OBJECTIVE: To compare the effects of portable superficial warmth with transcutaneous electrical nerve stimulation on pain in patients with fibromyalgia.
METHODS: The study had a randomized cross-over design. A total of 32 patients with fibromyalgia were randomly assigned to 2 groups. After instruction, the patients treated themselves using a portable device providing superficial warmth (42°C) or a transcutaneous electrical nerve stimulation apparatus. After 3 weeks the patients switched therapy. The patients rated pain intensity on a 0–100 numerical rating scale before and after each treatment. After 6 weeks, patients were questioned concerning therapy preference.
RESULTS: There was no difference in level of pain relief when comparing the 2 treatment modes. Median pain intensity in patients using warmth therapy decreased from 77.5 on the numerical rating scale before treatment to 62.5 after treatment and in patients using transcutaneous electrical nerve stimulation from 80 to 62.5. Ten patients reported a reduction of 20 units or more on the numerical rating scale after warmth therapy, as did 10 after transcutaneous electrical nerve stimulation. Seventeen of 32 patients preferred warmth therapy and 10 preferred transcutaneous electrical nerve stimulation.
CONCLUSION: Sensory stimulation with superficial warmth or transcutaneous electrical nerve stimulation yielded comparable temporary pain reduction in patients with fibromyalgia. Both procedures are self-administered, safe and inexpensive.
Key words: fibromyalgia, heat, pain, pain alleviation, physical therapy, physiotherapy, transcutaneous electrical nerve stimulation, TENS, treatment, warmth.
J Rehabil Med 2009: 41: 557–562
Correspondence address: Monika Löfgren, Department of Rehabilitation Medicine, Danderyd University Hospital, Building 39, Floor 3, SE-182 88 Stockholm, Sweden. E-mail: monika.lofgren@ ki.se
Submitted May 14, 2008; accepted February 20, 2009
INTRODUCTION
Fibromyalgia syndrome (FM) affects approximately 2% of the population in western countries; 85% of people with FM are women. Approximately 25% of those affected recover, but the remaining 75% live with pain, fatigue and other symptoms for many years. The generalized, migrating pain and tenderness that characterize patients with FM may be explained by alterations in the central nervous system (1), but peripheral mechanisms cannot be ruled out (2). Patients with FM have abnormal pain processing, with lowered mechanical and thermal pain thresholds (3) and altered temporal summation of pain stimuli (4).
Treatment of patients with FM aims to alleviate symptoms and help patients develop strategies for managing their situation despite the pain and other symptoms. Multi-professional rehabilitation that includes exercise and cognitive behavioural therapy has significant and lasting effects for patients with FM (5). A variety of pharmacological treatments are used to treat FM pain and other symptoms; the results are varied, and adverse events have sometimes been reported (6).
Few non-pharmacological studies on FM pain have been published. To our knowledge, only 2 methods of sensory stimulation have been studied: acupuncture and massage (7–9). The advantage of sensory stimulation techniques is that they have few unwanted side-effects. Transcutaneous electrical nerve stimulation (TENS) has been used for pain relief in acute and chronic pain for many years, but there is little evidence to support its use in FM pain. TENS is used at varying frequencies, most commonly (i) a steady, high frequency between 50 and 120 Hz or (ii) bursts of a high frequency (HF) delivered at a low frequency (LF) between 1 and 4 Hz.
Although not fully understood, it is thought that HF and LF TENS alleviate pain through somewhat different mechanisms of action. Both methods are thought to activate supraspinal and spinal mechanisms (10), which are involved in pain alleviation. Following LF TENS, increased levels of β-endorphin (11) and serotonin (12) were measured in the central nervous system. To activate the supraspinal inhibitory path ways, muscle contractions are required. In patients with FM, however, isometric muscle contractions increased pressure pain sensitivity, in contrast to healthy controls, where the pressure pain sensitivity decreased (13).
HF TENS has resulted in increased levels of β-endorphin, encephalin, dynorphin (11) and γ-amino-butyric acid (GABA) (14). Furthermore, when measured immediately after HF TENS, pain thresholds in healthy female volunteers, but not in males, have been improved (15), so HF TENS might be an option for women with FM.
Another method of pain alleviation that is well tolerated and has no serious side-effects is superficial warmth (16). The mechanisms of action are largely unknown. Thermotherapy has been shown to alleviate acute and sub-acute low-back pain effectively (17, 18), and paraffin wax baths have a beneficial short-term pain-relieving effect in patients with rheumatoid arthritis (19). Various kinds of balneotherapy (20, 21) and mud packs (16) have been reported to be beneficial in patients with FM pain.
Despite the small number of studies assessing the effects of TENS on persistent pain, the method is considered to be the ”gold standard” from a physiotherapeutic point of view. To our knowledge no portable device producing superficial warmth has been evaluated.
The aim of this study was to compare the effects of a new portable device producing superficial warmth with sensory stimulation with TENS on pain in patients with fibromyalgia. Our hypothesis was that warmth would be as effective as TENS in alleviating persistent pain due to fibromyalgia.
PATIENTS AND METHODS
Design overview
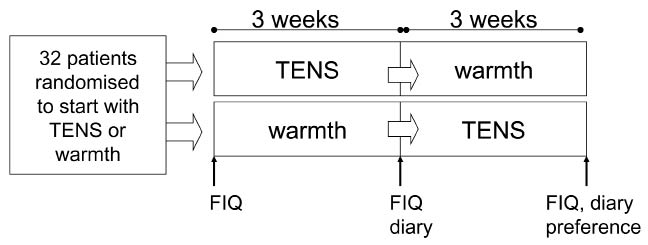
In this randomized cross-over study, patients with FM were randomized to approximately 3 weeks of TENS (n = 16) or superficial warmth stimulation (n = 16). The treatment period was then evaluated, and the patients began approximately 3 weeks of the other treatment modality, followed again by treatment evaluation (see Fig. 1). A cross-over design was chosen since we wanted to assess not only the effect on pain, but also the subjective experience of both methods. No wash-out period was included, since both treatment modalities are considered to act mainly through the gate-control mechanisms and therefore only have brief (minutes–hours) effects.
Fig. 1. Study design flow chart for collection of assessments using Fibromyalgia Impact Questionnaire (FIQ), diary and questionnaire on preferences. TENS: transcutaneous electrical nerve stimulation.
A physiotherapist (PT) at the rehabilitation department showed the patients where to place the 4 electrodes of each device and how to use the stimulator. Patients were further instructed to apply the electrodes to the areas that were most painful at each treatment session.
Patients
A total of 32 female patients with FM who were undergoing an individually based multiprofessional rehabilitation programme at a university hospital and who met the 1990 American College of Rheumatology criteria (22) participated in the study. The rehabilitation programme was based on cognitive behavioural theory and consisted of information, body awareness therapy, aerobic training, training in ergonomics, and group discussions.
Inclusion criteria were: (i) age between 18 and 60 years; (ii) no misuse of drugs; (iii) no serious psychiatric disease; and (iv) no previous experience of using TENS to alleviate musculoskeletal pain.
All participants signed an informed consent form. The Regional Ethics Approval Board in Stockholm, Sweden approved the study (no. 2006/982-31).
The participants’ mean age was 41 years (standard deviation (SD) 8.3). Mean time since diagnosis was 2.2 years (SD 4.2) and mean duration of pain or other symptoms before diagnosis 8.3 years (SD 6.8). At the time of inclusion,15 patients were working or studying: 2 patients worked full-time (40 h/week), 1 patient 75% of full-time, and 12 patients between 25% and 50% of full-time. Table I presents the patient characteristics in more depth.
| Table I. Characteristics of the 32 women included in the study |
| Age, years, median (range) | 41.5 (23–58) |
| Time since diagnosis, years, median (range) | 2.0 (0–13) |
| < 1, n (%) | 11 (34) |
| 1–2, n (%) | 16 (50) |
| 3–9, n (%) | 4 (13) |
| ≥ 10, n (%) | 1 (3) |
| Duration of symptoms, years, median (range) | 8.2 (1–26) |
| < 1, n (%) | 0 (0) |
| 1–2, n (%) | 3 (9) |
| 3–9, n (%) | 20 (63) |
| ≥ 10, n (%) | 9 (28) |
| Working status, n (%) | |
| Not working | 17 (53) |
| Working 25–50% | 12 (38) |
| Working 75–100% | 3 (9) |
| Profession, n (%) | |
| Healthcare/dental care professional | 11 (34) |
| Manager/receptionist | 6 (19) |
| Stockroom worker/cleaner/restaurant/shop | 7 (22) |
| Subway/taxi | 2 (6) |
| Birth attendant/vocational counsellor/social insurance officer/preschool teacher | 6 (19) |
| Level of education, n (%) | |
| 9-year compulsory school | 7 (22) |
| Secondary school | 21 (65) |
| University | 4 (13) |
| Married/co-habiting, n (%) | 22 (71) |
| Children, n (%) | 21 (65) |
Transcutaneous electrical nerve stimulation
The TENS apparatus used in this study (Fig. 2), a Cefar Primo stimulator (Cefar AB, Malmö, Sweden), has fixed frequencies. TENS treatment comprised HF stimulation at 80 Hz (P1) for at least 30 min. Participants were instructed to:
• Place the electrodes over painful sites and to increase the amplitude to a strong, but not unpleasant, level.
• Reduce the amplitude or move the electrodes to a less painful area if pain increased during treatment or burning or pricking sensations developed on the skin.
• Stop treatment for that session if stimulation was still painful.
• Use the TENS stimulator daily at home, at least 30 min/session and as often as needed on painful body areas.
Patients could vary stimulus duration (no less than 30 min) and intensity (amplitude) based on their experience of daily pain and treatment effect.
Portable superficial thermal stimulation
The thermal stimulator used in this study (Fig. 2) was a prototype developed by F. Nazerian (Appilox Meditech AB, Stockholm, Sweden). Intertek Semko AB, Medical Technology, Kista, Sweden was responsible for quality assurance of the device. Written instructions in Swedish were packaged with the stimulator.
Fig. 2. Stimulators for transcutaneous electrical nerve stimulation (TENS) (left) and superficial warmth (right).
The thermal stimulator produces comfortable warmth through 4 electrodes (3 × 8 cm) designed to be attached to any part of the body. Because the warmth delivered is only 40°C (SD 2°C), the stimulator can be used for several hours. The electrodes were attached to bare skin and held in place with surgical tape or tight-fitting clothes. Patients were instructed to place the electrodes on painful sites, which could vary from day to day, and to use the thermal stimulator for as long as they wished. The equipment was not fully developed at the time of the study; hence the maximum treatment time/day was 45 min to 2 h.
Data collection
All patients met a PT experienced in the alleviation of FM pain at the beginning of the study. The PT was responsible for delivering instructions, administrating the devices, and collecting data. The PT was not blinded to the therapy the patient was currently using. Patients completed the Fibromyalgia Impact Questionnaire (FIQ) (23) 3 times: (i) at base-line when the PT demonstrated the first pain alleviating method; (ii) 3 weeks later when the PT demonstrated the second pain alleviating method; and (iii) a further 3 weeks later at the conclusion of all treatment (Fig.1).
Primary outcome measure
Patients were instructed to rate pain intensity before and after treatment at all home treatment sessions on a 0–100 numerical rating scale (NRS) with the anchors “no pain” and “worst imaginable pain”. Patients were also instructed to note in their diaries how long pain reduction lasted (in minutes or hours). Diaries were collected after every 3-week period. Calculations of pain alleviation and duration of pain alleviation were based on diary ratings at the last treatment session recorded in the diaries. Patients who reported a decrease in pain intensity of 20 units or more on the NRS were considered responders, since a decrease of 2 units or more on a 0–10 NRS was found to be clinically significant (24).
Secondary outcome measure
After having completed both treatment modalities, patients completed a questionnaire on their preference of type of modality and therapy effectiveness.
Fibromyalgia Impact Questionnaire
The FIQ was developed to assess the health status of persons with FM (24). It was validated in Swedish for patients with FM (22), with an additional question on sick-leave. This study uses the English method to calculate the total score. The FIQ consists of 11 questions on physical function and 9 on how many days the patient felt good and how many days they had taken off work in the last week; the patient’s ability to work, including housework; amount of pain and fatigue; and degree of morning tiredness, stiffness, anxiety, and depression. Total score ranges from 0 to 100, with 100 indicating greatest impairment.
Because most of the patients participating in the present study were on sick-leave, the question on ability to work had a high number of non-responders; it was therefore excluded from the results and regarded as a missing value for all participants.
Statistical analysis
Data were analysed on an intention-to-treat (ITT) basis and on a per-protocol (PP) basis. Patient characteristics, such as age and time since diagnosis, are presented as mean values with SD. Ratings of pain intensity on the NRS are presented as median values with inter-quartile ranges (IQR). The Mann-Whitney U test was used to calculate between-group differences and the paired sign test within-group differences. The Mann-Whitney U test was also used to control for carry-over effects between the randomized groups. Total scores and sub-scores on the FIQ are presented as median values with IQR, and within-group differences were analysed with the paired sign test. The χ2 test was used to compare preferences between the 2 treatment modalities. Statistical analyses were carried out using Statistica 7 (StatSoft Scandinavia AB). The level of significance was set at p < 0.05.
RESULTS
Two patients dropped out of the study, i.e. did not complete either of the treatment modalities. The reasons for dropping out were unrelated to treatment.
Thirty patients completed at least one of the treatments, 28 of those completed the 3-week treatment period with superficial warmth and 29 completed the 3-week treatment period with TENS. Ratings on the NRS for the first and second treatment period are reported separately for each randomization group in Table II. No carry-over effects were detected (p = 0.812).
| Table II. Ratings of pain intensity on a numerical rating scale (NRS) before and after treatment with superficial warmth and transcutaneous electrical nerve stimulation (TENS). Data are presented in groups of randomization and treatment modality as median values with inter-quartile ranges (IQR). The difference between before and after treatment is presented for each group, with median and IQR |
| Random first modality | Modality | n | Before | After | Difference before–after |
| Median | IQR | Median | IQR | Median | IQR |
| TENS | TENS | 16 | 80 | 60–90 | 68 | 48–80 | 12 | 0–18 |
| | Warmth | 13 | 80 | 75–85 | 65 | 50–70 | 10 | 10–25 |
| Warmth | TENS | 12 | 75 | 58–85 | 58 | 30–70 | 18 | 5–22 |
| | Warmth | 11 | 75 | 50–90 | 45 | 20–75 | 20 | 10–25 |
Pain intensity and responders, intention-to-treat and per-protocol analyses
Between-group differences in pain intensity decrease were non-significant.
Twenty-four patients reported pain intensity before and after warmth therapy: median 77.5 (IQR 62.5; 85.5) and 62.5 (IQR 41; 72.5), respectively (p < 0.05). Twenty-eight patients reported pain intensity before and after TENS: median 80 (60; 90) and 62.5 (42.5; 72.5), respectively (p < 0.05).
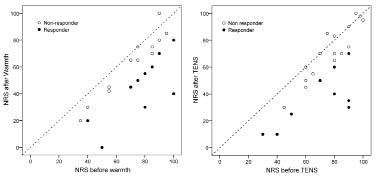
Thirty-two patients were included in the ITT analyses. Ten of these (31%) reported a reduction of 20 or more units on the NRS after warmth therapy, and 10 after TENS, and were thus considered responders (Fig. 3). In the PP analyses 42% of 24 patients using warmth therapy were responders to warmth therapy and 36% of 28 patients using TENS were responders to TENS (p = 0.66). Four patients were considered to be responders to both warmth therapy and TENS.
Fig. 3. Ratings on the numeric rating scale (NRS) before and after treatment with warmth or transcutaneous electrical nerve stimulation (TENS). Filled circles indicate responders (decrease more than 20 units on NRS), white circles indicate non-responders.
Duration of pain alleviation
No differences in duration of pain relief were reported between those receiving TENS and warmth. Mean duration of pain alleviation after warmth therapy treatment was 44 min and after treatment with TENS was 76 min.
Therapy effectiveness and treatment preference, intention-to-treat and per-protocol analyses
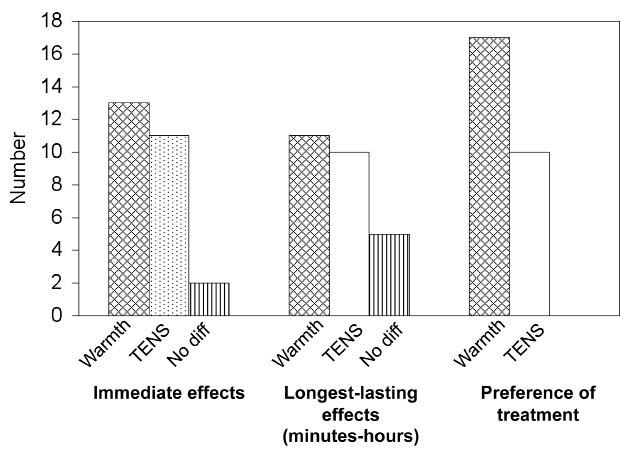
After 6 weeks, patients who had completed both therapies were asked which they would choose for continued treatment. According to the ITT analyses, 17 (53%) patients preferred warmth therapy and 10 (31%) TENS (p = 0.076) (Fig. 4). Information for one patient is missing. The PP analyses showed that 71% of the 24 patients using warmth therapy preferred warmth and 36% of the 28 patients using TENS preferred TENS (p > 0.05).
Fig. 4. Subjective reports of stimulation effectiveness and preference for treatment modality. Patients were asked to rate short- and long-term effectiveness of each treatment, as well as preference for treatment modality, after having completed both treatments. diff: difference.
At the evaluation of each treatment period patients rated the preference for each modality regarding: (i) immediate pain alleviation; and (ii) longest-lasting alleviation (min – h) (Fig. 4). According to the ITT analyses, 13 of the 32 patients (41%) included in the study reported that warmth therapy gave the best immediate pain relief and 11 (34%) TENS. Two patients found both modes equal in immediate pain relief, and one patient did not complete the questionnaire. Twenty-seven patients completed both treatments and the questionnaire.
Eleven patients (34%) reported that TENS gave the longest lasting relief and 10 (32%) warmth therapy. Five (16%) reported no difference between the therapies in long-lasting effects, and one patient did not complete the questionnaire.
Fibromyalgia Impact Questionnaire
The decrease in the “Physical function” score after warmth therapy, from a median of 6.0 to 5.4 (p < 0.05), was significant (Table III). The number of days the patients felt good tended to improve after superficial warmth therapy (median value decreased from 8.6 to 7.2 (p = 0.078)) but deteriorated after TENS (median value increased from 7.2 to 8.6 (p = 0.078)). Ratings of depression showed a strong tendency to increase after TENS (median value increased from 3.4 to 5.0 (p = 0.052)). There were no other between-group differences in the FIQ questions.
| Table III. Ratings on the Fibromyalgia Impact Questionnaire (FIQ) before and after treatment with superficial warmth (n = 26) and transcutaneous electrical nerve stimulation (TENS, n = 25). Data are presented as median values with interquartile ranges (IQR), and differences in treatment outcome are analysed with the paired sign test. The “Ability to work” item was excluded in the analyses and is not presented in this table. Only the “Physical function” item decreased significantly after superficial warmth therapy* |
| | Superficial warmth therapy | TENS |
| Before | After | p-value | Before | After | p-value |
| Median | IQR | Median | IQR | Median | IQR | Median | IQR |
| Total score | 71.5 | 58.7; 81.7 | 64.0 | 53.2; 82.2 | 0.845 | 66.2 | 52.6; 73.8 | 66.4 | 54.1; 82.0 | 1.000 |
| Physical function | 6.0 | 5.7; 7.5 | 5.4 | 5.0; 76.7 | 0.041 | 5.7 | 4.5; 6.3 | 6.0 | 5.1; 7.0 | 0.383 |
| Days felt good | 8.6 | 7.2; 10.0 | 7.2 | 5.7; 9.0 | 0.078 | 7.2 | 5.7; 10.0 | 8.6 | 5.8; 10.0 | 0.078 |
| Work missed | 10.0 | 6.1; 10.0 | 10.0 | 2.5; 10.0 | 0.687 | 10.0 | 0.0; 10.0 | 10.0 | 2.9; 10.0 | 0.250 |
| Pain | 8.0 | 6.4; 9.3 | 8.2 | 5.8; 9.0 | 0.307 | 7.0 | 5.6; 8.8 | 7.9 | 6.2; 8.8 | 1.000 |
| Fatigue | 9.2 | 8.4; 9.6 | 8.8 | 7.3; 9.6 | 0.307 | 9.0 | 7.8; 9.8 | 8.8 | 6.4; 9.4 | 0.832 |
| Morning tiredness | 9.0 | 8.4; 9.6 | 9.0 | 8.2; 9.6 | 1.000 | 8.9 | 7.8; 9.6 | 8.7 | 7.2; 9.2 | 0.832 |
| Stiffness | 8.5 | 6.3; 9.1 | 8.0 | 6.0; 9.3 | 1.000 | 8.1 | 6.8; 8.7 | 7.8 | 6.1; 8.6 | 1.000 |
| Anxiety | 5.5 | 4.0; 9.2 | 6.0 | 1.4; 8.8 | 0.541 | 5.5 | 1.4; 8.3 | 5.2 | 2.2; 8.4 | 0.678 |
| Depression | 5.0 | 2.4; 7.3 | 5.6 | 8.8; 7.6 | 0.678 | 3.4 | 0.7; 6.2 | 5.0 | 1.0; 7.6 | 0.052 |
| *26 of 28 patients completed both the period with superficial warmth and the FIQ, 25 of 29 completed both the period with TENS and the FIQ. |
DISCUSSION
Treatment with the portable superficial warmth device reduced pain as effectively as HF TENS. Both methods significantly reduced pain compared with base-line values. The number of responders to each treatment modality was the same: 10 patients (31%) reported a decrease in pain intensity of 20 units or more on the NRS following warmth therapy, as did 10 patients after TENS. Four patients were responders to both therapies. Duration of pain alleviation was greater after TENS than warmth therapy, but this difference was non-significant. After warmth therapy, the physical function score on the FIQ improved. Similar effects were also observed in the subjective reports of long-lasting and immediate effects of TENS and warmth therapy, but there was a trend, in that more patients would choose warmth therapy (53%) than TENS (31%) if they were to continue pain alleviation treatment.
Pharmacological treatment has been studied frequently in patients with FM. Tricyclic anti-depressants, serotonin and noradrenaline re-uptake inhibitors, tramadol, and pregabalin have been reported to reduce FM pain effectively (6). However, the use of these drugs is often associated with unwanted side-effects, which limits their use and results in patients discontinuing treatment.
Interest in the use of complementary treatments to treat pain in general and FM pain in particular, for which pain alleviation is unsatisfactory, is growing (6). Complementary treatment has been studied less than drugs, but low-intensity exercise has been reported to have positive results (26) and short-term pain-relieving effects have been observed after treatment with massage, ultrasound, and mineral baths (26). One study on balneotherapy and pool-based exercises reported lower pain in both groups (27) as did an exercise programme designed to enhance cardiovascular fitness, muscular endurance, and flexibility (28). In these studies, treatment compliance was high and no adverse events were reported.
Connective tissue massage increased pain during treatment but did not cause patients to drop out (7). Studies of acupuncture effects on FM pain were contradictory (8, 9, 29). One study reported increased pain as a side-effect of acupuncture, which caused participants to drop out (30). In our study, only 2 patients dropped out and did not complete either treatment; neither of these was due to unwanted side-effects. In this study few side-effects were reported. Three patients reported increased pain, 2 after TENS and one after warmth.
Our study found improvement in the physical function score on the FIQ. In other studies, the total FIQ score improved immediately after treatment with balneotherapy (16), pool-based exercise (31), connective tissue massage (7), and an exercise programme (32). The mechanism by which TENS modulates pain is known in part, but not the mechanism behind pain relief with superficial warmth. However, the pain-alleviating effects rendered by both treatments are short-lasting compared with the stimulation period, so significant changes in the FIQ total score might not have been detectable at the time of measurement. Also, our study comprised only 32 patients; a larger study with higher power might have detected differences in the FIQ total score.
No wash-out period was included in the study design. Both treatment modalities are considered to relieve pain using the gate control mechanisms, i.e. to have brief effect during and shortly after (min – h) the stimulation and therefore no carry-over effects were expected. The patients reported that the mean duration of pain alleviation after warmth therapy treatment was 44 min and after TENS, 76 min.
The treatment modalities we chose for our study are frequently used clinically with chronic-pain patients. Both types of sensory stimulation seemed to be equally effective for pain relief; but we did not control for placebo effects, so we cannot be sure that part of the measured effect was due to unspecific effects.
Also, both therapies had a similar number of responders (i.e. those who reported a decrease of at least 20 units on the NRS): TENS 34% and warmth 28%. These are slightly better results than those in a study on patients with spinal cord injury and neuropathic pain, where 21% reported a decrease of 2 units or more on the Borg CR-10 scale after TENS treatment (33). TENS has not been assessed in patients with FM, but LF TENS, was found to relieve hand pain in patients with rheumatoid arthritis (34). TENS has also been found to effectively relieve (at least peripheral) neuropathic pain; in this case, LF stimulation seems to be superior to HF, even though both have an effect on pain intensity (35). TENS effects on other chronic pain conditions are contradictory (36) and larger controlled studies are needed.
In conclusion, sensory stimulation consisting of superficial warmth or TENS stimulation yielded comparable temporary reduction of pain in patients with FM. Both procedures may be self-administered, are safe and inexpensive, and may be combined with other FM treatment.
ACKNOWLEDGEMENTS
This study was supported by the Swedish Rheumatism Association, the Department of Rehabilitation Medicine, Danderyd University Hospital and the Division of Rehabilitation Medicine, Karolinska Institutet, Department of Clinical Sciences, Danderyd Hospital, Stockholm, Sweden. The authors wish to thank Lisbet Broman for valuable help with statistical support.
REFERENCES