OBJECTIVE: To evaluate the effect of a single cystoscopic injection of botulinum toxin to the external urethral sphincter in treating detrusor external sphincter dyssynergia.
DESIGN: An open treatment trial with pre- and post-treatment evaluations.
SUBJECTS: A total of 20 suprasacral spinal cord injured patients with pure detrusor external sphincter dyssynergia.
METHODS: A single dose of 100 IU botulinum toxin A was applied into the external urethral sphincter via cystoscopy. Outcome measurements included maximal detrusor pressure, maximal urethral pressure, detrusor leak point pressure, integrated electromyography, and maximal pressure on static urethral pressure profilometry obtained 4 weeks post-treatment. Post-voiding residuals were checked pre- and post-injection.
RESULTS: There were significant reductions in integrated electromyography and static and maximal urethral pressure, but not in maximal detrusor pressure and detrusor leak point pressure after treatment. Post-voiding residuals were significantly decreased at all evaluation periods. In the sub-group analysis, patients who showed good effects of treatment had significantly lower baseline integrated electromyography (p < 0.05).
CONCLUSION: This study demonstrates the effect of a single cystoscopic injection of botulinum toxin in detrusor external sphincter dyssynergia. Integrated electromyography is a good evaluation tool for the net effect and dosage of botulinum toxin. Patients with severe spasticity over the external urethral sphincter may require repeated injections or higher doses.
Key words: botulinum toxin, urethral sphincter, urodynamic study, neurogenic bladder.
J Rehabil Med 2008; 40: 744–748
Correspondence address: Yu-Hui Huang, Department of Physical Medicine and Rehabilitation, Chung Shan Medical University; Department of Physical Medicine and Rehabilitation, Chung Shan Medical University Hospital, TW-1142 Section 3, Tay-Yuan Road, Taichung City 406, Taiwan. E-mail: yhhuang59@hotmail.com
Submitted January 15, 2008; accepted June 26, 2008
INTRODUCTION
Detrusor external sphincter dyssynergia (DESD), defined as an involuntary contraction of the external urethral sphincter during detrusor contraction, appears in 96% of patients with supra-sacral spinal cord injury (SCI) (1). It causes impaired micturition and high intra-vesical pressure that leads to potentially life-threatening urological complications, such as recurrent urinary tract infections, vesico-ureteral reflux, and hydronephrosis (2, 3). If left untreated, it can result in progressive damage to the kidneys (4). Different therapeutic strategies, including oral medication and surgery, have been proposed, but are usually ineffective or cause side-effects (5–8).
Botulinum toxin (BTX-A) is an inhibitor of acetylcholine release at the neuromuscular junction, which can decrease muscle contractility for 3–6 months (9). It has been used successfully in the treatment of focal dystonia and spasticity of skeletal muscles of the face, neck, pharynx and limbs (10–13). It was first reported as a treatment for DESD by Dykstra et al. (14) in 11 patients with SCI, with reduction of urethral pressure and post-voiding residuals (PVR). Schurch et al. (16), as well as several other researchers (14, 15, 17–22), also reported the effect of BTX-A injected either trans-urethral via cystoscopy or trans-perineal with electromyography (EMG). Most reported decreased urethral pressure (14–19). Its influence on detrusor pressure and PVR, however, remains controversial (18–20). It is difficult to compare previous reports due to different BTX-A dosages and treatment protocols. Some have raised the possibility of detrusor internal sphincter dyssynergia (DISD) as one of the reasons for poor treatment response (16, 22).
Reviewing the literature, the direct effects of BTX-A on the external sphincter are not clear because internal sphincter dyssynergia is not excluded and no quantitative data from sphincter EMG is available. Integrated EMG (IEMG) has been proposed to quantify muscle force production and is used in measuring isometric muscle torque, muscle force recovery from muscle relaxants, and the progression of motor blockade during lumbar epidural anaesthesia (23–25).
Reliable detection of patients who are at risk for distinct post-injection residua and choosing the right dose of BTX-A remain formidable challenges. The objectives of this study were to collect patients with SCI with pure DESD and evaluate the effect of single cystoscopic injection of 100 IU of BTX-A with IEMG of external urethral sphincter and other urodynamic parameters.
METHODS
The study subjects were patients in the rehabilitation department ward. Those over 18 years of age with supra-sacral SCI were screened for enrolment. The inclusion criteria were the presence of DESD, defined as inappropriate contractions of the external urethral sphincter with concomitant detrusor contractions determined by urodynamic studies, such as needle-electrode EMG, and voiding cystourethrometrography. All of the patients were neurologically stable (i.e. there was no progression of neurological symptoms in the previous 3 months) but suffered from post-voiding urine volume > 150 ml that was unresponsive to oral spasmolytic agents (hyoscine butylbromide), skeletal muscle relaxant (baclofen) and alpha blockers (doxazosin mesylate and terazosin).
Internal sphincter dyssynergia was excluded by evaluation of the bladder neck opening on cystography. The exclusion criteria also included pregnancy, coagulopathy disease, myasthenia gravis, aminoglycoside treatment, hypersensitivity to BTX-A, other causes of outlet obstruction (i.e. urethral stricture and benign prostatic hypertrophy), previous sphincterotomy, and BTX-A injection to the external urethral sphincter.
The study was approved by the ethics committee of Chung Shan Medical University Hospital. Each patient was fully informed about the procedure and written consent was obtained before the treatment.
A total of 20 patients were enrolled from August 2005 to October 2006. Basic demographic data are shown in Table I. There were 17 males and 3 females, mean age 37.9 (standard deviation (SD) 15.7) years. The distribution of SCI levels was: 12 (60%) cervical, 3 (15%) thoracic and 5 (25%) lumbar. The average period between the onset of spinal cord lesions and inclusion was 9.2 (SD 6.1) months (range: 3–24 months). International Standards of the Neurological and Functional Classification of Spinal Injuries, commonly known as the American Spinal Injury Association (ASIA) scale, were also measured and there were 11 (55%) Grade A, 2 (10%) Grade B, 4 (20%) Grade C, and 3 (15%) Grade D.
| Table I. Basic demographic data for the patients with supra-sacral spinal cord injury |
| Characteristics | |
| Total number of patients | 20 |
| Age, years, mean (SD) | 37.9 (15.7) |
| Injury duration, years, mean (SD) | 9.2 (6.1) |
| Gender, men/women, n (%) | 17 (85) / 3 (15) |
| Injury level, n (%) | |
| Cervical | 12 (60) |
| Thoracic | 3 (15) |
| Lumbar | 5 (25) |
| ASIA scale, n (%) | |
| Grade A | 11 (55) |
| Grade B | 2 (10) |
| Grade C | 4 (20) |
| Grade D | 3 (15) |
| SD: standard deviation; ASIA: American Spinal Injury Association. |
Thirteen of 20 patients voided with incomplete uncontrolled micturition who had PVR greater than 150 ml. Of the remaining 7 patients with complete urinary retention, 6 performed clean intermittent self-catheterization (CISC) and one had suprapubic cystostomy drainage for bladder emptying. Four patients complained of autonomic dysreflexia (AD) with headache, shivering and sweating while voiding. One patient presented with grade II left side vesico-ureteral reflux during video-urodynamic study (VUD).
The BTX-A urethral injection followed the procedure of Kuo (19) and was performed in the operating room. Equipment for vital sign monitoring was set up as routine general anaesthesia and intravenous sedation was given to all of the patients. The 100 IU BTX-A (Allergan, Irvine, California, USA) was diluted with 2 ml 0.9% saline. Using a rigid cystoscope and standard cystoscopic collagen injection needle, BTX-A was injected into the external urethral sphincter about 1–1.5 cm in depth at 3, 6, 9 and 12 o’clock positions in approximately equal aliquots. An additional 0.2 ml of normal saline was then injected to ensure that the maximum amount of toxin left in the needle was delivered. A Foley catheter was routinely in-dwelled for one day and any adverse event related to the injection was recorded.
VUD, performed with a Urodyn 5500 apparatus (Dantec Inc., Scovlunde, Denmark), was obtained from each subject before and 4 weeks after the BTX-A injection. This examination included filling cystourethrometrography (CUMG), sphincter EMG, and static urethral pressure profilometry (UPP). CUMG was performed by a triple lumen catheter that measured intra-vesical pressure and urethral pressure synchronously with continuous filling of isotonic saline at a rate of 30 ml/min. The measuring point of urethral pressure was at the level of maximal urethral pressure (26). Trans-perineal EMG of the external urethral sphincter was obtained via disposable concentric needle electrodes. The needle electrode placement in women was just lateral to the urethral meatus, at a depth of 15 mm. In males, the needle was inserted into the perineum midline about 1.5–2 cm anterior to the anus. A gloved finger in the rectum monitored the position of the prostate while the electrode was directed toward its apex. Final localization was made by monitoring the motor unit activity electromyographically and by examining the needle position fluoroscopically (27).
Integrated EMG was obtained at a rate of 30 Hz and an average of 30-sec duration around the maximal value was used for comparison. UPP was performed with a filling rate of 4 ml/min and catheter withdrawal rate of 1 mm/min. All descriptions and terminologies were in accordance with the recommendations of the International Continence Society. Maximal detrusor pressure (Pdet), maximal urethral pressure (dynamic Pure), and maximal detrusor leak point pressure (Plp) on CUMG, mean IEMG of the external urethral sphincter, and maximal urethral pressure on UPP (static Pure) were used for comparison.
The PVR was measured by catheterization pre- and in the 1st, 2nd, 3rd, and 6th month post-injection. Patients without spontaneous voiding and were relieved by CISC or in-dwelling catheters were defined to have PVR of 500 ml. Based on the longitudinal PVR data, patients were further divided into three sub-groups: good effect (PVR reduction ≥ 40% and effect lasting up to 6 months), poor effect (PVR reduction ≤ 20% and effect of no more than one month), and intermediate effect (all who did not meet the criteria of the previous two groups). The frequency and intensity of AD were also recorded.
Paired t-test was used to compare urodynamic parameters pre- and post-injection. Repeated measures analysis of variance (ANOVA) were used to analyse longitudinal data (PVR), while ANOVA with the Turkey test for post hoc analysis was used to compare baseline VUD parameters between efficacy sub-groups. The data was analysed using SPSS V8.0 with statistical significance set at p < 0.05.
RESULTS
All measurements from the VUD examination is presented in Table II. The VUD examination, performed at an average of 33.8 days post-injection, revealed significant reduction in static and dynamic Pure and IEMG compared with pre-injection values (mean reduction percentages (SD) were 20.6 (28.6), 17.3 (34.4), and 24.4 (35.6), respectively; p < 0.05). There was no significant change in Pdet. Thirteen patients with urine leakage in the first VUD had decreased Plp after treatment (mean reduction percentage 17.4 (31.4), p = 0.077).
| Table II. Videourodynamic parameters of the study subjects pre- and post-botulinum toxin injection. Data is shown as mean (standard deviation) |
| | Before | After | Mean reduction (%) | p-value |
| Pdet, cmH2O | 89.6 (48.6) | 80.4 (44.1) | 0 (31.6) | 0.193 |
| Plp, cmH2O | 78.6 (36.9) | 64.0 (42.5) | 17.4 (31.4) | 0.077 n = 13 |
| Dynamic pure, cmH2O | 107.5 (69.1) | 80.2 (35.7) | 17.3 (34.4) | 0.049* |
| IEMG, µV | 16.7 (13.6) | 12.5 (12.9) | 24.4 (35.6) | 0.023* |
| Static pure, cmH2O | 139.4 (40.5) | 104.8 (30.5) | 20.6 (28.6) | 0.004* |
| *p < 0.05. Pdet: detrusor pressure; Plp: detrusor leak point pressure; Dynamic pure: urethral pressure; IEMG: integrated electromyography; Static pure: urethral pressure on UPP (urethral pressure profilometry). |
There were 6 patients with CSIC and one with supra-pubic cystostomy catheter indwelling for bladder emptying. Their PVRs were measured as 500 ml as defined in our methodology. The PVR changes are summarized in Table III. PVR was significantly decreased after treatment in the 1st, 2nd, 3rd, and 6th month (mean reduction percentages were 41.2 (26.8), 32.6 (34.5), 24.6 (34.8), and 15.8 (28.1), respectively). In the subgroupings, there were 6 patients in the good effect (30%), 9 in the intermediate effect (45%), and 5 in the poor effect (25%). There were no differences in demographic data, including gender, age, injury level, ASIA scale, and onset duration among these subgroups. The differences of baseline VUD parameters and PVR were analysed (Table IV) and no differences were noted in PVR, maximal detrusor pressure, and maximal static and dynamic urethral pressures. However, the good effect sub-group had a significantly lower baseline IEMG compared with the poor effect sub-group (p < 0.05). Initial IEMG greater than 20 µV was noted in 4 patients (80%) of the poor effect sub-group. All of the patients in the good effect sub-group had initial IEMG lower than 20 µV.
| Table III. Post-voiding residuals of baseline and 1st, 2nd, 3rd, and 6th month post-botulinum toxin injection. Data is shown as mean (standard deviation) |
| | Original values (ml) | Mean reduction (%) |
| Baseline | 355 (125) | |
| 1st month | 207 (136)* | 41.2 (26.8) |
| 2nd month | 226 (155)* | 32.6 (34.5) |
| 3rd month | 243 (150)* | 24.6 (34.8) |
| 6th month | 281 (143)* | 15.8 (28.0) |
| *p < 0.05 compared with baseline. |
| Table IV. Comparison of baseline post-voiding residuals and videourodynamic parameters of efficacy sub-groups. Data is shown as mean (standard deviation) |
| | Good effect n = 6 | Intermediate effect n = 9 | Poor effect n = 5 | p-value of ANOVA |
| PVR, ml | 399 (103) | 308 (93) | 387 (152) | 0.159 |
| Pdet, cmH2O | 63.8 (59.6) | 98.4 (45.9) | 104.6 (33.4) | 0.308 |
| Dynamic pure, cmH2O | 86.0 (108.7) | 101.2 (41.4) | 144.6 (44.6) | 0.370 |
| Static pure, cmH2O | 137.3 (41.2) | 135.9 (35.8) | 148.0 (54.7) | 0.870 |
| IEMG, µV | 7.0 (7.8)* | 17.7 (7.6) | 26.5 (20.6)* | 0.049* |
| *p < 0.05 PVR: post-voiding residuals; Pdet: detrusor pressure; Dynamic pure: urethral pressure; Static pure: urethral pressure on UPP (urethral pressure profilometry); IEMG: integrated electromyography; ANOVA: analysis of variance. |
Four patients who had AD symptoms before treatment reported decreased frequency and intensity of AD. Left-sided grade II vesico-ureteral reflux noted pre-operatively in one patient also resolved after BTX-A injection. No serious side-effects related to BTX-A injection were noted and only two patients presented with mild haematuria for one day.
DISCUSSION
BTX-A injection seems to be a promising option for treating DESD, as shown since the first study of Dykstra et al. (14). Our finding that BTX-A injections can significantly reduce the static and dynamic urethral pressure is similar to previous reports (14–19). However, measuring urethral pressure is an indirect way of determining the effects of BTX-A, and the instability while measuring pressure seems to be a problem. Variations of up to 50% of the measured value can be seen with different orientations of the catheter (28). Static urethral pressure profile also has time variations that hinder the reproducibility and comparability of this measurement (29).
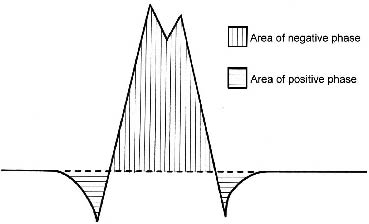
Direct measurement of the activity of the external urethral sphincter by EMG may be a more convincing way to evaluate the net effect of BTX-A. Several studies also report a reduction in EMG activity by observation of the appearance of raw EMG (15, 20, 21). To our knowledge, this study is the first report that quantifies the change of urethral sphincter activity by measuring IEMG and validates the effectiveness of BTX-A treatment. The original EMG waveform is a cluster of motor unit action potentials, which have upward and downward deflections due to the propagating muscle action potentials (Fig. 1), and is difficult to quantify directly. Increased muscle force output results in increased amplitude and discharge rate on the EMG, thus the area under the EMG waveform increased. The primary tools used to calculate the total area under this curve are the rectification and integration functions. Rectification is used to derive the absolute value of the original signal. Applying the absolute value function to a waveform folds the negative portion of the signal above zero reference to join the positive half of the signal, thus determining the total amount of energy (both positive and negative) contained by the waveform. Integration function is then used to calculate the area under the curve. The integral of a waveform increases in proportion to the amplitude, frequency, and duration of the original potential, usually relating linearly to the isometric tension up to the maximal contraction (30).
Fig. 1. Typical appearance of motor unit action potentials with tri-phasic waveform (positive-negative-positive).
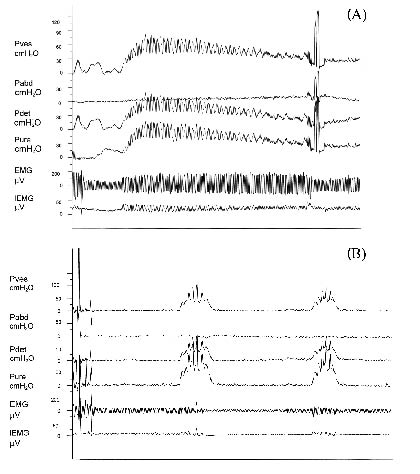
In our observation, some patients with strong and continuous DESD initially may still have intermittent sphincter activity after this procedure (Fig. 2). This finding can partly explain our result that the maximal detrusor pressure was still high after BTX-A injection. Another explanation may be obtained from two previous studies. Cote et al. in 1981 (31) reported that long-term bladder outlet obstruction by benign prostatic hypertrophy is often associated with detrusor hyper-reflexia that could persist up to 3 months after resolution of the obstruction. De Seze et al. (18) also proposed that after BTX-A injection to patients with SCI with DESD, detrusor maximal pressure decreases later than the maximal urethral pressure.
Although the maximal detrusor pressure did not decrease, the duration of high pressure shortened and urine leaked at a relative lower pressure in our patients (leak point pressure decreased with a reduction percentage of 17). This could decrease the risk of upper genitor-urinary tract complications in SCI patients (32). Furthermore, Schurch et al. (33) also proposed the treatment option of concomitant BTX-A injection to the detrusor and external urethral sphincter for those with both DESD and detrusor hyper-reflexia.
Fig. 2. Curves of cystourethrometrography from a patient before and after botulinum toxin A injection. (A) Pre-injection. (B) Post-injection. Pves: intravesical pressure; Pabd: intraabdominal pressure; Pdet: detrusor pressure; Pure: urethral pressure; EMG: original electromyography signal; IEMG: integrated EMG.
In a review of literature regarding the clinical effect on PVR by BTX-A injection, the results were debatable. There was no significant change in PVR reported in the studies by Kuo (19) and Gallien (20). Schurch et al. (16) proposed that 9 of 24 patients had decreased PVR volumes from 450 to 50 ml but it remained high in 8 patients. Phelan et al. (22) reported that 14 (67%) of 21 patients had significant subjective improvement in PVR but BTX-A injection still failed to improve PVR in 33% of their patients. Bladder neck dyssynergia is independent of DESD and is suggested to be one of the reasons why some patients do not improve their PVR (16). Compared with previous studies, the relative high PVR noted in our patients was due to the 7 patients (35%) who emptied their bladder with CISC or suprapubic catheter drainage.
To investigate other causes influencing PVR, the patients were further divided into sub-groups for analysis according to the PVR values and effect durations. The baseline EMG activity of the good effect group was significantly lower than that of the poor effect group. All of the good effect subjects had initial IEMG lower than 20 µV. Most (80%) of the poor effect patients had initial IEMG greater than 20 µV. This means that those who had lower urethral sphincter spasticity had greater responsiveness to single 100 IU BTX-A injection. From our preliminary results, we propose that a single cystoscopic injection of 100 IU BTX-A may be beneficial to selected SCI patients with pure DESD for about 6 months. Repeat or higher doses may be warranted for SCI patients with more severe DESD (average IEMG greater than 20 µV).
We should recognize that there are some limitations in this study. We have only 20 patents and this makes sub-group analysis difficult. Larger, randomized, and placebo-controlled studies are warranted to investigate the influencing factors of clinical effect. Another limitation is that VUD is conducted only in the first month and we do not observe significant drop of detrusor pressure after relief of outlet obstruction by BTX-A injection at this early stage. Future study with long-term follow-up of VUD will more clearly demonstrate the response of detrusor pressure in the efficacious period of BTX-A injection.
In conclusion, this study clearly demonstrates the effect of a single cystoscopic injection of 100 IU BTX-A for DESD in SCI patients without DISD. IEMG is a useful evaluation tool for the net effect of BTX-A, and can help to identify further the types of patients who may benefit most from the correct dosages of BTX-A. Those with severe spasticity over the external urethral sphincter (average IEMG greater than 20 µV) may need repeated injections or higher doses.
ACKNOWLEDGEMENTS
This study was supported by a research grant (Grant No. NSC 942314B040005) from the National Science Council of the Republic of China.
REFERENCES
1. Blaivas JG, Sinha HP, Zayed AA, Labib KB. Detrusor-external sphincter dyssynergia. J Urol 1981; 125: 542–544.
2. Blaivas JG, Barbalias GA. Detrusor-external sphincter dyssynergia in men with multiple sclerosis: an ominous urologic condition. J Urol 1984; 131: 91–94.
3. Thomas DG, editor. Spinal cord injury. New York: Churchill Livingstone; 1984, p. 259–272.
4. Lin, VW, editor. Spinal cord medicine: principles and practice. New York: Demos Medical Publishing, Inc.; 2003, p. 299.
5. Yang CC, Mayo ME. External urethral sphincterotomy: long-term follow-up. Neurourol Urodyn 1995; 14: 25–31.
6. Chancellor MB, Rivas DA, Linsenmeyer T, Abdill CA, Ackman CF, Appell RA, et al. Multicenter trial in North America of UroLume urinary sphincter prosthesis. J Urol 1994; 152: 924–930.
7. Chancellor MB, Erhard MJ, Rivas DA. Clinical effect of alpha-1 antagonism by terazosin on external and internal urinary sphincter function. J Am Paraplegia Soc 1993; 16: 207–214.
8. Perkash I, Giroux J. Clean intermittent catheterization in spinal cord injury patients: a followup study. J Urol 1993; 149: 1068–1071.
9. Leippold T, Reitz A, Schurch B. Botulinum toxin as a new therapy option for voiding disorders: current state of the art. Eur Urol 2003; 44: 165–174.
10. Scott AB, Kennedy RA, Stubbs HA. Botulinum A toxin injection as a treatment for blepharospasm. Arch Ophthalmol 1985; 103: 347–350.
11. Tsui JK, Calne DB. Botulinum toxin in cervical dystonia. Adv Neurol 1988; 49: 473–478.
12. Schneider I, Thumfart WF, Pototschnig C, Eckel HE. Treatment of dysfunction of the cricopharyngeal muscle with botulinum A toxin: introduction of a new, noninvasive method. Ann Otol Rhinol Laryngol 1994; 103: 31–35.
13. Hesse S, Lucke D, Malezic M, Bertelt C, Friedrich H, Gregoric M, et al. Botulinum toxin treatment for lower limb extensor spasticity in chronic hemiparetic patients. J Neurol Neurosurg Psychiatry 1994; 57: 1321–1324.
14. Dykstra DD, Sidi AA, Scott AB, Pagel JM, Goldish GD. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988; 139: 919–922.
15. Dykstra DD, Sidi AA. Treatment of detrusor-sphincter dyssynergia with botulinum A toxin: a double-blind study. Arch Phys Med Rehabil 1990; 71: 24–26.
16. Schurch B, Hauri D, Rodic B, Curt A, Meyer M, Rossier AB. Botulinum-A toxin as a treatment of detrusor-sphincter dyssynergia: a prospective study in 24 spinal cord injury patients. J Urol 1996; 155: 1023–1029.
17. Petit H, Wiart L, Gaujard E, Le Breton F, Ferriere JM, Lagueny A, et al. Botulinum A toxin treatment for detrusor-sphincter dyssynergia in spinal cord disease. Spinal Cord 1998; 36: 91–94.
18. de Seze M, Petit H, Gallien P, de Seze MP, Joseph PA, Mazaux JM, et al. Botulinum a toxin and detrusor sphincter dyssynergia: a double-blind lidocaine-controlled study in 13 patients with spinal cord disease. Eur Urol 2002; 42: 56–62.
19. Kuo HC. Botulinum A toxin urethral injection for the treatment of lower urinary tract dysfunction. J Urol 2003; 170: 1908–1912.
20. Gallien P, Robineau S, Verin M, Le Bot MP, Nicolas B, Brissot R. Treatment of detrusor sphincter dyssynergia by transperineal injection of botulinum toxin. Arch Phys Med Rehabil 1998; 79: 715–717.
21. Wheeler JS, Jr, Walter JS, Chintam RS, Rao S. Botulinum toxin injections for voiding dysfunction following SCI. J Spinal Cord Med 1998; 21: 227–229.
22. Phelan MW, Franks M, Somogyi GT, Yokoyama T, Fraser MO, Lavelle JP, et al. Botulinum toxin urethral sphincter injection to restore bladder emptying in men and women with voiding dysfunction. J Urol 2001; 165: 1107–1110.
23. Sleivert GG, Wenger HA. Reliability of measuring isometric and isokinetic peak torque, rate of torque development, integrated electromyography, and tibial nerve conduction velocity. Arch Phys Med Rehabil 1994; 75: 1315–1321.
24. Weber S, Muravchick S. Monitoring technique affects measurement of recovery from succinylcholine. J Clin Monit 1987; 3: 1–5.
25. Chamberlain DP, Crawford RD. Integrated electromyographic measurement of abdominal motor blockade during bupivacaine epidural anesthesia for lower abdominal and pelvic surgery. Anesth Analg 1987; 66: 57–63.
26. Abrams P, Feneley R, Torrens M, editors. Urodynamics. Berlin: Springer-Verlag; 1983, p. 60.
27. Yalla SV, McGuire EJ, Elbadawi A, Blaivas JG, editors. Neurourology and urodynamics: principles and practice. New York: MacMillan Publishing Co.; 1988, p. 49.
28. Mundy AR, Stephenson TP, Wein AJ, editors. Urodynamics: principles, practice and application. 2nd edn. New York: Churchill Livingstone; 1994, p. 148.
29. Plevnik S, Janez J. Urethral pressure variations. Urology 1983; 21: 207–209.
30. Kimura J, editor. Electrodiagnosis in diseases of nerve and muscle: principles and practice. 3rd edn. New York: Oxford University Press, Inc.; 2001, p. 35 and 326.
31. Cote RJ, Burke H, Schoenberg HW. Prediction of unusual postoperative results by urodynamic testing in benign prostatic hyperplasia. J Urol 1981; 125: 690–692.
32. McGuire EJ, Cespedes RD, O’Connell HE. Leak-point pressures. Urol Clin North Am 1996; 23: 253–262.
33. Schurch B, Stohrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol 2000; 164: 692–697.