OBJECTIVE: Patients with chronic pain and severe disuse syndrome have pain with physiological, psychological and social adaptations. The duration and severity of complaints, combined with previously failed treatments, makes them unsuitable for treatment in primary care.
DESIGN: A prospective waiting list controlled study.
Patients: A total of 32 patients with chronic pain for at least one year and severe disuse syndrome were included in an inpatient multidisciplinary cognitive behavioural treatment.
METHODS: Patients were assessed before the waiting list period, before the clinical phase, after the clinical phase and after follow-ups of 6 months and one year. The visual analogue scale for pain and fatigue were assessed. Muscle strength of the arms and legs, arm endurance and a 6-minute walking test were used to assess physical outcome. The Symptom Checklist-90, RAND-36, pain cognition list and the Tampa scale for kinesiophobia were used to assess psychological outcome.
RESULTS: Long-term significant (p < 0.001) improvements were found for pain, fatigue, walking distance, muscle strength, anxiety, depression, somatization, negative self-efficacy, and catastrophizing in the intervention period.
CONCLUSION: An inpatient multidisciplinary cognitive behavioural programme is beneficial for patients with chronic pain and a severe disuse syndrome.
Key words: chronic pain, cognitive behavioural treatment, disuse syndrome, prospective study.
J Rehabil Med 2009; 41: 122–128
Correspondence address: C. P. van Wilgen, University Center for Sport, Exercise and Health, University Medical Center Groningen, University of Groningen, PO Box 30.001, NL-9700 RB Groningen, The Netherlands. E-mail: c.p.van.wilgen@sport.umcg.nl
Submitted December 7, 2007; accepted August 21, 2008
INTRODUCTION
Chronic pain is defined as pain without apparent biological value that has persisted beyond the normal tissue healing time (1). The concept of disuse was introduced by Bortz (2); it refers to a behavioural adaptation leading to physical inactivity. Verbunt et al. (3) later described disuse syndrome as a physiological, psychological and social adaptation to long-lasting pain. In the fear avoidance model of Vleayen & Linton (4) disuse has a prominent place in the expression of chronic pain. Some patients with chronic pain develop a severe disuse syndrome, such as sitting in a wheelchair, being restricted to bed or not being able to move a body part. In addition to the physical disuse, severe pain behaviour, such as severe medication (over)use, psychosocial dysfunction, depression, restrictions in social activities and social adaptation, such as not being able to work, are often present (3, 5). In addition, these patients do not respond to standard medical or primary care and as a result have many failed treatment experiences. The severity of the complaints and the previous failed treatments makes them unsuitable to participate in treatment groups or in an outpatient setting. These patients are regarded as very difficult to treat and largely regarded as “untreatable”. One possibility is to treat this patient group with an individually tailored inpatient cognitive behavioural treatment (CBT) programme; however, few studies have been published concerning inpatient CBT programmes.
CBT is the treatment of choice for chronic pain. It has superior effects compared with a heterogeneous collection of other treatment modes in reducing pain experience, increasing positive cognitive coping, reducing behavioural expression of pain (6, 7), reducing pain intensity, improving functioning, and reducing depression (8), and it has shown better long-term effects (9). Multidisciplinary CBT programmes for chronic pain are more successful than mono-disciplinary programmes in reducing pain and increasing functioning (10). Although CBT is often evaluated, most studies do not describe the content of the treatment (11), which makes it difficult to learn from these studies and introduce new clinical treatment strategies. No published studies were found describing this specifically difficult patient group. A few studies have been performed on inpatient programmes for chronic pain. Better long-term results for physical performance and psychological functioning for an inpatient group compared with an outpatient group, both receiving CBT, were described by Williams et al. (12). No differences between inpatient and outpatient groups were found by Altmaier et al. (13). This study, which had a 6-month follow-up, compared a standard 3-week inpatient rehabilitation programme including education, support and exercise with a standard regime combined with psychological treatment (training in relaxation and coping skills) for patients with chronic back pain. Eccleston et al. (14) described significant improvements in levels of school absenteeism and in physical and psychological outcome following a 3-week interdisciplinary cognitive behavioural inpatient programme, with a 3-month follow-up, consisting of education, activities and cognitive therapy for adolescents with chronic pain. No differences in effects were found between the inpatient and outpatient groups described by Peters & Large (15). The study, without follow-up, described a 4-week inpatient programme, a 9-week outpatient programme for patients with chronic pain, and a non-treated control group. It can be concluded that inpatient studies are scarce and the results conflicting. Most studies do not describe the content of the CBT, which makes it difficult to interpret the results.
The aim of this study was to describe an inpatient multidisciplinary CBT programme for patients with chronic pain with severe disuse syndrome, and to analyse the long-term effects of the programme on symptoms, physical outcome and psychological outcome.
METHODS
Participants
Patients were referred by their general practitioner or by a medical specialist to the multidisciplinary Pain Centre of the University Medical Centre Groningen (the Netherlands). On referral a complete medical history was requested from the patient’s general practitioner, which had to include a recent examination by a neurologist; if this was not available, the neurologist was added to the assessment. Before admission to the Pain Centre patients completed a questionnaire about personal data and an extensive pain assessment questionnaire. Assessment was performed by a physical therapist, a physician and a psychologist, following which a diagnosis was made during a consensus meeting. The physician then explained the diagnosis and the proposed treatment to the patients, i.e. chronic pain, no further medical examinations, no somatic treatment objective, treatment advice being clinical CBT. The following inclusion criteria were used for clinical CBT: chronic pain longer than one year, not suitable for treatment in primary care, a severe disuse syndrome, full agreement of the patient and the multidisciplinary team after the pre-clinical phase with the diagnosis and treatment proposal, and informed consent of the patient. The exclusion criteria were: ongoing medical treatment at the time of the study, nociceptive pain, persistent cognition of a somatic cause for pain, or requests (demands) for additional (advanced) medical diagnostics.
If the patient agreed with the diagnosis and the treatment proposal and met the inclusion criteria for clinical treatment an appointment was made with the physiotherapist and the psychologist involved in the assessment. The physiotherapist and psychologist began the pre-clinical procedure and were responsible for further treatment. For those patients who did not agree with the proposal, contact was ended and they were not registered in the study.
Programme
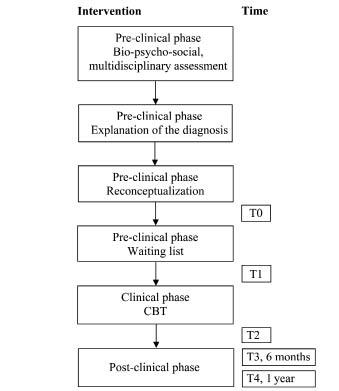
The programme was performed in 3 phases: pre-clinical, clinical and post-clinical (Fig. 1).
Fig. 1. Study overview: interventions and time of assessments. CBT: cognitive behavioural therapy.
Preclinical phase. In the preclinical phase the goal of intervention was education and to introduce the content of the clinical CBT (16). Education focuses on reconceptualization or changing the bio-medical explanation for pain into a bio-psycho-social explanation. The interaction of behavioural, psychological and social factors specific for the experience of the patient’s pain was explained. The sensitization model, which combines these factors, was often used for this explanation (17). This sensitization model explains chronic pain as the result of reactions to a physical cause of pain and its related changes in the central nervous system. This model was not used in all patients. In some patients a simpler model was used, describing the imbalance in patient’s capacities, all physical, cognitive and emotional characteristics of the patient and the patient’s load and capacity. The model that best fitted the patient, according to the multidisciplinary team members, was used. In this preclinical phase a close family member or friend of the patient was present and was also informed about the bio-psycho-social explanation of the pain. At least 3 appointments were used for education and reconceptualization and to prepare patients for the clinical phase. The number of appointments (total time) in the pre-clinical phase differed between patients. Many patients had somatic beliefs about their pain, and full agreement between the patient, the family member or friend of the patient and team members about the bio-psycho-social explanation of their pain complaints had to be reached. When agreement was achieved regarding the explanation of the pain and the purpose of treatment, patients were informed orally and in writing about the content of the clinical CBT. During this stage patients were asked to set their personal goals for the clinical programme. These goals had to be realistic for the clinical phase; physical goals were described in terms of time or distance. After setting the goals, patients were put on the waiting list. This period acted as a control period. The time they spent on the waiting list before the clinical phase depended on the availability of a hospital bed, but also on personal factors, such as family commitments, holidays, the need for extra time to prepare, etc.
Clinical phase. Individual CBT was given in the clinical phase. The duration of the clinical phase ranged from 3 to 6 weeks. The programme was run by a physiotherapist, a physician and a psychologist, all experienced in the treatment of patients with chronic pain. All team members focused on changing behaviour and cognitions related to their own professional field. For instance, the physiotherapist focused on activities, exercises, and day schedules, but also on interpretations of bodily sensations and fear of movement. The psychological treatment consisted of CBT as part of the multidisciplinary approach.
The programme was individually tailored; psychological treatment approximately 4 h/week, physiotherapy approximately 5 h/week and counselling by a physician approximately 2 h/week. Patients practised autonomously during the day according to a programme set out by the psychologist and physiotherapist. The programme consisted of several treatment modalities, including operant behavioural treatment, medication reduction, reconceptualization, bed-rest, desensitization, exercises, time-management, pacing, self-efficacy and respondent treatment, as described below.
• Operant treatment: during the clinical phase the treatment was based on operant conditioning principles (18). Healthy behaviour was positively reinforced by all team members. Physical exercises were time contingent performed and consisted of graded activity or graded exposure and functional activities.
• Medication reduction: all analgesics and pain-related drugs were reduced from the first day of admission according to a time contingent schedule. The reduction schedule was planned by the physician together with the patient, with the aim of reaching a target of no medication within 2 weeks.
• Reconceptualization: this started during the preclinical phase and was continued in the clinical phase. It involved dealing with unrealistic thoughts about bodily sensations, the use of medications, altered self-image, lack of control of movements and/or the performance of physical exercises. New coping strategies, which are based on functional cognitions, were discussed before being instructed.
• Bed-rest: although rest is known to be potentially harmful (19), especially in patients with chronic pain and severe disuse syndrome, the clinical CBT programme commenced with a 3-day rest period. Patients were allowed self-care, meals and, if they desired, smoking. The rest period was intended to decrease arousal, restore neuromuscular co-ordination and help patients to re-learn and shape normal movement by means of easy-to-perform bed exercises. This period allows exercising at a very low intensity but high frequency (every hour).
• Desensitization: the desensitization programme is a time contingent programme in which a stimulus, such as pressure or movement, which leads to a painful sensation was gradually increased. During the clinical phase desensitization was scheduled in a daily timetable. In patients with severe allodynia, desensitization started at a low intensity, with a short duration but a relatively high frequency. For example, by the patient touching the painful part with the whole hand for 1 sec every hour.
• Exercises, stretching, physical training: patients performed a graded activity programme starting with exercises that could be performed easily. The exercises were chosen by the patient and coached by the physical therapist, and were aimed at relaxation, flexibility and muscle co-ordination. Patients were taught that the exercises should be performed in a non-strenuous way and that they were intended to enable the relearning of normal movements in a smooth, relaxing and easy to perform way. When quality of movements was normalizing, the intensity and duration of the exercises was gradually increased and physical training was started consisting of cycling (on an exercise bicycle), walking or swimming.
• Time-management, pacing and self-efficacy: during the clinical phase patients were taught to use timetables, which enabled visualization of the accomplished improvements and prompt learning of pacing principles. Time management and pacing was also meant as a tool to transpose behavioural changes to the patients’ own social environment after discharge. Self-efficacy was strengthened by making timetables, by giving the patient insight that exercising is not harmful and that it is possible to control pain despite increasing exercise intensity and expanding physical activities. During the programme patients became increasingly responsible for their progress and time management.
• Respondent treatment: relaxation techniques according to Jacobson & Schultz or autogenic training were used. Exercises were supported by the use of an audio-CD. Distraction strategies were also taught.
• Participation of significant other: in the clinical phase a close family member or friend of the patient was informed about the programme and, if necessary, they were engaged in the programme. At discharge it was a requisite of the programme that the family member or friend was present at an evaluation meeting with the patient and team members.
Post-clinical phase. Before discharge a personal post-clinical programme was compiled by the patient together with the members of the multidisciplinary team. Such a programme may consist of psychological treatment, physical therapy, and/or a physical training programme. The content depended on the patient’s individual problems and goal setting. The data concerning the post-clinical phase were gathered during routine control visits to the multidisciplinary Pain Centre.
Data collection
The following data were collected from the medical records: age, gender, medication use, number of specialists consulted, duration of pain and employment status. The following symptoms were assessed: pain before and after a 6-min walking test and fatigue (0–100 visual analogue scale (VAS) on both) over the last week, as well as muscle strength, arm endurance test and a 6-min walking test. All data were gathered by the physiotherapists and analysed by the first author. Muscle strength was measured according to a standardized protocol using a hand-held dynamometer (Microfet, Hoggan Health Industries, West Jordan, USA) using the “make-method” in which patients were encouraged to produce a maximal contraction (20). The average muscle strength of each arm (biceps, triceps and 3-point grip/3) and each leg (quadriceps and hamstrings/2) was calculated. The reliability of muscle testing in patients with chronic pain depends on which muscle is tested, test modes, and standardization of the procedure (21). To determine whether a real change in outcome has occurred a change of at least the smallest detectable difference (SDD) must be met. Although no criteria for SDD for muscle strength have been described, a change of 10% of the total measured muscle strength seems acceptable (21). In the arm endurance test the time patients could hold both arms horizontally while standing was measured (maximally 3 min) (22). The reliability of this test in patients with chronic pain is unknown. In the 6-min walking test, patients were asked to walk as far as possible in 6 min. The walking distance (without walking aids) in metres was measured while walking a corridor, running was not allowed and no encouragement was offered. Walking tests have been described in patients with heart failure and chronic obstructive pulmonary disease (COPD), but are also thought to be appropriate for patients with chronic pain (23).
To assess the psychological outcome the following self-reported questionnaires, all Dutch language versions, were used: Symptom Checklist (SCL)-90, RAND-36, Pain Cognition List (PCL) and the Tampa Scale for Kinesiophobia (TSK). The SCL-90 is a multidimensional self-report inventory to assess current psychological symptoms, and yields 9 symptom domains, of which the domains anxiety, depression, somatization, hostility and quality of sleep were assessed and used in this study. Patients were asked to rate on a 5-point scale from 1 (not at all) to 5 (extreme) how much each item had distressed or bothered them during the last 7 days, including the day of the examination. The psychometric properties were found to be sufficient (24). The RAND-36 is a self-administered questionnaire measuring general health and health-related quality of life, similar to the SF-36, extended with the domain health changes. It consists of 9 domains: physical functioning, social functioning, role limitations due to physical problems, role limitations due to emotional problems, general mental health, vitality, bodily pain, general health perception, and health changes. All raw scores were converted to a 0% (poor health) to 100% (excellent health) scale. The psychometric properties of the RAND-36 were found to be sufficient (25, 26). The PCL is an inventory with 77 questions. The questionnaire consists of 5-point Likert answer category (total agreement – total disagreement). For this study the domains catastrophizing and negative self-efficacy were used. Catastrophizing reflects an extremely negative experience of pain and non-realistic beliefs. Negative self-efficacy reflects the impact of pain in daily activities. The PCL is a reliable and valid instrument in patients with chronic pain (27, 28). The TSK is a self-administered questionnaire assessing 17 items, concerning beliefs regarding the relationship between pain, activities, injuries and re-injuries (29). The total score was analysed. The psychometric properties of the TSK have been found to be sufficient (30).
Data analysis
Statistical analysis was performed using SPSS, version 14.0. Descriptive statistics were applied and, to determine the effects of the clinical CBT, a repeated measures analysis of variance (ANOVA) was performed for the waiting list period (T0), start intervention (T1) after intervention (T2), 6-month follow-up (T3) and 1-year follow-up (T4). Sphericity was assumed, if Mauchly’s W was significant the Greenhouse-Geisser adjustment was used. If a significant overall time effect was found in the analysis, a post hoc analysis of consecutive phases (T0–T1, T1–T2, T2–T3, T3–T4, T4–T5) was performed to determine in which phase (significant) change had occurred. Alpha was set at 0.001.
RESULTS
A total of 32 patients were included in the clinical CBT programme, of whom 26 (76%) completed the study. Six patients dropped out of the study; 2 because of a positive result. One of these 2 improved after the pre-clinical phase and no longer needed clinical CBT. The other patient was free of pain after CBT and was no longer willing to participate in the follow-up. Two patients dropped out during the clinical CBT due to conflict with the multidisciplinary team; one patient was in conflict about the treatment approach and the other patient demanded spinal cord stimulation after having met a patient who had received this treatment in the same department. Psychiatric problems became obvious in one patient during the clinical CBT and this patient was transferred to a psychiatric clinic. One patient received spinal cord stimulation during follow-up and was therefore excluded from the study (Table I). Prior to the study all patients had received physiotherapy, and had visited (many) medical specialists. All patients had received invasive therapy or surgery for their pain. All patients had used medication and 92% still used medication for their pain (Table I). Severe disuse was reflected by the fact that 50% used medical equipment, and in the domain limitations due to physical problems in the RAND-36 the average score was 15%. The waiting list prior to the clinical phase was, on average, 3 months (range 1–6 months); no significant changes in outcome were found during this control period. The mean duration of the clinical phase was 3.5 weeks, the duration for patients with Complex Regional Pain Syndrome Type I (CRPS-I) was 6 weeks. The outcomes for symptoms and physical functioning are shown in Table II. Pain, fatigue and the physical outcome, except arm endurance and arm muscle strength on the right side, improved significantly during treatment (Table II). Muscle strength increased more than 10% during the intervention period. Patients had a reduction in pain of 37% before the 6-min walking test and a reduction of 45% after the walking test. After one year of follow-up 2 patients had an increase in pain, 16 had a decrease and 8 patients were free of pain. Three domains of the SCL; anxiety, depression and somatization, improved significantly during the clinical phase and the effects remained stable after one year of follow-up. Hostility and sleep decreased, although not significantly, during the clinical phase and they remained lower in the year of follow-up than in the control period (Table III). The RAND-36 domains, physical functioning, general health perception and health changes improved significantly during the clinical phase. All other domains, except for social functioning, increased considerably. All 9 domains improved between T0 and T4. The mean increase in the domains of the RAND-36 was 17%. (Table III) Negative self-efficacy, catastrophizing and kinesiophobia decreased significantly during the clinical phase and remained stable in the 1-year follow-up (Table IV).
| Table I. Descriptive data of study group (n = 26) |
| Variables | |
| Age, years, mean (SD) | 42 (11) |
| Pain duration, years, mean (SD) | 8 (7) |
| Number of medical specialists consulted for this pain problem prior to consulting, before CBT, mean (SD) | 5 (2) |
| Gender, n (%) | |
| Female | 19 (73) |
| Male | 7 (27) |
| Side of pain*, n (%) |
| Back and leg pain | 10 (38) |
| Total body or body side | 5 (19) |
| Back pain and or neck pain | 4 (15) |
| Foot and leg | 2 (8) |
| Neck, shoulder and arm | 5 (20) |
| Work status, n (%) |
| Disability pension | 14 (54) |
| Housewife | 4 (15) |
| Working | 5 (19) |
| Unemployed | 1 (4) |
| Sick leave | 1 (4) |
| Retired | 1 (4) |
| Medication, n (%) |
| NSAIDs + benzodiazepines and/or tricyclic antidepressants | 11 (42) |
| NSAIDs (including paracetamol) | 6 (23) |
| Opioids + benzodiazepines or tricyclic antidepressants | 4 (15) |
| NSAIDs + opioids + benzodiazepines | 3 (12) |
| No medication | 2 (8) |
| Medical equipment used, n (%) |
| Wheelchair | 3 (12) |
| Arm-sling | 3 (12) |
| Corset/brace | 3 (12) |
| Elbow crutches | 2 (8) |
| Lying in bed | 2 (8) |
| None | 13 (50) |
| Drop-outs (n = 6), n (%) |
| After preclinical phase; no longer need for a clinical phase | 1 (4) |
| Conflicts in clinical phase and psychiatric problems | 3 (12) |
| After CBT in post-clinical phase included in SCS programme | 1 (4) |
| After CBT pain-free, no longer willing to participate in the study | 1 (4) |
| *Included 4 patients with severe CRPS I. CBT: cognitive-behavioural treatment; SD: standard deviation; NSAIDs: non-steroidal anti-inflammatory drugs; SCS: spinal cord stimulation. |
| Table II. Results of the physical outcome variables, parameters pain, fatigue, 6-minute walking test (6MWT) distance, arm endurance and strength, mean scores and standard deviations (SD) are presented, an analysis of variance (ANOVA) repeated measures was performed for the waiting list period (T0), start of intervention (T1), after intervention (T2), 6-month follow-up (T3) and 1-year follow-up (T4) |
| Physical measures | T0 Mean (SD) | T1 Mean (SD) | T2 Mean (SD) | T3 Mean (SD) | T4 Mean (SD) | p | Significant between* |
| Pain VAS (0–100) Before 6MWT Pain VAS (0–100) After 6MWT | 52 (34) 64 (32) | 60 (31) 66 (31) | 34 (27) 40 (28) | 37 (32) 41(35) | 33 (32) 35 (33) | < 0.001 < 0.001 | T1–T2 T1–T2 |
| Fatigue VAS (0–100) | 54 (20) | 52 (16) | 37 (17) | 36 (22) | 46 (24) | < 0.001 | T1–T2 |
| 6MWT, m | 346 (185) | 324 (195) | 412 (153) | 426 (152) | 492 (210) | < 0.001† | T1–T2 |
| Arm endurance, sec | 85 (76) | 85 (81) | 98 (69) | 113 (60) | 106 (65) | 0.208† | – |
| Strength Left arm Right arm Left leg Right leg | 93 (58) 109 (51) 125 (75) 106 (63) | 87 (55) 100 (53) 103 (68) 97 (72) | 101 (57) 114 (49) 137 (83) 139 (72) | 97 (55) 115 (48) 141 (68) 132 (74) | 93 (54) 114 (46) 146 (67) 133 (72) | 0.120† 0.085 0.001† < 0.001 | T1–T2 – T1–T2 |
| p: significance of the factor time. Data were checked for sphericity. *Only consecutive periods (T0–T1, T1–T2, T2–T3, T3–T4) were analysed for their significance; p < 0.001 in the post hoc analyses. †If data did not fulfil criteria for sphericity a Greenhouser Geisser correction was applied for the degrees of freedom. VAS: visual analogue scale. |
| Table III. Results of the SCL-90 and the RAND-36, mean scores and standard deviations (SD) are shown, an analysis of variance (ANOVA) repeated measures was performed for the waiting list period (T0), start intervention (T1), after intervention (T2), 6-month follow-up (T3) and 1-year follow-up (T4) |
| | T0 Mean (SD) | T1 Mean (SD) | T2 Mean (SD) | T3 Mean (SD) | T4 Mean (SD) | p | Significant between* |
| SCL-90† | | | | | | | |
| Anxiety | 17 (7) | 16 (5) | 13 (4) | 14 (5) | 13 (4) | 0.003 | – |
| Depression | 31 (14) | 31 (13) | 23 (8) | 24 (7) | 23 (6) | 0.001 | – |
| Somatization | 28 (8) | 29 (7) | 21 (6) | 23 (7) | 23 (8) | < 0.001 | T1–T2 |
| Hostility | 9 (2) | 8 (3) | 7 (1) | 8 (2) | 8 (2) | 0.004 | – |
| Sleep | 9 (3) | 10 (3) | 9 (3) | 7 (3) | 7 (2) | 0.003 | – |
| RAND-36‡ | | | | | | | |
| Physical functioning | 36 (22) | 40 (24) | 58 (26) | 57 (22) | 55 (25) | < 0.001 | T1–T2 |
| Social functioning | 54 (29) | 56 (26) | 56 (26) | 69 (28) | 67 (21) | 0.011 | – |
| Limitations due to physical problems | 15 (33) | 30 (35) | 48 (38) | 50 (36) | 45 (39) | 0.001 | – |
| Limitations due to emotional problems | 64 (44) | 68 (41) | 74 (36) | 75 (42) | 70 (41) | 0.924 | – |
| General mental health | 61 (18) | 66 (16) | 67 (18) | 71 (21) | 75 (17) | 0.019 | |
| Vitality | 45 (11) | 48 (17) | 55 (16) | 56 (17) | 55 (17) | 0.028 | – |
| Bodily pain | 37 (19) | 39 (18) | 49 (19) | 50 (20) | 51 (21) | 0.003 | – |
| General health perception | 51 (23) | 52 (17) | 68 (19) | 62 (21) | 60 (22) | < 0.001 | T1–T2 |
| Health changes | 35 (30) | 37 (26) | 65 (32) | 75 (25) | 61 (37) | < 0.001 | T1–T2 |
| p: significance of the factor time. †Lower scores indicate less psychopathology. ‡Higher scores indicate a better quality of life. *Only consecutive periods (T0–T1,T1–T2,T2–T3,T3–T4) were analysed for their significance; p < 0.001 in the post hoc analysis. |
| Table IV. Results of the Pain Cognition list and the Tampa Scale for Kinesiophobia, mean scores and standard deviations (SD) are shown, an analysis of variance (ANOVA) repeated measures was performed for the waiting list period (T0), start intervention (T1), after intervention (T2), 6-month follow-up (T3) and 1-year follow-up (T4) |
| | T0 Mean (SD) | T1 Mean (SD) | T2 Mean (SD) | T3 Mean (SD) | T4 Mean (SD) | p | Significant between* |
| Negative self-efficacy | 50 (11.8) | 54 (9.0) | 36 (9.0) | 36 (11.9) | 37 (11.0) | < 0.001 | T1–T2 |
| Catastrophizing Kinesiophobia | 50 (10.7) 38 (6.4) | 46 (12.1) 36 (7.3) | 36 (10.5) 30 (6.7) | 38 (11.8) 31 (6.7) | 38 (13.8) 32 (4.8) | < 0.001 < 0.001 | T1–T2 T1–T2 |
| p: significance of the factor time. *Only consecutive periods (T0–T1,T1–T2,T2–T3,T3–T4) were analysed for their significance p < 0.001 in the post hoc analysis. Higher scores indicate a better quality of life. |
DISCUSSION
Multidisciplinary inpatient CBT resulted in significant long-term improvements in this difficult to treat patient group. No significant changes were found during the waiting list period. Significant improvements following treatment were found in pain, fatigue, physical outcome and psychological outcome; these improvements were maintained up to one year after treatment. Improvements in physical and psychological outcome demonstrate an almost identical pattern, suggesting a strong relationship between all bio-psycho-social factors. Clinical CBT seems capable of interfering in the vicious circle of chronic pain. The effects are not only significant, but also clinically relevant, since patients gained on average 17% on the quality of life domains.
Explanation of chronic pain and reconceptualization of beliefs concerning the cause of pain were conducted before admission to the clinical CBT. This procedure might have influenced baseline assessments. Patients might have improved on the basis of this explanation, since reduction in organic-pain-beliefs is associated with improvements in disability in patients with chronic pain (16). In the explanation of chronic pain the sensitization model was often used. This model explains how pain can exist without apparent tissue damage. The explanation given to the patient was that pain is present because of changes in the nervous system. A metaphor was often used. Within the reconceptualization of beliefs, psychosocial and behavioural factors were explained as factors that enhance sensitization.
Besides the sensitization model a simpler model was used describing the imbalance in patient’s behaviour, cognitive and emotional characteristics and the patient’s load and capacity. This choice of educational model using a metaphor was used by the physiotherapist and psychologist. Choosing a model that suits the patient seems essential to enable patients to understand the explanations of pain. We did not analyse on what exact criteria the decision was made to choose a specific model or metaphor; future studies could focus on this process.
The explanation in the pre-clinical phase is, in fact, a motivational assessment. Patients who were not able to accept the explanation, or who did not accept behavioural and psychosocial interference, were not accepted for treatment. Agreement between patients and healthcare professionals concerning diagnosis and treatment is of major importance, especially in this group of patients who have had many negative experiences with former treatments and often have specific demands for treatment modalities.
Patients in the current study had severe and multifactorial problems. Most patients had (excessive) drug abuse and an inpatient programme for medication reduction had to be applied, especially in the group taking opioids and benzodiazepines. Reduction in the inappropriate use of medication, taking side-effects into account, is of importance in the treatment and is also an important reason for clinical treatment. The pain reduction found in this study is even more remarkable considering the reduction in medication.
The clinical phase started with rest. Bed-rest is described as potentially harmful for patients with chronic pain (19). The purpose of this bed-rest, however, was not pain reduction, but to change behavioural patterns and decrease arousal. This specific patient group showed disturbed quality of movements or complete non-use of a body part. Bed-rest gave patients the opportunity to restore neuromuscular co-ordination and regain normal quality of movement. Although rest is difficult at first for most of these patients it focuses them on undertaking exercise with a high frequency. Most patients had severe allodynia and hyperalgesia, and the desensitization programmes had to start at a very low intensity, short duration and with a high frequency.
Pain decreased during intervention and follow-up. CBT and operant treatment programmes in patients with chronic pain use a time contingent approach in which pain reduction is not a treatment goal. The results show that, for patients with neuropathic pain who sometimes have severe allodynia, a multidisciplinary approach can result in pain reduction, and desensitization is possible within 3–6 weeks. The advantage of a clinical programme is that patients can focus completely on their treatment; this seems to be essential, especially in the case of a desensitization programme.
Patients with severe disuse also have decreased muscle strength. Muscle strength in patients with chronic pain is associated with pain intensity (20). Arm muscle strength did not improve significantly, while leg muscle strength improved in both legs. The increase in muscle strength can be attributed to regaining muscle strength, behavioural changes, pain reduction or a learning effect. Some patients experienced a maximal muscle strength test as fearful; they fear pain or the damaging of body structures. Through a decrease in fear a learning effect could have occurred.
Psychologically these patients, according to the norm score of the patients with chronic pain on the SCL-90, score averagely, which discriminates them from the group of psychiatric patients who have much higher scores on the SCL-90 (24). CBT is one of the most extensively described and used forms of psychotherapy in several psychological disorders (31, 32). CBT for chronic pain is not a uniform treatment; it is often a combination of operant, cognitive and respondent treatment approaches, combined with physical exercises. CBT is applied as a multidisciplinary treatment, involving a psychologist, a physician, physiotherapist and/or occupational therapist. In the current study CBT consisted of psychological interventions using cognitive, operant and respondent techniques combined with physical therapy and medication reduction. The individual treatment approach for this difficult patient group demands substantial experience from all healthcare professionals involved. All team members used the same operant approach, explanations and worked from a bio-psycho-social model. The team members held a weekly patient meeting and regular informal meetings to discuss the treatment.
All patients were extremely inactive or disabled, as was reflected by the low score on the physical domains of the RAND-36 and the physical tests. Although 5 patients were working, none of them was able to have a full-time job. Half of the patients used one or more items of medical equipment. Patients improved on the RAND-36 predominantly on those domains on which they perceived the most problems prior to the CBT; physical functioning, limitations due to physical problems, vitality and bodily pain. Although patients improved, quality of life did not improve to the values of the Dutch norm population (26).
The results of our study are promising, but not all patients with chronic pain are in need of an expensive inpatient CBT. From the studies of inpatient programmes described and from our clinical impression we conclude that inpatient programmes are especially suitable for patients with severe behavioural and psychosocial adaptations and inappropriate use of medication, who are not responding to standard care. The additional value of an inpatient programme seems to be extensive counselling and motivational strategies enabling a strong treatment relationship between patients and healthcare professionals. This relationship may lead to an increase in the confidence of the patient in the therapists and the treatment approach. This confidence may be important in order to facilitate behavioural and cognitive changes and may increase patients’ self-efficacy. Having confidence in the treatment is difficult for patients with a long history of failed treatments. Another advantage of an inpatient programme is that the influence of the social environment is strongly reduced, enabling changes in behaviour and cognitions. A drawback might be that attention must be given to the generalization of patient’s behaviour in his own social environment. Relapse prevention after the clinical phase must be encouraged by involving a significant other, and allowing weekend leaves with behavioural experiments during the CBT.
Strengths of this study were that it had follow-ups of 6 months and one year and multimodal outcome variables were assessed, both questionnaires and physical measurements. The measurements during follow-up at 6 months were chosen in order to monitor behavioural changes and changes in symptoms in the year after the clinical phase. Positive effects were found on almost all outcome variables, symptoms, physical outcome as well as psychological outcome after the intervention. The effects were maintained during the 1-year follow-up. It therefore seems unlikely that these effects are non-specific effects of the intervention. Patient files were analysed retrospectively and it was found that the majority of the patients reduced their pain medication; this should be investigated in more detail in further studies, in addition to study of the side-effects of a detoxification programme.
Weaknesses of the study were the limited number of patients with severe behavioural adaptations who were motivated to undergo CBT. In the current study regular care was evaluated in a pre–post design. Patients were selected on clinical criteria and not on strict research criteria. Unfortunately, the number and clinical characteristics of the patients who did not accept the bio- psycho-social explanation of their complaints was not recorded.
In conclusion, clinical multidisciplinary CBT is successful for patients with chronic pain with a severe disuse syndrome. This individual clinical approach breaks the vicious bio-psycho-social circle of chronic pain.
REFERENCES
1. Merskey H, Bogduk N, editors. Classification of chronic pain; descriptions of chronic pain syndromes and definitions of pain terms, 2nd edn. International Association for the Study of Pain. Seattle: IASP Press; 1994.
2. Bortz WM. The disuse syndrome. West J Med 1984; 141: 691–694.
3. Verbunt JA, Seelen HA, Vleayen JW, van de Heijden GJ, Heuts PH, Pons K, et al. Disuse and deconditioning in chronic low back pain: concepts and hypotheses on contributing mechanisms. Eur J Pain 2003; 7: 9–21.
4. Vlaeyen JWS, Linton SJ. Fear avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 2000; 85: 317–332.
5. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007; 133: 581–624.
6. Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999; 80: 1–13.
7. Hoffman BM, Papas RK, Chatkoff DK, Kerns RD. Meta analysis of psychological interventions for chronic low back pain. Health Psychol 2007; 26: 1–9.
8. Turner JA, Mancl L, Aaron LA. Short- and long-term efficacy of brief cognitive behavioural therapy for patients with chronic temporomandibular disorder pain: a randomized, controlled trail. Pain 2006; 121: 181–194.
9. Williams A. Cognitive behavioural treatment. In: Dostrovsky JO, Carr DB, Koltzenburg M, editors. Proceedings of the 10th world congress on pain. Seattle: IASP Press; 2003, p. 825–837.
10. Guzman J, Esmail R, Karjalainen K, Guzman J, Esmail R, Karjalainen K. Multidisciplinary rehabilitation for chronic low back pain: systematic review. BMJ 2001; 322: 1511–1516.
11. Tulder van MW, Ostelo R, Vlaeyen JWS, Tulder van MW, Ostelo R, Vlaeyen JWS. Behavioural treatment for chronic low back pain: a systematic review within the framework of the Cochrane Back Review Group. Spine 2000; 25: 2688–2699.
12. Williams AC, Richardson PH, Nicholas MK, Pither CE, Harding VR, Ridout KL, et al. Inpatient vs. outpatient pain management: results of a randomised controlled trial. Pain 1996; 66: 13–22.
13. Altmeier EM, Lehmann TR, Russel DW, Weinstein JN, Kao CF. The effectiveness of psychological interventions for the rehabilitation of low back pain: a randomized controlled trail evaluation. Pain 1992; 49: 329–335.
14. Eccleston C, Malleson PN, Clinch J, Connel H, Sourbut C. Chronic pain in adolescents: evaluation of a programme of interdisciplinary cognitive behaviour therapy. Arch Dis Child 2003; 88: 881–885.
15. Peters JL, Large RG. A randomised control trail evaluating in- and outpatient pain management programmes. Pain 1990; 41: 283–293.
16. Walsh DA, Radcliffe JC. Pain beliefs and perceived physical disability of patients with chronic low back pain. Pain 2002; 97: 23–31.
17. Wilgen van CP, Keizer D. The sensitization model; a method to explain chronic pain to a patient [Het sensitisatiemodel: een methode om een patiënt uit te leggen wat chronische pijn is]. Ned Tijdschr Geneeskd 2004; 148: 2535–2538 (in Dutch).
18. Fordyce WE, editor. Behavioural methods for chronic pain and illness. St Louis, MO: Mosby; 1976.
19. Allen C, Glasziou P, Del Mar C. Bed rest: a potentially harmful treatment needing more careful evaluation. Lancet 1999; 9: 1229–1233.
20. Wilgen van CP, Akkerman L, Wieringa J, Dijkstra PU. Muscle strength in patients with chronic pain, Clin Rehabil 2003; 17: 885–889.
21. Smidt N, van der Windt DA, Assendelft WJ, Mourits AJ, Devillé WL, de Winter AF, et al. Interobserver reproducibility of the assessment of severity of complaints, grip strength, and pressure pain threshold in patients with lateral epicondylitis. Arch Phys Med Rehabil 2002; 83: 1145–1150.
22. Harding VR, Williams AC, Richardson PH, Nicholas MK, Jackson JL, Richardson IH, et al. The development of a battery of measures for assessing physical functioning of chronic pain patients. Pain 1994; 58: 367–375.
23. Pankoff BA, Overend TJ, Lucy SD, White KP. Reliability of the six-minutes walk test in people with fibromyalgia. Arthritis Care Res 2000; 13; 291–295.
24. Arrindel WA, Ettema JHM, editors. SCL-90: manual of a multidimensional psychopathology indicator. Lisse: Swets & Zeitlinger; 2003.
25. Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, et al. Translation, validation and norming of the Dutch language version of the SF-36 Health survey in community and chronic diseases populations. J Clin Epidemiol 1998; 51: 1055–1068.
26. van der Zee KI, Sanderman R, editors. RAND-36 manual. Groningen: Northern Centre for Health Care Research; 1993.
27. Vlaeyen JWS, Geurts SM, Kole-Snijders AMJ, Schuerman JA, Groenman NH, Eek van H. What do chronic pain patients think of their pain? Towards a cognition questionnaire. Br J Clin Psychology 1990; 29: 383–394.
28. Vlaeyen JWS, Geurts SM, Eek van H, Snijders AM, Schuerman JA, Groenman NH, editors. Pain cognition list manual. Lisse: Swets & Zeitlinger; 2000.
29. Vlaeyen JWS, Kole-Snijders AMJ, Rotteveel AM, Ruesink R, Heuts PHT. The role of fear of movement/(re)injury in pain disability. J Occup Rehab 1995; 5: 235–252.
30. Roelofs J, Goubert L, Peters ML, Vlaeyen JWS, Crombez G. Tampa scale for kinesiophobia: further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. Eur J Pain 2004; 8: 495–502.
31. Butler CB, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioural therapy: a review of meta analyses. Clin Psychol Rev 2006; 26: 17–31.
32. Wessely S, Nimnuan C, Sharp M. Functional syndromes: one or many? Lancet 1999; 354: 936–939.