Lynne Turner-Stokes, DM, FRCP
From the School of Medicine, Department of Palliative Care, Policy and Rehabilitation, King’s College London, London, UK
Lynne Turner-Stokes, DM, FRCP
From the School of Medicine, Department of Palliative Care, Policy and Rehabilitation, King’s College London, London, UK
OBJECTIVE: To assimilate the published evidence for the effectiveness of multidisciplinary rehabilitation following acquired brain injury in adults of working age.
DESIGN: The evidence derived from 2 contrasting approaches to systematic evaluation of the published literature is compared.
METHODS: A synthesis of best evidence compiled from a Cochrane Review of randomized controlled trials is compared with literature assembled for the UK National Service Framework for long-term neurological conditions, using a new typology based on evaluation of research quality irrespective of study design.
RESULTS: The trial-based studies provided “strong evidence” that more intensive programmes are associated with more rapid functional gains, and “moderate evidence” that continued outpatient therapy can help to sustain gains made in early post-acute rehabilitation. However, they failed to address the impact of early or late rehabilitation, the effect of specialist programmes (e.g. vocational or neuro-behavioural rehabilitation), or cost-effectiveness. In contrast, the non-trial-based studies provided strong evidence in all these areas, as well as evidence for the cost-benefits of rehabilitation.
CONCLUSION: There is now a substantial body of high-quality research evidence for the effectiveness, and indeed the cost-effectiveness, of rehabilitation. This review highlights the importance of looking beyond the somewhat restrictive set of trial-based evidence.
Key words: systematic review, rehabilitation, brain injuries, effectiveness, cost-benefits.
J Rehabil Med 2008; 40: 691–701
Correspondence address: Lynne Turner-Stokes, Regional Rehabilitation Unit, Northwick Park Hospital, Watford Road, Harrow, Middlesex, HA1 3UJ, UK. E-mail: lynne.turner-stokes @dial.pipex.com
Submitted May 26, 2008; accepted August 21, 2008
INTRODUCTION
Few would now dispute the need to gather robust evidence to inform best clinical practice. Questions remain, however, about how this should be done – what sort of evidence should be taken into account, and how it should be assimilated.
Rehabilitation following acquired brain injury (ABI) is a complex intervention. It poses several major challenges for clinical research that tend to confound traditional randomized controlled trial designs.
• The numbers are comparatively small, and there is marked heterogeneity with respect to the patient group, the intervention and setting. Also to the outcomes that are relevant at each stage of recovery.
• There are often ethical considerations, as many patients with ABI may lack the mental capacity to give fully informed consent to participate in research. Moreover, the expanding body of evidence for effectiveness of multi-disciplinary rehabilitation in many conditions (particularly stroke) makes it increasingly unethical to randomize patients to "no treatment" or even "standard" care.
• The timescale over which rehabilitation may have its effects (often months or years) is usually longer than any funded research project and hinders the use of "wait-list" control groups.
The Cochrane Library is widely cited as a source of robust systematic reviews and research syntheses that draw together the evidence available from randomized controlled clinical trials (RCTs), tested further by meta-analysis. Although there is a reasonably strong evidence base for the effectiveness of brain injury rehabilitation using this methodology (1), it is increasingly recognized that RCTs cannot be applied to address all the questions that need to be answered (2).
Other methods have been developed for assimilating published literature to include a broader range of "evidence". These encompass other research designs, qualitative studies and different techniques that allow the evaluation of individual experience in addition to controlled experimental data. One such method is the research typology that was developed for the UK National Service Framework (NSF) for Long Term Neurological Conditions (3) and used to evaluate the evidence base that was assembled to underpin the NSF standards (4).
This article will briefly review the evidence base for rehabilitation in ABI (of any cause) in working-age adults, and discuss the different information that derives from these 2 sources, to examine the strength of recommendations that can be made with respect to clinical management, based on the current evidence for benefits and cost-effectiveness of intervention.
RCT-BASED EVIDENCE – THE COCHRANE APPROACH
A Cochrane Review entitled “Multi-disciplinary rehabilitation for acquired brain injury in adults of working age” (1) was first published in 2005, and is currently in the process of being updated. Its focus was on adults of working age, to reflect the principal case-load of specialist neurorehabilitation services in the UK. “Multidisciplinary” rehabilitation was defined as intervention from at least 2 disciplines. Because brain injury rehabilitation services are increasingly defined by the needs of patients, rather than by the underlying pathology (i.e. disease or diagnosis), the review took a broad approach to the definition of “acquired brain injury” to include all causes (vascular, traumatic, inflammatory, toxic anoxic, etc.). It also took an inclusive approach to trial design – including all RCTs and also quasi-randomized and quasi-experimental designs, providing they met the quality criteria. Full details of the search strategy and methodology may be found in the review (1).
The review sought to address the following specific questions:
• Does organized multi-disciplinary rehabilitation achieve better outcomes than the absence of such services for this group of patients?
• Does a greater intensity (time and/or expertise) of rehabilitation lead to greater gains?
• Which type of programmes are effective and in which setting?
• Which specific outcomes are influenced (dependency, social integration, mood, return to work, etc.)?
• Are there demonstrable cost-benefits of multi-disciplinary rehabilitation?
It was anticipated that the trials would be heterogeneous with respect to patient group, trial design and outcomes measured, and that pooling of data for meta-analysis would not be possible. This indeed proved to be the case. Instead, the review took a rigorous approach to the evaluation of trial quality, and performed a synthesis of best evidence according to methods described by van Tulder and colleagues in the Cochrane Back Review Group (5).
From an initial list of over 2300 articles, 14 trials were initially identified that met the criteria for selection: 10 were of good methodological quality and a further 4 of lower quality. A further trial and an update report have been added so far in the recent update, which is still on-going. The principal characteristics of the trials are listed in Table I.
| Table I. Trials included in the Cochrane Review: multidisciplinary rehabilitation following acquired brain injury in adults of working age | ||||
| Authors | Design Trial numbers | Treatment | Control | Quality score |
| Trials of rehabilitation in the milder ambulatory group (n = 1330) | ||||
| Wade et al. 1997 (7) All severities | Single blind RCT n = 478 | Advice + Treatment as needed | Standard services | 14 |
| Wade et al. 1998 (6) All severities | Single blind RCT n = 218 | Advice + Treatment as needed | Standard services | 14 |
| Paniak et al. 1998/2000 (9, 10) Moderate to severe | Single blind RCT n = 119 | Advice + Treatment as needed | Information only | 15 |
| Salazar et al. 2000 (8) Moderate to severe | Unblinded RCT n = 120 | Intensive 8-week programme | Telephone advice only | 14 |
| Elgmark et al. 2007 (11) Mild | Single blind RCT n = 395 | Advice + Treatment as needed | Standard services | 13 |
| Trials of outpatient (OP) rehabilitation programmes (n = 182) | ||||
| Smith et al. 1981 (12) Stroke | Unblinded RCT n = 133 | OP physio and O/T 6 months (2 levels of intensity) | Self exercise at home | 14 |
| Werner & Kessler 1996 (13) Stroke at least one year on | Single blind, Quasi RCT n = 49 | OP physio and O/T 3 months | No treatment | 9 |
| Trials of community multi-disciplinary (MD) rehabilitation programmes (n = 207) | ||||
| Powell et al. 2002 (14) Moderate to severe TBI | Single blind RCT n = 111 | Outreach MD team 6 months 2 visits/week | Written information only | 14 |
| Bowen et al. 2001 (15) Carers of TBI patients | Unblinded, Quasi RCT n = 96 | Head Injury Neuro-rehabilitation team | Standard services | 11 |
| Trials of inpatient specialist rehabilitation programmes (n = 111) | ||||
| Semlyen et al. 1998 (16) TBI | Unblinded, Quasi-experimental n = 51 | Specialist brain injury rehabilitation | Other rehabilitation local district services | 9 |
| Ozedemir et al. 2001 (17) Stroke | Unblinded, Quasi RCT n = 60 | Inpatient programme | Home exercise | 9 |
| Trials of intensity of rehabilitation (n = 381) | ||||
| Kwakkel et al. 1999 (18) Stroke | Single blind RCT n = 101 | Intensive arm/leg training | Inflatable splint | 16 |
| Shiel et al. 2001 (19) TBI | Unblinded RCT n = 51 | Added intensity rehabilitation | Standard regimen | 12 |
| Slade et al. 2002 (20) Mixed ABI | Single blind RCT n = 161 | Added intensity rehabilitation | Standard regimen | 14 |
| Zhu et al. 2001 and 2007 (21, 22) TBI | Single blind RCT n = 68 | Added intensity rehabilitation | Standard regimen | 15 |
| RCT: randomized controlled trial; OP: outpatient; O/T: occupational therapy; physio: physiotherapy; MD: multidisciplinary; TBI: traumatic brain injury; ABI: acquired brain injury of any cause. | ||||
WHAT DOES THIS SYNTHESIS OF TRIALS TELL US?
Five trials (6–11) (with a total of 1330 subjects) were primarily concerned with outcomes at the level of participation in ambulatory patients with mild traumatic brain injury. From these trials, there was “strong evidence” to suggest that the majority of patients make a good recovery with the provision of appropriate information, but without the need for any additional specific intervention. However, within the sub-group with moderate to severe injury (Post-Traumatic Amnesia (PTA) > 1 h < 7 days), there was “strong evidence” for benefit from formal intervention, and also evidence that they may not present themselves for rehabilitation unless routine follow-up after the acute phase is provided.
The other 10 trials enrolled patients already presenting to rehabilitation. This was therefore a more severely damaged population, and the outcomes tended to be focused on reducing disability. Six trials focused on 3 different models of rehabilitation: outpatient rehabilitation (2 trials; total n = 182) (12, 13); community multidisciplinary team approaches (2 trials; total n = 207) (14, 15); and specialist inpatient rehabilitation (2 trials; total n = 111) (16, 17). The remaining 5 trials (total n = 381) (18–22) addressed the benefits of increased intensity of rehabilitation. From these there was:
• "strong evidence" that more intensive rehabilitation programmes are associated with more rapid function gains, once patients are fit to engage – with no evidence of a ceiling effect in therapeutic intensity;
• "moderate evidence" that outpatient therapy improves functional gain, with "limited evidence" that more intensive treatment regimens are associated with better outcomes;
• "limited evidence" that specialist inpatient rehabilitation and/or specialist multi-disciplinary community rehabilitation may provide additional functional gains and reduce carer distress;
• "indicative evidence" (from 1 outpatient study) that rehabilitation may be effective more than 1 year after the onset of brain injury.
No trial-based evidence could be found to confirm or refute the cost-effectiveness of rehabilitation. None of the studies undertook a direct analysis of cost-effectiveness, and although there was "moderate evidence" that more intensive rehabilitation leads to reduced length of stay, this was frequently affected by external confounders (such as the lack of a suitable place to discharge the patient to, or lack of community support for a patient otherwise ready for discharge).
There were many methodological challenges – in particular with regard to heterogeneity. Worthy attempts to increase the population base through multi-centre collaboration were thwarted by unanticipated differences in practice and population, which limited the assimilation of data. The trials also served to highlight the practical and ethical restraints on randomization of severely affected individuals for whom there are no realistic alternatives to specialist intervention. These will continue to impose limitations on the application of traditional research methodologies in this particular group of patients, and there is therefore a need to explore and understand the literature from other research designs.
THE EVIDENCE FROM THE NATIONAL SERVICE FRAMEWORK TYPOLOGY
Within the UK National Health Service, a series of National Service Frameworks (NSFs) have been developed since 2001 to define clear standards and targets for implementation of evidence-based practice. The NSF for Long Term Neurological Conditions (3) took a highly person-centred approach to setting standards for life-long care from diagnosis to death. A new typology of evidence was developed to underpin these standards (4). The typology places value on the experience of individuals and their family who live with a long-term condition, by including the expert opinion of users/carers and professionals – expressed through consultation or consensus processes – alongside evidence gathered through formal research. Its evaluation of research evidence focuses on the quality of research, and the appropriateness of research design to answer the question in hand, as opposed to restricting evidence to any single type of design. Importantly the quality assessment is designed to be applicable across both quantitative and qualitative research designs, and to be simple – so that it may be applied by any clinician seeking to gather evidence within the context of clinical practice.
Each piece of research-based evidence is awarded a rating based on 3 categorizations: design, quality and applicability.
• Research design is categorized as shown in Table II.
| Table III. Evidence for rehabilitation assimilated according to the National Service Framework typology | ||||
| Authors | Design | Programme | Outcomes | Quality Score |
| Early and post-acute rehabilitation (n = 3780) | ||||
| Cope & Hall 1982 (23) TBI | Cohort analysis n = 36 | Early rehabilitation (< 35 days) vs late (> 35 days) | Reduced length of stay and morbidity in early group | 6 P1 Medium direct |
| Mackay et al. 1992 (24) TBI | Cohort analysis n = 36 | Admissions receiving earlier formalized rehabilitation vs those with standard care | Reduced length of stay and better cognitive outcomes in early group | 7 P1 High direct |
| Khan et al. 2002 (25) TBI | Cohort analysis n = 1875 | Retrospective comparison of performance before and after introduction of an integrated TBI programme in a level 1 trauma centre | Length of stay reduced from 30 days to 12.5 days with total cost savings of $21.8 million over 6 years | 6 P1 Medium direct |
| Musicco et al. 2003 (26) Stroke | Cohort analysis n = 1716 | Early rehabilitation (< 7 days) vs late (delayed > 1 month) | Improved return to independence (FIM) in early group | 9 P1 High direct |
| Engberg et al. 2006 (27) Severe TBI | Cohort analysis n = 117 | Centralized early subacute rehabilitation. vs pre-centralization | Improved outcomes after centralization (GOS) | 6 P1 Medium direct |
| Specialist inpatient rehabilitation for severe or very severe (n = 963) | ||||
| Cope et al. 1991 (28) TBI | Cohort analysis n = 145 | Specialist inpatient MD rehabilitation Comparison of groups of severity for cost-efficiency | Reduction in length of stay and long-term care needs and costs | 5 P1 Medium direct |
| Spivack et al. 1992 (29) TBI | Cohort analysis n = 95 | Specialist inpatient MD rehabilitation for “catastrophic TBI” | More disabled patients had longer lengths of stay and required more intensive rehabilitation but crossed over to reach higher outcomes | 7 P1 High direct |
| Whitlock 1992 (30) TBI | Cohort analysis n = 23 | Specialist inpatient MD rehabilitation for “very severe TBI” – the group “regarded by many physicians as beyond hope” | One-third achieved “good” to ‘moderate” outcomes on the GOS, and half were discharged home | 7 P1 High direct |
| Semlyen et al. 1998 (16) TBI | Quasi experimental n = 51 | Specialist inpatient MD rehabilitation vs standard care | Improved gains in independence evident up to one year | 7 P1 High direct |
| Gray 2000 (31) ABI | Cohort analysis n = 349 | Specialist inpatient MD rehabilitation for patients not responding to standard programmes | Significant functional gains (FIM+FAM) were still made | 8 P1 High direct |
| Turner-Stokes et al. 2006 and 2007 (32, 33) ABI | Cohort analysis n = 297 | Specialist inpatient MD rehabilitation for complex brain injury Comparison of groups of different severity for cost-efficiency | Rehabilitation cost effective in reducing long-term care costs, especially in highly dependent group with longer lengths of stay | 9 P1 High direct |
| Behaviour modification programmes (n = 140) | ||||
| Eames et al. 1995 (34) TBI | Cohort analysis n = 64 | Inpatient behavioural modification programme At least one year post-discharge | Improved functional skills an social behaviour | 7 P1 High direct |
| Wood et al. 1999 (35) TBI | Cohort analysis n = 76 | Community-based post-acute neuro-behavioural programme. At least one year post-discharge | Improved social activity, reduced needs for support with savings in ongoing cost of care | 8 P1 High direct |
| Residential programmes – transitional living units (TLU) (n = 105) | ||||
| Johnston 1991 (36) TBI | Cohort analysis n = 82 | Comprehensive TLU one year follow-up | Reduced institutionalization and supervision, increased employment | 8 P1 High direct |
| Harrick et al. 1994 (37) TBI | Longitudinal cohort n = 21 | Comprehensive TLU one and 3 year follow-up | Benefits from Johnston 1991 study maintained at 3 years | 6 P1 Medium direct |
| Willer et al. 1999 (38) TBI | CCT matched case design n = 23 | Residential community re-entry programme vs standard home care | Gains in motor skills and cognitive abilities | 6 P1 Medium direct |
| Day centre programmes (n = 280) | ||||
| Klonoff et al. 2001 (39) (Prigatano) TBI | Longitudinal cohort n = 145 | Comprehensive Day treatment programme 11 years follow-up | 67% in employment. No decline in productivity since discharge | 9 P1 High direct |
| Malec 2001 (40) TBI | Longitudinal cohort n = 96 | Comprehensive Day treatment programme one year follow-up | Reduction in unemployment and need for supervision sustained at one year | 6 P1 Medium direct |
| Sarajuuri et al. 2005 (41) TBI | Non-randomized CCT n = 39 | Comprehensive Day treatment programme 2 year follow-up | Improved productivity in the intervention group (89%) compared with controls (55%) at 2 years follow-up | 5 P1 Medium direct |
| Table III contd | ||||
| Authors | Design | Programme | Outcomes | Quality Score |
| Outpatient programmes (n = 162) | ||||
| Prigatano et al. 1984 (42) TBI | Quasi-experimental study n = 35 | Co-ordinated MD neuropsychological programme | Improved productivity and reduced emotional distress in intervention group at discharge from programme | 8 P1 High direct |
| Malec et al. 1993 (43) TBI | Longitudinal cohort analysis n = 29 | Outpatient group-based MD rehabilitation one year follow-up | Reduction in unemployment and need for supervision sustained at one year | 6 P1 Medium direct |
| Ben-Yishay et al. 1987 (44) TBI | Cohort analysis n = 94 | Outpatient group-based holistic MD rehabilitation | Improved productivity and return to competitive employment | 8 P1 High direct |
| Late rehabilitation (n = 506) | ||||
| Tuel et al. 1992 (45) TBI | Cohort analysis n = 49 | Late inpatient rehabilitation At least one year post-injury | Significant gains in independence (BI) for about half the patients | 4 P1 Medium direct |
| Gray & Burnham 2000 (46) TBI | Cohort analysis n = 349 | Inpatient rehabilitation Mean 1.5 years post-injury | Significant gains in independence (FIM+FAM) | 8 P1 High Direct |
| Malec 2001 (40) TBI | Cohort analysis n = 60 (of 96 in full study) | Day centre rehabilitation: Time since injury ranging from 1 to > 10 years | Reduction in unemployment and need for supervision sustained at one year | 10 P1 High direct |
| Powell et al. 2002 (14) TBI | Cohort analysis (from an RCT) n = 48 | Home-based MD rehabilitation Mean 4 years since injury | Gain in independence (BI) and community integration (BICRO-39) | 10 P1 High direct |
| Vocational rehabilitation: Specialist Work Support programmes (n = 433) | ||||
| Abrams et al. 1993 (47) TBI | Cohort analysis n = 142 | Supported work programme 6 month follow-up Cost-benefit analysis | Improved return to work with overall gain to taxpayers | 8 P1 High direct |
| Wehman et al. 2003 (48) TBI | Longitudinal prospective cohort analysis n = 59 | Supported work programme Cost-benefit analysis | Improved return to work with calculated cost-benefits | 8 P1 High direct |
| Murphy et al. 2006 (49) Mixed ABI | Cohort analysis n = 232 | Supported work programme Evaluation at exit of programme | Achieved 72% productivity – 41% in paid employment, 31% voluntary or education | 6 P1 Medium direct |
| Longer term outcomes (n = 256) | ||||
| Olver et al. 1996 (50) TBI | Longitudinal prospective cohort analysis. Follow-up study at 2–5 years n = 103 | Inter-disciplinary inpatient programme with outpatient follow-up to assist maximal community re-integration | Between 2 and 5 years, continue to increase independence in personal, domestic and community ADL. But one-third of patients employed at 2 years were unemployed at 5 years | 8 P1 High direct |
| Hoofien et al. 2001 (51) TBI | Cohort analysis (follow-up study) mean 14 years post-injury n = 76 | Patients discharged from national institute of rehabilitation, followed up through structured interviews and neuropsychological tests | TBI had substantial impact on psychiatric symptomatology, family and social domains, compared with only moderate influence on cognitive functioning and independence | 7 P1 High direct |
| Possl et al. 2001 (52) TBI | Cohort analysis (follow-up study) 7–8 years post-injury n = 43 | Comprehensive rehabilitation programme with specific emphasis on vocational re-entry | Mixed outcomes. One-third reported stable work retention at the pre-morbid level, but 16% at a lower level, and 19% had persistent difficulties. | 7 P3 High direct |
| Sander et al. 2001 (53) TBI | Longitudinal prospective cohort analysis. Follow-up at one year and at 2–5 years n = 34 | Inpatient post-acute rehabilitation programme | Gains in independence generally maintained but some drop-off of employment between years 1 and 5 | 7 P1 High direct |
| CCT: controlled clinical trial; MD: multidisciplinary; TLU: Transitional Living Unit; FIM: Function Independence Measure; FIM+FAM: Functional Assessment Measure, BI: Barthel Index; BICRO: Brain Injury Community Rehabilitation Outcome Scale; TBI: traumatic brain injury; ABI: acquired brain injury of any cause; ADL: activities of daily living; GOS: Glasgow Outcome Score; P1: Primary research using quantitative approaches; P3: Primary research using mixed methods (qualitative and quantitative). | ||||
• Quality rating is based on the 5 quality items shown in Table II. “High quality” research studies are those which score at least 7/10; “medium quality” studies score 4–6/10 and “poor quality” studies score 3/10 or less.
• Applicability is determined by whether the research was derived directly from the population of people with long-term neurological conditions (direct evidence) or extrapolated from other conditions (indirect evidence).
In this way, each study carries a typology and quality rating (e.g. P1 High Direct – meaning a high quality quantitative study of direct applicability). Synthesis of research evidence is then achieved by combining the relevant studies according to Table II – grade A being "strong" evidence, grade B "moderate" and grade C "limited" evidence. The typology was refined and evaluated as part of the development of the NSF. Inter-rater reliability was shown to be acceptable through independent quality ratings (4).
| Table II. National Service Framework (NSF): research design, quality rating and grades of evidence | |||
| Categories of research design within the NSF Typology | |||
| Primary Research-based Evidence | |||
| P1 | Primary research using quantitative approaches | ||
| P2 | Primary research using qualitative approaches | ||
| P3 | Primary research using mixed methods (qualitative and quantitative) | ||
| Secondary Research-based Evidence | |||
| S1 | Meta-analysis of existing data analysis | ||
| S2 | Secondary analysis of existing data. | ||
| Review-based Evidence | |||
| R1 | Systematic reviews of existing research; | ||
| R2 | Descriptive or summary reviews of existing research | ||
| Quality rating within the NSF Typology | |||
| Quality Criteria | |||
| Are the research question/aims and design clearly stated? | |||
| Is the research design appropriate for the aims and objectives of the research? | |||
| Are the methods clearly described? | |||
| Is the data adequate to support the authors’ interpretations/ conclusions? | |||
| Are the results generalizable? | |||
| Total score = max 10 | |||
| Each quality item is scored as follows: 2 = Yes, 1 = In part, 0 = No. | |||
| Grades of evidence for the NSF Typology | |||
| Research Grade A: | • More than one study of high quality score (≥ 7/10) and • At least one of these has direct applicability | ||
| Research Grade B: | • One high quality study or • More than one medium quality study (4–6/10) and • At least one of these has direct applicability Or • More than one study of high quality score (≥ 7/10) of indirect applicability | ||
| Research Grade C: | • One medium quality study (4–6/10) or • Lower quality (2–3/10) studies or • Indirect studies only | ||
The synthesis of research evidence that was used to underpin the NSF standards was based on an extensive literature search by the NSF Research and Evidence group, and 2 dedicated researchers. Instead of a one-time single search strategy, this synthesis included a broad-based, multi-source search covering research databases representing both medical and social sciences literature. It drew on reference lists and the knowledge of the expert working group to cover evidence across the range of long-term neurological conditions, and was revisited on a number of occasions over several years. Due to space limitations in the NSF, the intention was not to provide an exhaustive list of articles, but to select the best quality evidence available. All identified articles were subjected to independent evaluation by at least 2 researchers to create a synthesis of best evidence for each standard, based on the NSF typology (4). From a total set of 304 selected articles covering the 11 NSF quality requirements, 26 high- and medium-quality non-RCT studies (mainly cohort analyses) relating to multidisciplinary rehabilitation of working-aged adults following ABI were included in the original synthesis. This set has subsequently been updated through a search strategy based on the Cochrane review strategy (minus the design qualifiers) to include 5 further studies of high or medium quality. Low quality studies were excluded. The main findings are summarized in Table III, and the combined evidence from the 2 approaches is illustrated in Fig. 1.
Fig. 1. Summary of evidence for effectiveness of rehabilitation from the 2 systematic analyses. RCT: randomized control trial; LOS: length of stay; OP: outpatient; TLU: Transitional Living Unit.
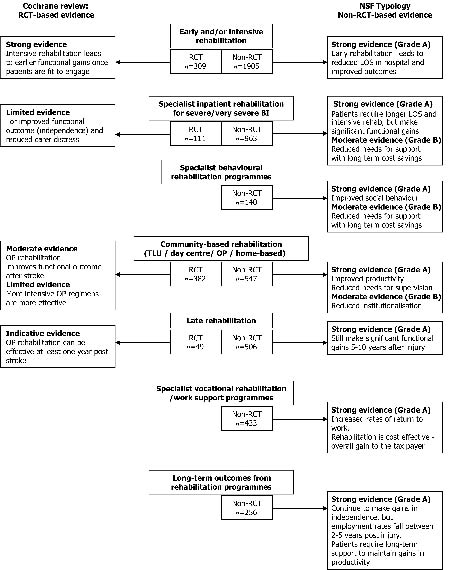
WHAT DOES THIS ALTERNATIVE SYNTHESIS OF TRIALS TELL US?
With respect to inpatient rehabilitation there was:
• strong (grade A) evidence from 5 studies (23–27) of early post-acute rehabilitation (total n = 3780) that early co-ordinated multidisciplinary rehabilitation leads to better outcomes and reduced length of stay in hospital, although severity or injury and co-morbidity were inevitable confounders;
• grade A evidence from 6 studies (n = 963) (16, 28–33) for the effectiveness of specialist inpatient rehabilitation. Highly dependent patients with severe or very severe brain injury (who are often regarded by many physicians as “beyond hope”) still made significant functional gains, although they required longer lengths of stay and more intensive treatment.
Managing unwanted behaviours is perhaps the most challenging area of rehabilitation. Two longitudinal cohort studies of behavioural rehabilitation programmes (total n = 140) (34, 35) provide grade A evidence that such interventions can lead to enhanced independence and social activity.
Back in the community, evidence for milieu-based rehabilitation was available for residential programmes in transitional living units (2 studies, n = 105) (36–38), day centre programmes (3 studies n = 280) (39–41) and outpatient programmes (3 studies n = 162) (42–44). Taken together, these 8 studies (n = 547) provide:
• grade A evidence for hard outcomes including increased productivity and reduced levels of supervision;
• grade A evidence for softer outcomes including improved societal participation and neuropsychological adjustment, and for stability of these benefits for up to 3 years post-injury (with grade B evidence for stability up to 11 years) (39).
Four studies (total n = 506) (14, 40, 45, 46) of late rehabilitation between them offer grade A evidence that organized rehabilitation can still make significant gains more than one year after the initial injury – and in some cases even 10–20 years afterwards. Although not seen in all patients, these gains have potential for cost impact (for example return to work and reduced use of healthcare) as well as improving quality of life for individuals and their families (see below).
With regard to return to work, the picture is somewhat mixed.
• Three studies of specialist vocational or work support programmes (47–49) (n = 433), provide grade A evidence for the effectiveness of supported employment.
• There was also grade A evidence that comprehensive community programmes can achieve improved productivity and return to paid employment, at least for a proportion of patients (36, 40, 42, 44).
However, the rates of employment remain disappointing overall (ranging from 27% (36) to 39% (40)) suggesting that careful patient selection is required.
Four studies with a total of 256 patients examined the longer term outcomes from rehabilitation (50–53). In general patients continued to make gains in independence and community integration between 2 and 5 years post-injury. However productivity rates were less well maintained. Although one high quality study (39) reported a high level of work stability at 11 years, others demonstrated a drop off of employment between 2 and 5 years post-injury (50, 53), suggesting that continued community support may be required even for a decade or more after injury.
Cost-effectiveness has been addressed in a number of ways.
• There was moderate (grade B) evidence that savings can accrue to health service providers through reduction in length of stay due to early, intensive and co-ordinated rehabilitation (23, 25, 54).
• Taking evidence from specialist inpatient services and specialist inpatient behavioural units together, there was strong (grade A) evidence that rehabilitation can reduce the needs for ongoing care with potential cost savings that offset the initial investment in rehabilitation (28, 32, 33, 35, 55), and this was particularly so in the more dependent group of patients (32, 33).
• There was also grade A evidence for cost-benefits of return to paid employment, in that the salaries from paid employment exceed the cost of intervention (49), with overall gain to the tax-payer (48).
HOW MIGHT WE PUT THESE TOGETHER TO FORMULATE RECOMMENDATIONS?
So we have evidence from both RCT and non-RCT-based research to support the effectiveness of rehabilitation for adults with ABI, but how can we put this together to support recommendations for clinical practice? There are many different ways of grading evidence and the strength of recommendations (56), and a recent drive to establish a common system has been proposed by the GRADE Working Group (Grading of Recommendations Assessment, Development and Evaluation) (57). The GRADE system offers 2 grades of recommendation based on the balance between desirable and undesirable effects of an intervention. The system carries a number of advantages. Whilst its “quality of evidence” rating is still based crudely on experimental design (58), it does offer the opportunity to up- or down-grade the evidence rating according to the quality of the research and strength of findings. Moreover, in the formulation of recommendations for management, this system collates not only the quality of evidence, but also the balance between benefits and harms or risks. These may be judged both at the level of the individual, and at the level of society; for example, the balance between costs of the intervention and potential for cost-savings to society as a whole. Table IV illustrates how the evidence derived from our assimilation of the literature might be put together under the GRADE system.
| Table IV. Summary of evidence to underpin recommendations according to the GRADE system (Grading of Recommendations Assessment, Development and Evaluation) | ||||||
| Intervention | Patients with ABI – particular categories | Outcomes from intervention | Quality of evidence | Potential for cost savings | Harms/risks | Strength of recommendation |
| Early rehabilitation | Severe ABI requiring inpatient hospital treatment | Earlier gains in independence Reduced LOS in hospital | Moderate | + | – | Recommended |
| Intensive rehabilitation | Severe ABI – fit to engage in intensive rehabilitation | Earlier gains in independence Reduced LOS in hospital | High | + | – | Strongly recommended |
| Specialist rehabilitation | Severe/very severe ABI with complex rehabilitation needs | Improved independence Reduced needs for on-going care support Demonstrated cost savings | Moderate/ High | ++ | – | Strongly recommended |
| Behavioural management programmes | ABI patients with severe behavioural problems | Improved social behaviour Reduced needs for on-going care support | Low/ moderate | + | – | Recommended |
| Community rehabilitation programmes | Moderate/severe ABI requiring support for community integration | Improved productivity Reduced need for institutionalization /support | Moderate | ++ | – | Recommended |
| Specialist vocational programmes | Moderate/severe ABI with potential for return to work | Gains in productivity Demonstrated cost savings with net gains to tax payer | Moderate/ High | ++ | – | Strongly recommended |
| Late and ongoing rehabilitation | Moderate/severe ABI with continued disability | Maintenance of independence and integration including productivity | Low/ Moderate | +/- | – | Conditionally recommended (in selected cases) |
| ABI: acquired brain injury of any cause; LOS: length of stay. | ||||||
On the basis of the research evidence available and demonstrated potential for cost-benefits, the strongest recommendations under the GRADE classification would be for (early) intensive rehabilitation; specialist programmes for those with complex needs; and specialist vocational programmes for those with potential to return to work. Although there is encouraging data from non-RCT studies to support the benefits of behavioural management programmes, community rehabilitation and longer-term interventions, this evidence is not yet sufficient to support strong recommendations for management, and more work is required in particular with respect to demonstrating cost-effectiveness, and to identifying those patients most likely to benefit.
DISCUSSION
These two analyses of the literature serve to demonstrate that there is now a substantial body of high-quality research evidence for the effectiveness, and indeed the cost-effectiveness, of rehabilitation. They also highlight the importance of looking beyond the somewhat restrictive set of trial-based evidence.
The studies included in the Cochrane Review explored a number of different rehabilitation models. They examined key issues of intensity, and the benefits of following up mild brain injury. However, they failed to address the impact of early or late rehabilitation, or to examine the effect of specialist programmes such as vocational or neuro-behavioural rehabilitation. Critically they provided no evidence on cost-effectiveness. By contrast, the non-trial-based evidence examined all these areas and produced an evaluation of cost-benefits on several different levels. In addition, it provided information on long-term outcomes over a timescale that is hardly ever forthcoming from the trial-based literature.
Some may argue that the additional set of non-RCT evidence is inferior, soft evidence. On the other hand, the data are predominantly derived from cohort analyses, and so represent the systematic collection of over 6600 cases treated under “real life” conditions. Moreover, the source articles have been submitted to close inspection and quality evaluation, and only those meeting acceptable quality criteria are included. A significant limitation in both syntheses, however, is the heterogeneity of outcome measures, which makes it difficult to combine data from different studies into a single meta-analysis.
The combination of these 2 research syntheses to support recommendations under the GRADE system serves to highlight both some strengths and some weaknesses in that system. The language of GRADE and most of the examples proffered by the working group are still primarily focused on single therapeutic interventions – mainly drug prescription – and its application in this context was not straightforward. The opportunity to up- and down-grade evidence from different trial designs according to the quality and strength of the findings is welcome, but does not go far enough towards recognizing the relative contributions of different study designs in the context of complex interventions. The balance between benefits and harms is also problematic. At individual level, the risks of intervention are very small in comparison with many therapeutic interventions. Clinical experience suggests that, if offered the choice, many patients would express a preference for ongoing rehabilitation even in the absence of demonstrable gain. The costs of intervention are often considerable, however, so the cost-benefits to society as whole become an important consideration. The critical questions for future research are not so much whether an intervention is effective overall, but how to target the limited resources available to achieve the maximum benefit and value for money. These questions are unlikely to be answered by RCTs.
In the USA, Horn & Gassaway (59) and de Jong et al. (60) have argued that it is not “evidence-based practice” we need now, but “practice-based evidence” in rehabilitation. They submit that the real proof of effectiveness comes from the systematic collection of prospective data (the “clinical practice improvement” approach), which provides information about what works for which patients in real-life clinical practice.
In the USA, payers such as the Centers for Medicare and Medicaid Services require all rehabilitation facilities to report a minimum data-set for each case episode, including data on length of stay, functional status and discharge destination, which are collated in one or other of the national data systems (principally eRehabData.com or the Uniform Data System for Medical Rehabilitation). These large data-sets bring uniformity to the collection of data, providing an opportunity for comparison between centres. However, they lack the depth of detail to describe the complexity of an individual’s needs for rehabilitation, or the rehabilitation interventions provided. Moreover, they collect information only on admission and discharge, so that everything that happens between is unknown (60). The Post Stroke Rehabilitation Outcomes Project (60) is a large multi-centre prospective cohort study designed to collect sufficiently detailed data to evaluate the impact of each rehabilitation intervention, individually and collectively, on the outcome at discharge, and hence to open the black box of stroke rehabilitation. Although a step in the right direction, even this is limited in the outcomes it collects and as yet it does not provide information on longer term outcomes.
The international agreement of a common core data-set for brain injury rehabilitation, which includes an evaluation of needs, inputs and outcomes from rehabilitation, would seem to be the next logical step to understanding what works for whom in brain injury rehabilitation. However, the challenge lies in defining a data-set that provides the relevant information, and is feasible for collection in the course of routine clinical practice. In the early 1990s, a prospective 175-item data-set for traumatic brain injury was developed through the European Brain Injury Society, with the intention of building a multi-national database to provide systematic data-gathering over 5 years post-injury. However, although the data document has been translated into several languages and there are isolated reports in the literature (61), uptake has been limited due to the length of time needed to administer the document (62) and further work is still required to develop and validate a manageable data-set.
In summary, this review highlights the importance of including a wide range of research designs in the analysis of evidence for effectiveness of rehabilitation. Whilst experimental designs and neurobiological research continue to provide an important contribution to the understanding of effective interventions in rehabilitation, this review emphasizes the need for systematic data collection in the course of real life clinical practice, as well as long-term follow-up and evaluation of health-related economic outcomes. Future research should focus on identifying which approaches work best for which patients, or opening the “black box” of rehabilitation, to fill in the gaps in our current knowledge.
ACKNOWLEDGEMENTS
I would like to acknowledge my co-authors for the Cochrane Review, in particular Ajoy Nair for assistance with evaluation and assimilation of the trial-based literature. Thanks are also due to Barbara Sullivan, Judith Sergeant and Richard Harding for their help in evaluations using the NSF typology. Funding for the original Cochrane Review was provided by the UK Department of Health, and financial support for preparation of this manuscript was kindly provided by the Dunhill Medical Trust and the Luff Foundation.
REFERENCES
1. Turner-Stokes L, Nair A, Disler P, Wade D. Cochrane review: multi-disciplinary rehabilitation for acquired brain injury in adults of working age. The Cochrane Database of Systematic Reviews. Oxford: Update Software 2005; Issue 3.
2. Whyte J. Traumatic brain injury rehabilitation: are there alternatives to randomised controlled clinical trials? Arch Phys Med Rehabil 2002; 83: 1320–1322.
3. Do H, editor. The National Service Framework for Long Term Conditions. London: Department of Health; 2005.
4. Turner-Stokes L, Harding R, Sergeant J, Lupton C, McPherson K. Generating the evidence base for the National Service Framework (NSF) for Long Term Conditions: a new research typology. Clin Med 2006; 6: 91–97.
5. van Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Method guidelines for systematic reviews in the Cochrane Back Review group for Spinal Disorders. Spine 1997; 22: 2323–2330.
6. Wade DT, King NS, Wenden FJ, Crawford S, Caldwell FE. Routine follow-up after head injury: a second randomised controlled trial. J Neurol Neurosurg Psych 1998; 65: 177–183.
7. Wade DT, Crawford S, Wenden FJ, King NS, Moss NE. Does routine follow-up after head injury help? A randomised controlled trial. J Neurol Neurosurg Psych 1997; 62: 478–484.
8. Salazar AM, Warden DL, Schwab K, Spector J, Braverman S, Walter J, et al. Cognitive rehabilitation for traumatic brain injury: a randomized trial. JAMA 2000; 283: 3075–3081.
9. Paniak C, Toller Lobe G, Durand A, Nagy J. A randomized trial of two treatments for mild traumatic brain injury. Brain Inj 1998; 12: 1011–1023.
10. Paniak C, Toller-Lobe G, Reynolds S, Melnyk A, Nagy J. A randomized trial of two treatments for mild traumatic brain injury: 1 year follow-up. Brain Inj 2000; 14: 219–226.
11. Elgmark Andersson E, Emanuelson I, Bjorklund R, Stalhammar DA. Mild traumatic brain injuries: the impact of early intervention on late sequelae. A randomized controlled trial. Acta Neurochirurgica 2007; 149: 151–159.
12. Smith D, Goldenberg E, Ashburn A, Kinsella G, Sheikh K, Brennan PJ, et al. Remedial therapy after stroke: a randomised controlled trial. BMJ 1981; 282: 517–520.
13. Werner RA, Kessler S. Effectiveness of an intensive outpatient rehabilitation program for postacute stroke patients. Am J Phys Med Rehabil 1996; 75: 114–120.
14. Powell J, Heslin J, Greenwood R. Community based rehabilitation after severe traumatic brain injury: a randomised controlled trial. J Neurol Neurosurg Psych 2002; 72: 193–202.
15. Bowen A, Tennant A, Neumann V, Chamberlain MA. Neuropsychological rehabilitation for traumatic brain injury: do carers benefit? Brain Inj 2001; 15: 29–38.
16. Semlyen JK, Summers SJ, Barnes MP. Traumatic brain injury: efficacy of multidisciplinary rehabilitation. Arch Phys Med Rehabil 1998; 79: 678–683.
17. Ozdemir F, Birtane M, Tabatabaei R, Kokino S, Ekuklu G. Comparing stroke rehabilitation outcomes between acute inpatient and nonintense home settings. Arch Phys Med Rehabil 2001; 82: 1375–1379.
18. Kwakkel G, Wagenaar RC, Twisk JW, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. Lancet 1999; 354: 191–196.
19. Shiel A, Burn JPS, Henry D, Clark J, Wilson BA, Burnett ME, et al. The effects of increased rehabilitation therapy after brain injury: results of a prospective controlled trial. Clin Rehabil 2001; 15: 501–514.
20. Slade A, Chamberlain MA, Tennant A. A randomised controlled trial to determine the effect of intensity of therapy on length of stay in a neurological rehabilitation setting. J Rehabil Med 2002; 34: 260–266.
21. Zhu XL, Poon WS, Chan CH, Chan SH. Does intensive rehabilitation improve the functional outcome of patients with traumatic brain injury? Interim result of a randomized controlled trial. Br J Neurosurg 2001; 15: 464–473.
22. Zhu XL, Poon WS, Chan CC, Chan SS. Does intensive rehabilitation improve the functional outcome of patients with traumatic brain injury (TBI)? A randomized controlled trial. Brain Inj 2007; 21: 681–690.
23. Cope N, Hall K. Head Injury rehabilitation: benefits of early intervention. Arch Phys Med Rehabil 1982; 63: 433–437.
24. Mackay LE, Bernstein BA, Chapman PE, Morgan AS, Milazzo LS. Early intervention in severe head injury: long-term benefits of a formalized program. Arch Phys Med Rehabil 1992; 73: 635–641.
25. Khan S, Khan A, Feyz M. Decreased length of stay, cost savings and descriptive findings of enhanced patient care resulting from and integrated traumatic brain injury programme. Brain Inj 2002; 16: 537–554.
26. Musicco M, Emberti L, Nappi G, Caltagirone C. Early and long-term outcome of rehabilitation in stroke patients: the role of patient characteristics, time of initiation, and duration of interventions. Arch Phys Med Rehabil 2003; 84: 551–558.
27. Engberg AW, Liebach A, Nordenbo A. Centralized rehabilitation after severe traumatic brain injury – a population-based study. Acta Neurol Scand 2006; 113: 178–184.
28. Cope DN, Cole JR, Hall KM, Barkan H. Brain injury: analysis of outcome in a post-acute rehabilitation system. Part 2: Subanalyses. Brain Inj 1991; 5: 127–139.
29. Spivack G, Spettell CM, Ellis DW, Ross SE. Effects of intensity of treatment and length of stay on rehabilitation outcomes. Brain Inj 1992; 6: 419–434.
30. Whitlock JA, Jr. Functional outcome of low-level traumatically brain-injured admitted to an acute rehabilitation programme. Brain Inj 1992; 6: 447–459.
31. Gray DS. Slow-to-recover severe traumatic brain injury: a review of outcomes and rehabilitation effectiveness. Brain Inj 2000; 14: 1003–1014.
32. Turner-Stokes L, Paul S, Williams H. The efficiency of specialist rehabilitation in reducing dependency and costs of continuing care for adults with complex acquired brain injuries. J Neurol Neurosurg Psych 2006; 77: 634–639.
33. Turner-Stokes L. Cost-efficiency of longer-stay rehabilitation programmes: can they provide value for money? Brain Inj 2007; 21: 1015–1021.
34. Eames P, Cotterill G, Kneale TA, Storrar AL, Yeomans P. Outcome of intensive rehabilitation after severe brain injury: a long-term follow-up study. Brain Inj 1995; 10: 631–650.
35. Wood RL, McCrea JD, Wood LM, Merriman RN. Clinical and cost effectiveness of post-acute neurobehavioural rehabilitation. Brain Inj 1999; 13: 69–88.
36. Johnston MV. Outcomes of community re-entry programmes for brain injury survivors. Part 1: independent living and productive activities. Brain Inj 1991; 5: 141–154.
37. Harrick L, Krefting L, Johnston J, Carlson P, Minnes P. Stability of functional outcomes following transitional living programme participation: 3-year follow-up. Brain Inj 1994; 8: 439–447.
38. Willer B, Button J, Rempel R. Residential and home-based postacute rehabilitation of individuals with traumatic brain injury: a case control study. Arch Phys Med Rehabil 1999; 80: 399–406.
39. Klonoff PS, Lamb DG, Henderson SW. Outcomes from milieu-based neurorehabilitation at up to 11 years post-discharge. Brain Inj 2001; 15: 413–428.
40. Malec JF. Impact of comprehensive day treatment on societal participation for persons with acquired brain injury. Arch Phys Med Rehabil 2001; 82: 885–895.
41. Sarajuuri JM, Kaipio ML, Koskinen SK, Niemela MR, Servo AR, Vilkki JS. Outcome of a comprehensive neurorehabilitation program for patients with traumatic brain injury. Arch Phys Med Rehabil 2005; 86: 2296–2302.
42. Prigatano GP, Fordyce DJ, Zeiner HK, Roueche JR, Pepping M, Wood MC. Neuropsychological rehabilitation after closed head injury in young adults. J Neurol Neurosurg Psych 1984; 47: 505–513.
43. Malec JF, Smigielski JS, DePompolo RW, Thompson JM. Outcome evaluation and prediction in a comprehensive-integrated post-acute outpatient brain injury rehabilitation programme. Brain Inj 1993; 7: 15–29.
44. Ben-Yishay Y, Silver S, Piasetsky E, Rattock J. Relationship between employability and vocational outcome after intensive holistic cognitive rehabilitation. J Head Trauma Rehabil 1987; 2: 35–48.
45. Tuel SM, Presty SK, Meythaler JM, Heinemann AW, Katz RT. Functional improvement in severe head injury after readmission for rehabilitation. Brain Inj 1992; 6: 363–372.
46. Gray DS, Burnham RS. Preliminary outcome analysis of a long-term rehabilitation program for severe acquired brain injury. Arch Phys Med Rehabil 2000; 81: 1447–1456.
47. Abrams D, Barker LT, Haffey W, Nelson H. The economics of return to work of survivors of traumatic brain injury: Vocational services are worth the investment. J Head Trauma Rehabil 1993; 8: 59–76.
48. Wehman P, Kregel J, Keyser-Marcus L, Sherron-Targett P, Campbell L, West M, et al. Supported employment for persons with traumatic brain injury: a preliminary investigation of long-term follow-up costs and program efficiency. Arch Phys Med Rehabil 2003; 84: 192–196.
49. Murphy L, Chamberlain E, Weir J, Berry A, Nathaniel-James D, Agnew R. Effectiveness of vocational rehabilitation following acquired brain injury: preliminary evaluation of a UK specialist rehabilitation programme. Brain Inj 2006; 20: 1119–1129.
50. Olver JH, Ponsford JL, Curran CA. Outcome following traumatic brain injury: a comparison between 2 and 5 years after injury. Brain Inj 1996; 10: 841–848.
51. Hoofien D, Gilboa A, Vakil E, Donovick PJ. Traumatic brain injury (TBI) 10–20 years later: a comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Inj 2001; 15: 189–209.
52. Possl J, Jurgensmeyer S, Karlbauer F, Wenz C, Goldenberg G. Stability of employment after brain injury: a 7-year follow-up study. Brain Inj 2001; 15: 15–27.
53. Sander AM, Roebuck TM, Struchen MA, Sherer M, High WM Jr. Long-term maintenance of gains obtained in postacute rehabilitation by persons with traumatic brain injury. J Head Trauma Rehabil 2001; 16: 356–373.
54. Slade A, Chamberlain MA, Tennant A, editors. Enhancing therapy: does it make a difference? In: Society for Research and Rehabilitation; 1998; Southampton, UK: Clin Rehabil; 1998.
55. Aronow H. Rehabilitation effectiveness with severe brain injury: translating research into policy. J Head Trauma Rehabil 1987; 2: 24–36.
56. Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res 2004; 22: 38.
57. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso- Coello P, et al. Rating quality of evidence and strength of recommendations: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926.
58. Atkins D, Briss PA, Eccles M. Systems for grading the quality of evidence and the strength of recommendations II: a pilot study of a new system for grading the quality of evidence and the strength of recommendations. BMC Health Serv Res 2005; 5: 25.
59. Horn SD, Gassaway J. Practice-based evidence study design for comparative effectiveness research. Med Care 2007; 45 (suppl 2): S50–S57.
60. DeJong G, Horn SD, Conroy B, Nichols D, Healton EB. Opening the black box of post-stroke rehabilitation: stroke rehabilitation patients, processes, and outcomes. [comment]. Arch Phys Med Rehabil 2005; 86 Suppl 2: S1–S7.
61. Avesani R, Salvi L, Rigoli G, Gambini MG. Reintegration after severe brain injury: a retrospective study. Brain Inj 2005; 19: 933–939.
62. Cudmore S, Pentland B. Early experience of the utility of the European Head Injury Evaluation Chart. Brain Inj 1996; 10: 517–529.
