This was done by means of a custom-made questionnaire on secondary complications. Physicians were instructed that only clinically important infections, not a simple nose or head cold, were to be counted as RI.
Personal and lesion characteristics
Age, gender, body weight, body mass index (BMI) (i.e. body weight (kg)/height (m2)), smoking status, level and completeness of lesion were established at discharge from inpatient rehabilitation. A person was defined as a smoker when he or she smoked prior to the onset of SCI. Level and completeness of lesion were determined according to the International Standards for Neurological and Functional Classification of Spinal Cord Injury (15). Persons were diagnosed with tetraplegia in case of impairment or loss of function at the cervical segments of the spinal cord, and with paraplegia in case of impairment or loss of function at the thoracic, lumbar or sacral segments. The lesion was defined as complete when motor function was absent (American Spinal Injury Association (ASIA) category A or B), and incomplete when motor function was preserved in the lowest sacral segment (ASIA category C or D).
Statistical analyses
Analysis was performed with SPSS 12.0. Data are presented as mean and standard deviation. Data on the incidence of RI were analysed in a descriptive way. Based on the occurrence of RI (yes/no), 2 subgroups were created. These subgroups were tested on differences in person-related and lesion-related characteristics (Phi-Cramers’ V for nominal and Mann-Whitney U test for continuous values). Differences in PF between the group with and without RI were tested with the Mann-Whitney U test. p < 0.05 was considered significant.
Receiver operating characteristic (ROC) curves were constructed for all PF parameters, considering the occurrence of RI as the dependent parameter (16, 17). The accompanying area under the curve (AUC) was calculated to determine how accurately the different PF parameters could predict the occurrence of RI one year after rehabilitation discharge. A parameter with AUC = 100% was considered a perfect predictor, 90 < AUC ≤ 100% highly accurate, 70 < AUC ≤ 90% moderately accurate, 50 < AUC ≤ 70% less accurate, and AUC = 50% a non-informative predictor (18). Subsequently, for each parameter a cut-off value was determined (based on the highest sum of sensitivity and specificity), and the corresponding sensitivity, specificity and positive predictive value (PPV) and negative predictive value (NPV) were calculated.
RESULTS
At discharge from inpatient rehabilitation, 199 persons were still enrolled in the Dutch multicentre study. Pulmonary function at discharge was missing in 20 persons and 39 persons did not participate in the measurements one year after discharge. As a result, in 140 persons PF at discharge and RI one year after discharge from inpatient rehabilitation was known and used for analyses in the present study. Analysis showed no significant differences in person-related and lesion-related characteristics, between persons who were tested at discharge for PF and persons who were not. Also, there were no significant differences found in PF, person-related and lesion-related characteristics, between persons who were used for the final analyses of this study and persons who dropped out because they did not participate in the measurements one year after discharge. Table I shows the patient characteristics; none of these persons were ventilator dependent.
|
Table I. Population characteristics and comparison of age, body weight, body mass index (BMI), gender, smoking status, level and completeness of lesion (all measured at discharge) in persons with and without respiratory infection (RI) in the first year after discharge from inpatient rehabilitation
|
|
|
Total population
n = 140
|
Persons with RI, n = 14
|
Persons without RI, n = 126
|
p*
|
|
Age, years, mean (SD)
|
39.9 (13.8)
|
44.3 (14.1)
|
39.4 (13.7)
|
0.224
|
|
Body weight, kg, mean (SD)
|
74.7 (14.8)
|
76.4 (14.2)
|
74.6 (14.9)
|
0.677
|
|
BMI, mean (SD)
|
23.6 (4.1)
|
25.2 (4.6)
|
23.4 (4.0)
|
0.130
|
|
Gender, male, %
|
72.1
|
50.0
|
74.6
|
0.051
|
|
Smokers, %
|
45.3
|
42.9
|
45.5
|
0.849
|
|
Level of lesion, tetraplegia, %
|
36.7
|
57.1
|
34.4
|
0.094
|
|
Completeness of lesion, complete, %
|
67.2
|
76.9
|
66.2
|
0.430
|
|
*Differences between subgroups were tested with Phi-Cramers’ V (nominal values) and the Mann-Whitney U test (continuous values).
SD: standard deviation.
|
Fourteen of the 140 persons (10%) experienced RI in the first year after discharge from inpatient rehabilitation; 9 persons stayed in bed for at least one day (1, 2, 3, 5, 10, 10, 14, 18 and 40 days) because of RI. Of these, 2 were hospitalized. Gender and level of lesion tended to be different between the groups (relatively more females and more tetraplegia in the RI group). The RI group did not differ from the non-RI group with respect to age, weight, BMI, smoking, or completeness of lesion (Table I).
All PF parameters were significantly (p < 0.05) lower in persons who experienced RI in the first year after discharge from inpatient rehabilitation than in those who did not (Table II).
Almost all PF parameters had an AUC between 70% and 90% and therefore can be considered moderately accurate predictors; according to our definitions only percentage predicted FVC was a less accurate predictor (Table III). Fig. 1 presents the ROC curve and the AUC of FEV1.
|
Table II. Comparison of pulmonary function at discharge in persons with and without respiratory infection (RI) in the first year after discharge from inpatient rehabilitation
|
|
|
Total population
(n = 140)
|
Persons with RI
(n = 14)
|
Persons without RI
(n = 126)
|
p*
|
|
FVC, l
|
3.91 (1.28)
|
3.08 (1.53)
|
4.00 (1.22)
|
0.003
|
|
FVC, % predicted†
|
84.24 (22.86)
|
71.04 (23.83)
|
85.70 (22.36)
|
0.026
|
|
FEV1, l
|
3.17 (1.01)
|
2.35 (1.00)
|
3.27 (0.98)
|
0.001
|
|
FEV1, % predicted†
|
82.34 (21.44)
|
65.29 (17.95)
|
84.23 (21.01)
|
0.001
|
|
PEF, l/sec
|
5.65 (1.88)
|
3.96 (1.60)
|
5.84 (1.82)
|
0.001
|
|
PEF, % predicted†
|
63.92 (19.37)
|
47.73 (16.93)
|
65.72 (18.84)
|
0.001
|
|
Data are presented as mean (standard deviation).
*Differences between subgroups were tested with the Mann-Whitney U test.
†Based on able-bodied persons of same age, gender, and height.
FVC: forced vital capacity; FEV1: forced expiratory volume in 1 sec; PEF: peak expiratory flow.
|
|
Table III. Predictors of respiratory infection in the first year after discharge from inpatient rehabilitation, with area under the curve (AUC) and cut-off point based on highest sum of sensitivity and specificity. Sensitivity (Se), Specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) are presented for the given cut-off points
|
|
|
AUC (%)
|
Cut-off point
|
Se (%)
|
Sp (%)
|
PPV (%)
|
NPV (%)
|
|
FVC, l
|
74
|
3.2
|
78.6
|
73.8
|
25.0
|
96.9
|
|
FVC, % predicted
|
68
|
77
|
71.4
|
66.7
|
19.2
|
95.5
|
|
FEV1, l
|
78
|
2.5
|
78.6
|
75.4
|
26.2
|
96.9
|
|
FEV1, % predicted
|
76
|
70
|
71.4
|
74.6
|
23.8
|
95.9
|
|
PEF, l/sec
|
78
|
4.7
|
78.6
|
73.0
|
24.4
|
96.8
|
|
PEF, % predicted
|
76
|
51
|
64.3
|
79.4
|
25.7
|
95.2
|
|
FVC, FEV1 and PEF were measured at discharge and expressed in absolute values and percentage of predicted (% predicted) values based on able-bodied persons of same age, gender, and height.
FVC: forced vital capacity; FEV1: forced expiratory volume in 1 sec; PEF: peak expiratory flow.
|
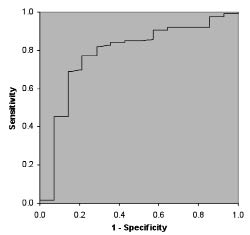
Fig. 1. Receiver operator characteristics (ROC) curve with accompanying area under the curve (AUC) of forced expiratory volume in 1 sec (l).
DISCUSSION
This study shows that PF at discharge from inpatient rehabilitation can be used to identify persons with SCI at risk of developing RI within the first year after discharge. Persons who developed RI had significantly lower PF than the others, and most PF parameters can be considered moderately accurate predictors of RI.
Our results are in agreement with other studies exploring respiratory complications in patients with neuromuscular disorders other than SCI (19, 20). Both of these last studies identified inspiratory vital capacity (IVC) as one of the predictors (besides peak cough flow and maximal inspiratory pressure, respectively) of severe chest infection in children with neuromuscular disorders, and sleep-disordered breathing in adults with neuromuscular disorders, respectively. IVC is a measure of vital lung capacity and is comparable to FVC in our study. In the study of Dohna et al. (19) and Ragette et al. (20), IVC was a stronger predictor than in our study, probably because persons in their study groups had lower mean PF and a higher incidence of illness.
Respiratory problems and complications are often thought to be a problem for persons with tetraplegia only. Although we found a (non-significant) tendency for more persons with tetraplegia than persons with paraplegia to experience RI, the occurrence of RI was not restricted to tetraplegia. Therefore, the results of our study indicate that PF is a stronger predictor of RI than lesion level, and programmes aimed at the prevention and treatment of RI should target not only persons with tetraplegia but also those with paraplegia and low PF.
In the RI group we were surprised to find a tendency for relatively more females than males. Because healthy females have lower PF (absolute values) compared with males (21), a similar percentile decrease following SCI might cause PF to reach a certain absolute cut-off value and therefore lead to an earlier increased risk of RI in females compared with males. Other possible determinants of RI (e.g. age, body weight, BMI, smoking and completeness of lesion) showed no significant difference between the RI and non-RI group. Other factors that we did not explore (e.g. the level of physical activity) might also have influenced the risk of RI.
In this study we also investigated which PF parameter can best be used to predict RI. However, no single PF parameter appeared to be clearly superior to the other PF parameters in predicting RI. As in previous studies (22), all PF parameters in the present study were highly correlated with each other; correlation coefficients ranged from 0.48 to 0.92 (p < 0.001). All the PF parameters showed a significant difference between the RI and non-RI group, and the ROC curves were similar. Most of the PF parameters had an AUC between 70% and 90%, which makes them “moderately accurate” predictors (18). Only percentage predicted FVC appeared to be a weaker predictor, indicated by an AUC below 70%.
To explain the predictive characteristics of the PF parameters, we used FEV1 (litres) as an example. An FEV1 with a cut-off point of 2.5 l has a good sensitivity and a good specificity in predicting RI. Also the positive predictive value is high, meaning that just over one-quarter (26.2%) of persons with an FEV1 below 2.5 l actually experienced RI within one year after discharge. However, 21.4% (1-Se) of persons who experienced RI would have been missed. Since RI is a serious complication, possibly leading to hospitalization or even death, a higher cut-off point can be considered in future treatment protocols (16). A cut-off point of 2.8 l FEV1, for example, will lead to higher sensitivity (85.7%) and lower specificity (67.5%). Although, more persons will be falsely identified as being at risk of developing RI, more importantly, fewer at risk will be missed.
A limitation of our study is that the incidence of RI was determined retrospectively by anamnestic information, without precise criteria. RI is a rather wide diagnostic term, which includes upper and lower tract infection. Even though the participating physicians were instructed that only clinically important infections were to be counted as RI, differences in interpretation between physicians may have occurred in the less serious cases. A former study, including only radiographically confirmed pneumonia (infection of lung tissue) and/or atelectasis (airlessness within the lung), showed a lower (3.5 vs 10%) incidence one year after discharge (2). This supports the idea that, in some cases, less severe infections are also included in our study. To investigate if this could have confounded our results, we also performed analyses in which only persons who stayed in bed at least one day because of infection were defined as having RI. These analyses showed an incidence of 6.4%, which is closer to that reported by McKinley et al. (2). Moreover, the earlier described results became more profound; differences in PF between the RI and non-RI groups were significant and the ROC curves improved, resulting in higher AUCs. Thus, PF at discharge from inpatient rehabilitation might be an even stronger predictor for the more severe RI in SCI after discharge.
A second limitation of the present study is the small number of persons with RI in the study group; this is attributed to the relatively low incidence of this complication, and the short follow-up period. Subgroups of approximately equal size would have enabled the use of multi-factorial analysis that included several factors at the same time (including PF, lesion characteristics, and perhaps physical activity), which might have resulted in a more accurate prediction model.
Finally, of 199 persons still enrolled in the Dutch multicentre study at discharge from inpatient rehabilitation, we excluded 59 persons because PF was missing or they did not participate in the measurements one year after discharge. Analysis between groups did not show significant differences between persons used for the present study and the persons excluded. A considerable part of the loss to follow-up was due to the small number of persons that participated in the measurements after discharge from the rehabilitation centre (12). Although the precise reasons for not participating after discharge are unknown, they were widely diverse. Verbal reports ranged from being too busy with work and/or social life to make time for the measurements, to not feeling well enough to travel to the rehabilitation centre and perform the tests. Therefore, we have no indication whether the loss to follow-up after discharge led to a specific selection and thereby influenced the results of the study.
In conclusion, PF at discharge from inpatient rehabilitation can be used as a predictor of respiratory infection in the first year after discharge in SCI. FVC (l), FEV1 (l or % predicted) and PEF (l/sec or % predicted) are moderately accurate predictors. We recommend testing PF during primary rehabilitation, to identify persons at risk of developing RI. Not only persons with tetraplegia, but also persons with paraplegia and low PF (e.g. FEV1 < 2.5 l) should be instructed in preventive strategies and monitored after discharge for changes in pulmonary status.
Future studies should focus on decreasing the risk of long-term respiratory complications by improving PF with specific training. At present, a follow-up study of the current cohort is in progress; this study will investigate changes in PF and respiratory complications 5 years after discharge from inpatient rehabilitation.
ACKNOWLEDGEMENT
The multicentre study was supported by the Netherlands Organisation for Health Research and Development, ZON-Mw Rehabilitation program, Grant no. 1435.0003.
REFERENCES



