OBJECTIVE: To investigate the feasibility of cervical spine mobilization in elderly dementia patients with dysphagia, and its effect on swallowing capacity.
METHODS: Fifteen nursing home residents (9 women, 6 men, age range 77–98 years) with severe dementia (median Mini Mental State Examination score = 8/30, percentile (P)25–75 = 4–13) and known dysphagia participated in a randomized controlled trial with cross-over design. Cervical spine mobilization was administered by trained physiotherapists. Control sessions consisted of socializing visits. Feasibility (attendance, hostility, complications) and maximal swallowing volume (water bolus 1–20 ml) were assessed following one session and one week (3 sessions) of treatment and control.
RESULTS: Ninety percent of cervical spine mobilization sessions were completed successfully (3 sessions could not be carried out due to the patient’s hostility and 2 due to illness) and no complications were observed. Swallowing capacity improved significantly after cervical spine mobilization (from 3 ml (P25–75 = 1–10) to 5 ml (P25–75 = 3–15) after one session p = 0.01 and to 10 ml (P25–75 = 5–20) (+230%) after one week treatment p = 0.03) compared with control (no significant changes, difference in evolution after one session between treatment and control, p = 0.03).
CONCLUSION: Cervical spine mobilization is feasible and can improve swallowing capacity in cognitively impaired residents in nursing homes. Given the acute improvements following treatment, it is probably best provided before meals.
Key words: dementia, dysphagia, musculoskeletal manipulation, cervical spine, paratonia, cervical spine mobilization.
J Rehabil Med 2008; 40: 755–760
Correspondence address: Ivan Bautmans, Frailty in Ageing Research Group, Vrije Universiteit Brussel, Laarbeeklaan 103, BE-1090 Brussels, Belgium. E-mail: ivan.bautmans@vub.ac.be
Submitted December 19, 2007; accepted May 19, 2008
INTRODUCTION
Dysphagia is a common disorder in elderly persons presenting dementia, frequently leading to severe complications (e.g. aspiration pneumonia) (1, 2). It has been shown that swallowing deficiency and the risk of aspiration pneumonia worsens with increasing severity of dementia (3). Elderly patients with severe dementia often live in nursing homes, where dysphagia is a well-known issue (4). Besides swallowing dysfunctions directly induced by dementia-related central nervous disorders, poor positioning and bad posture are recognized as significant correlates with severe dementia and dysphagia in elderly residents in nursing homes (5).
In addition to cognitive decline, dementia (Alzheimer’s disease) is associated with different motor signs, which worsen with increasing severity of dementia (6). Typical for dementia patients is the occurrence of paratonic rigidity, defined as “resistance to passive movement of a joint whereby the degree of resistance varies depending on the speed of movement” (resistance increases when the joint is moved rapidly and decreases or even disappears when it is moved more slowly) (7). Recently, a consensus definition for paratonia has been proposed (8), differentiating this dementia-related phenomenon from other well-known motor signs in central nervous diseases (e.g. clasp-knife phenomenon, Parkinsonism, etc.) (7, 8).
It can be assumed that paratonic rigidity is at least partly responsible for postural changes that become obvious in severe and end-stage dementia patients (foetal posture). It has been demonstrated that the posture of the head and cervical spine is closely related to oropharyngeal swallowing capacity (9). Steele et al. (5) reported that poor positioning was identified in 33% of 349 nursing home residents screened for mealtime difficulties, and concentrated among residents requiring high levels of physical care and those with severe cognitive impairment. In addition, Kayser-Jones & Pengilly (4) reported improper positioning in a qualitative study of nutritional problems in elderly residents with dysphagia in nursing homes. Correction of swallowing posture is one of the recommendations formulated by the American Gastroenterological Association in the Medical Position Statement on Management of Oropharyngeal Dysphagia (10). Although it is not clear whether passive mobilization can improve paratonic rigidity (8), it can be assumed that correction of the head and neck posture of these patients can improve oropharyngeal swallowing. In fact, there is a lack of specific guidelines regarding manoeuvres to correct head posture and their application, especially for elderly patients with dementia. The aim of this study was to investigate the feasibility of cervical spine mobilization in frail elderly dysphagic nursing home residents who have severe dementia, and its effect on swallowing capacity.
METHODS
Participants
All residents of a nursing home (capacity 460 beds) aged 65 years or older, cognitively impaired due to Alzheimer’s dementia (mini mental state examination (MMSE) < 24/30), presenting paratonia with altered neck posture (cervical anteroposition, extension or kyphosis) and known dysphagia (speech therapy report in the medical record) were eligible for participation. Patients were excluded when presenting other known central nervous conditions that could influence swallowing (e.g. Parkinson’s disease or Huntington’s disease, hemiplegia, tetraplegia), when they were acutely ill (fever, acute infections) or when they were fed by a catheter or nasogastric tube. Finally, 16 residents (10 women, 6 men, age range 77–98 years) were included in the study. The study protocol was approved by the local ethics committee and informed consent was obtained for all participants from the patient’s legal representative, when appointed, or from close relatives. None declined consent to participate.
Study design and randomization
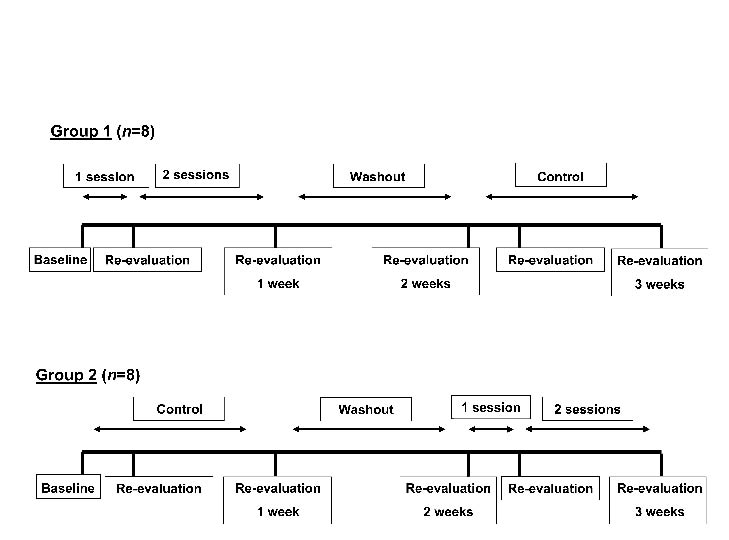
Since the study involved frail elderly residents in a nursing home who have severe cognitive impairments, we expected a large inter-individual heterogeneity for the study outcomes. Therefore, in order to increase the power of the study, a randomized controlled trial with cross-over design was planned, in which each participant was his or her own control. The 16 participants were divided randomly into 2 groups: group one started with one week of cervical spine mobilization, followed by one week wash-out and one week as control. The other group started with one week as control, followed by one week wash-out and one week of cervical spine mobilization (Figs 1 and 2). For each patient, all measurements and interventions took place at the same time of day in order to avoid diurnal bias.
Intervention
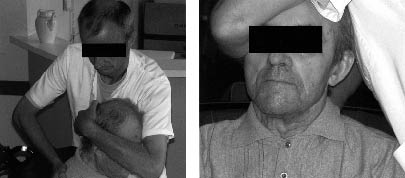
During the mobilization week, the resident’s cervical spine was gently mobilized using manual techniques performed by a physiotherapist (11). The participant was seated comfortably with his or her head supported against the chest of the therapist, who maintained the head in his hand and arm (Fig. 3). The therapist gently mobilized the head and cervical spine in order to correct the patient’s posture (i.e. centring the head in a neutral position above the shoulders). The mobilization consisted of free passive movements of the head without active participation of the patients and without supplementary traction or other components. Mobilization was performed within the available range of movement, without eliciting muscular defence or complaints from the patients. Three sessions were performed during the mobilization week (every 2 days), each session lasting approximately 20 min. The control sessions were identical in planning and duration, but consisted of a socializing visit by the physical therapist. During the wash-out period, no supplementary intervention was performed. All therapists (n = 5) had clinical responsibilities in the nursing home and were familiar with the participating residents. The therapists were instructed during 2 training sessions of 1.5 h. The intervention did not interfere with the current medical and paramedical treatment of the participating residents (standard physical therapy consisting of daily sessions of gait/transfer rehabilitation (if relevant) and/or exercises for the limbs; no cervical spine mobilization), which was supported by different therapists who were unaware of group assignment. No changes were applied to the seating devices during the study period.
Fig. 3. Gentle cervical spine mobilization. The participant was seated comfortably with his head supported against the chest of the therapist, who maintained the head in his hand and arm. The therapist gently mobilized the head and cervical spine in order to correct the posture (i.e. centring the head in a neutral position above the shoulders). The mobilization consisted of free passive movements of the head without active participation of the patients and without supplementary traction or other components. Mobilization was performed within the available range of movement, without eliciting muscular defence or complaints from the patients.
Measurements
Primary outcome measures were feasibility (attendance, hostility to therapy, complications) and dysphagia limit (maximal volume of water (0–20 ml) that can be swallowed in a single movement) (12).
After inclusion, all patients were evaluated for physical dependency as described by Katz et al. (13) on a scale ranging from 6 (completely independent for activities of daily living) to 24 (dependent for washing, clothing, use of the toilet, transfer and incontinence and eating). Height and weight were also measured and body mass index was calculated as weight/height2.
Feasibility. Following each session (mobilization or control) the physiotherapist recorded the patients’ availability, hostility (verbal or non-verbal resistance to the intervention) and any complications.
Dysphagia limit. Dysphagia limit, defined as the maximal bolus of water that can be swallowed in a single movement, was evaluated using an adapted approach described by Ertekin et al. (12). The different evaluation times or the dysphagia limit are shown in Fig. 1. The dysphagia limit was evaluated before and after the first session (intervention and control, within 2 h) and after one week (intervention and control, within 2 h following the last session). All evaluations were performed by an independent investigator who did not participate in the treatments and who was blinded for group assignment and intervention. During the examination, participants were seated on a chair with the head in a neutral position. Subjects were presented gradually increasing boli of 1, 3, 5, 10, 15 and 20 ml water in a stepwise manner and were asked to swallow each bolus in a single movement. The investigator recorded by observation the number of swallowing movements necessary for each bolus, coughing and if any water was spilled out of the mouth. The swallowing movements were monitored simultaneously by surface electromyography (sEMG) of the submandibular (SM) muscles, in order to validate the number of observed swallowing movements.
Fig. 1. Randomized controlled trial with cross-over design. All participants (n = 16) were randomly divided into 2 groups. Group 1 started with one week mobilization, followed by one week wash-out and one week control. Group 2 started with one week control, followed by one week wash-out and one week mobilization.
Electrophysiological recording. Two self-adhesive pre-gelled electrodes (silver chloride (AgCl), type T3404, Bio-Medical Instruments Inc, USA, 1.1 mm in diameter, 25 mm inter-electrode distance) were placed under the chin over the SM muscles (mylohyoid, geniohyoid and anterior digastric muscles) and 1 reference electrode on the lateral epicondyl of the left elbow (skin was cleaned using pure alcohol and shaved when necessary). All raw sEMG signals were simultaneously sampled at 2500 Hz by a universal amplifier (Bimec, IDEE/Maastricht Instruments, Maastricht, The Netherlands) and stored on a personal computer. During sampling, the operator added a digital flag to the signal at each visually observed swallowing movement of the subject (by inspection of laryngeal movements). Filtering (Butterworth 4th order, high-pass 5 HZ and notch-filtered) and signal-processing (amplification and rectification) was performed using data-acquisition software (IdeeQ version 1.92, IDEE/Maastricht Instruments, Maastricht, The Netherlands). These electrophysiological evaluations have been reported to be reproducible and reliable for the assessment of dysphagia (14).
Statistical analysis
Statistical analysis was performed using SPSS for Windows (release 15.0.1). Median values and percentiles 25–75 are given. Since the number of participants was small and the primary outcome-measure was scored on an ordinal scale, non-parametric techniques (with exact testing) were used. Baseline values between both groups were compared using the Mann-Whitney U test. Changes in dysphagia limit were computed following one session and following one week intervention (3 sessions). Differences in evolution and changes over time were analysed using the Wilcoxon signed-rank test. The significance level was set at 2-sided p < 0.05.
RESULTS
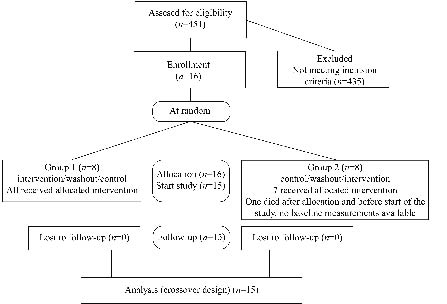
One female patient (age 80 years) became severely ill (with lung emboli) after the randomization procedure and before the start of the baseline measurements and intervention. Her clinical condition did not allow any evaluation of dysphagia limit and she died 2 weeks after randomization. Since this event was unrelated to the planned intervention, this patient was excluded from all further analyses (see Fig. 2). The characteristics of the remaining participants are shown in Table I. Twelve patients presented cervical anteroposition (C0–C3 extended and C3–C7 flexed) and 3 showed cervical kyphosis (C0–C7 flexed).
Fig. 2. Study flow diagram.
| Table I. Participants’ characteristics. Group 1 started with one week of cervical spine mobilization, followed by one week wash-out and one week as control. Group 2 started with one week as control, followed by one week wash-out and one week of cervical spine mobilization (see Figs 1 and 2). |
| Parameter | Group 1 | Group 2 |
| Women (n = 5) | Men (n = 3) | Women (n = 4) | Men (n = 3) |
| Age, years, median (25%–75%) | 91.0 (83.0–93) | 82.0 (77.0–86.0) | 85.0 (82.0–89.5) | 83.0 (82.0–88.0) |
| MMSE, score/30, median (25%–75%) | 7.0 (4.0–9.0) | 12.0 (8.0–21.0) | 10.5 (5.0–13.5) | 7.0 (0.0–13.0) |
| Weight, kg, median (25%–75%) | 54.9 (52.5–56.8) | 64.0 (59.0–86.7) | 57.0 (50.15–62.4) | 58.5 (57.5–79.0) |
| Height, cm, median (25%–75%) | 151.0 (148.0–156.5) | 163.0 (154.0–169.0) | 153.0 (145.5–155.5) | 170.0 (164.0–174.0) |
| Body mass index, median (25%–75%) | 21.4 (20.9–27.2) | 27.0 (22.2–30.4) | 24.2 (22.8–26.6) | 21.8 (19.9–26.1) |
| Physical dependency*, score/24, median (25%–75%) | 23.0 (21.0–24.0) | 19.0 (18.0–19.0) | 23.5 (22.0–24.0) | 24.0 (19.0–24.0) |
| Head and neck posture, n |
| Anteroposition | 4 | 2 | 3 | 3 |
| Kyphosis | 1 | 1 | 1 | 0 |
| Co-morbidity, n | | | | |
| Osteoporosis | 4 | 1 | 3 | 2 |
| Osteoarthritis | 0 | 2 | 3 | 1 |
| Type-2 diabetes mellitus | 0 | 0 | 1 | 0 |
| Chronic heart failure | 1 | 0 | 1 | 2 |
| Arterial hypertension | 1 | 0 | 1 | 0 |
| Chronic obstructive pulmonary disease | 2 | 0 | 2 | 1 |
| Gastrointestinal disorder | 3 | 2 | 4 | 2 |
| Gout | 0 | 1 | 0 | 0 |
| *Physical dependency following Katz et al. (13), with higher scores indicating worse physical dependency. MMSE: Mini-Mental State Examination. |
Feasibility
Ninety percent of the cervical spine mobilization sessions were performed successfully (i.e. the patient was available for treatment and showed no hostility to the intervention) and no complications were observed. In all participants, the first mobilization session was performed successfully, but 5 of the follow-up sessions were unsuccessful. Three of these sessions were impossible due to patient’s hostility (second and third session in one patient, third session in another patient) and 2 due to illness (second and third session in one patient due to exacerbation of pressure ulcers and fever).
Dysphagia limit
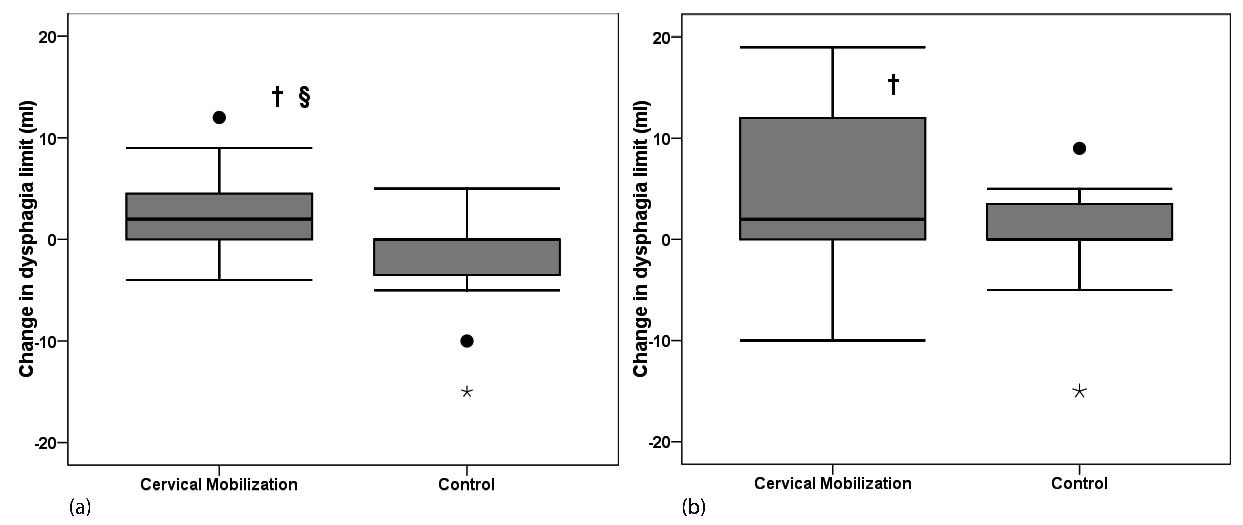
At baseline, no significant difference was observed between both groups (p = 0.29). As shown in Fig. 4, the dysphagia limit improved significantly following one session of cervical spine mobilization (p = 0.01) compared with control (no change p = 0.27, difference in evolution p = 0.03). After one week of cervical spine mobilization, the dysphagia limit remained improved (p = 0.03), but did not change significantly following one week control condition (p = 0.42, difference in evolution p = 0.12).
Fig. 4. Improvement of dysphagia limit following cervical spine mobilization. (a) After one session. (b) After one week intervention. °Outlier, *extreme, †significant improvement (p < 0.05), §significant difference in evolution between mobilization and control (p < 0.05).
DISCUSSION
This randomized controlled trial investigated the feasibility and benefits of gentle manual mobilization of the cervical spine for dysphagia in elderly residents in a nursing home presenting severe cognitive decline. Only 2 patients showed hostility during the treatment and no complications were observed, thus highlighting the feasibility of the procedure in these frail elderly patients. The results indicate that cervical spine mobilization can attenuate dysphagia. In fact, the dysphagia limit improved significantly following only one session of manual mobilization (p = 0.01) compared with control (no change p = 0.27, difference in evolution p = 0.03), indicating rapid benefits following postural correction of the cervical spine. After one week of cervical mobilization (3 sessions), the dysphagia limit remained improved (p = 0.03), while there were no significant changes following a one week control (p = 0.42, difference in evolution p = 0.12). The fact that in 3 patients the follow-up sessions were impossible due to hostility or illness has reduced the statistical power of the analysis, which may have contributed to the absence of a statistically significant difference between the improvements following one week of treatment compared with one week control.
The cross-over-design of the study, allowing every patient to be his or her own control, was chosen in order to reduce possible bias due to heterogeneity between participants and thus optimize study power. Post hoc power calculations (15) showed that the observed changes in dysphagia limit with alpha = 0.05 presented a power of 80% for the changes after one mobilization session, 77% for the changes after one week intervention, 79% for the difference in evolution between intervention and control after one session, and 40% for the difference in evolution between intervention and control after one week. Although a type-1 error cannot be excluded completely, the significant changes in dysphagia limit that we found in our study present sufficient statistical power (77–80%). The absence of a significant difference in evolution between intervention and control after one week (p = 0.12) might also be due to a type-2 error given the low power of that analysis (40%). Also, a relatively short study-duration per participant (total of 3 weeks) was adopted given the unstable clinical character of frail elderly residents in nursing homes, illustrated by the fact that within this short period one subject died and another became ill. For the same reason a minimal duration was chosen for the wash-out period (one week). Given the limited learning effects that can be expected in elderly patients with dementia, we hypothesized that after one week the treatment effects would have been washed out. It cannot be excluded that a longer treatment and wash-out period would have increased the power of the difference in changes between intervention and control over one week. A longer follow-up period in future studies might be interesting in order to investigate the long-term effects of cervical spine mobilization on dysphagia in frail elderly residents in nursing homes, although a much larger population should be recruited given the high risk for drop-out and interference with the unstable health condition of these patients.
In our study, the dysphagia limit as described by Ertekin et al. (12) was used as measure for dysphagia in our participants. Previous reports have demonstrated that the dysphagia limit is valid for the detection of oropharyngeal dysphagia (specificity and sensitivity 100% and 95.4%, respectively, using 20 ml as the threshold value (12)), and is responsive to changes following treatment (12) and changes in head and neck posture (9). Several studies found a strong relationship between the onset of SM activity and biomechanical events (antero-superior displacement of the hyoid), allowing an objective evaluation of the number of swallowing events during fluid ingestion (16–18). We chose this approach as it is not invasive and is feasible in frail elderly patients with severe cognitive decline. One patient reached the dysphagia limit of 20 ml (threshold value for normal deglutition (12)) after one session (dysphagia limit at baseline = 15 ml) and 4 patients after one week (dysphagia limit at baseline = 1 ml (in 2 patients), 3 ml and 10 ml). The median improvements of the dysphagia limit following manual mobilization of the cervical spine (from 3 ml (percentile (P)25–75 = 1–10) to 5 ml (P25–75 = 3–15) after one session and from 3 ml (P25–75 = 1–10) to 10 ml (P25–75 = 5–20) after one week) seem clinically significant and it can be assumed that this might have had beneficial effects during eating in our patients. However, the effects on nutritional intake and body mass were not assessed in our study, and these are issues that need to be explored further in future studies.
Despite their high age and cognitive decline, all participating patients were fed orally. The burden of complications (e.g. aspiration pneumonia) due to dysphagia is well documented in elderly patients with dementia (1–4). From an ethical perspective, a conservative intervention that is able to increase the swallowing capacity, and thus comfort during eating, in dysphagic elderly patients with dementia should be considered as valuable. Moreover, as stated recently in a consensus report for designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons, exclusion of frail elderly persons with cognitive decline and excessive safeguards may halt the development of new care strategies that may improve their health and quality of life (19). The cervical spine mobilization that was applied in our study consisted of gentle, slow, free passive movements of the head with low force and within the available range of movement. Contrary to the high-velocity thrust techniques, the type of mobilization techniques used in our study are considered to be safe, even in patients presenting osteoporosis (20–22). The cervical spine mobilization is pain-free and does not involve mechanical devices.
Besides cognitive dysfunction, Alzheimer’s disease is associated with important motor impairments. In particular, bradykinesia, postural alterations and paratonia are described in these patients (6–8). Paratonia differs from other neurological motor signs such as rigidity (as seen in Parkinson’s disease) by the fact that the resistance observed during passive mobilization is related to the speed of the movement (higher speed = higher resistance) (7, 8). Although cortical activity is involved in oropharyngeal swallowing (15), here we hypothesized that postural alteration of the neck and head due to paratonia contributes (at least partly) to dysphagia in frail elderly nursing home residents with severe dementia. Since our patients were not able to exercise consciously due to their severe dementia, we choose gentle passive manual mobilization of the cervical spine as a rehabilitation tool in order to improve posture and attenuate dysphagia. The influence of head and neck posture on oropharyngeal swallowing has been previously demonstrated by Ertekin et al. (9), with worse dysphagia limits in a “chin-up” position. This position (extended upper cervical segments) corresponds well to the postural alterations seen in our patients (mostly cervical anteroposition). Therefore, it can be assumed that the improved dysphagia limits in our study were induced by correcting the head and neck posture during cervical spine mobilization. However, we did not quantify changes in neck and head posture following treatment in our patients, and more research is needed in this area in order to identify the mechanisms by which cervical spine mobilization can improve dysphagia in frail and dementing elderly patients. All patients participating in our study presented dysphagia without known underlying neurological disorders except Alzheimer’s disease. The effects of cervical spine mobilization in nursing home residents with dysphagia due to other (neurological) origins remains unclear.
Our study has several limitations. First, despite the cross-over design and sufficient power for most statistical analyses, the sample size was low. Secondly, as discussed previously, a short treatment and wash-out period was chosen, which might have affected the power of the analysis of changes over one week intervention. Thirdly, the effects of cervical spine mobilization on posture, nutritional intake and body composition were not assessed.
From the results of this study we conclude that gentle cervical spine mobilization in elderly dysphagic nursing home residents with severe cognitive impairment is feasible and can improve swallowing capacity. Postural correction of the cervical spine therefore merits a broader application in the management of these patients. Given the acute improvements following treatment, cervical spine mobilization is probably best provided before meals. Future research is necessary in order to identify the mechanism by which cervical spine mobilization improves dysphagia, as well as its impact on eating and drinking.
REFERENCES
1. Kalia M. Dysphagia and aspiration pneumonia in patients with Alzheimer’s disease. metabolism (aging: beneficial effects on patients from recent advances in genetics, neurobiology, and physiology). Metabolism 2003; 52 Suppl 2: 36–38.
2. Janssens J-P, Krause K-H. Pneumonia in the very old. Lancet Infect Dis 2004; 4: 112–124.
3. Wada H, Nakajoh K, Satoh-Nakagawa T, Suzuki T, Ohrui T, Arai H, et al. Risk factors of aspiration pneumonia in Alzheimer’s disease patients. Gerontology 2001; 47: 271–276.
4. Kayser-Jones J, Pengilly K. Dysphagia among nursing home residents. Geriatric Nursing 1999; 20: 77–83.
5. Steele CM, Greenwood C, Ens I, Robertson C, Seidman-Carlson R. Mealtime difficulties in a home for the aged: not just dysphagia. Dysphagia 1997; 12: 43–50.
6. Scarmeas N, Hadjigeorgiou GM, Papadimitriou A, Dubois B, Sarazin M, Brandt J, et al. Motor signs during the course of Alzheimer disease. Neurology 2004; 63: 975–982.
7. Kurlan R, Richard IH, Papka M, Marshall F. Movement disorders in Alzheimer’s disease: more rigidity of definitions is needed. Mov Disord 2000; 15: 24–29.
8. Hobbelen JS, Koopmans RT, Verhey FR, Van Peppen RP, de Bie RA. Paratonia: a Delphi procedure for consensus definition. J Geriatr Phys Ther 2006; 29: 50–56.
9. Ertekin C, Keskin A, Kiylioglu N, Kirazli Y, On AY, Tarlaci S, et al. The effect of head and neck positions on oropharyngeal swallowing: a clinical and electrophysiologic study. Arch Phys Med Rehabil 2001; 82: 1255–1260.
10. American Gastroenterological Association medical position statement on management of oropharyngeal dysphagia. Gastroenterology 1999; 116: 452–454.
11. van der El A, Lunacek P, Wagemaker A, editors. Manuele therapie deel II: Wervelkolom behandeling [Manual therapy part II: Spinal treatment]. 2nd edn. Rotterdam: Uitgeverij Manuwel; 1993.
12. Ertekin C, Aydogdu I, Yuceyar N. Piecemeal deglutition and dysphagia limit in normal subjects and in patients with swallowing disorders. J Neurol Neurosurg Psychiatry 1996; 61: 491–496.
13. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963; 185: 914–919.
14. Ertekin C, Aydogdu I, Yuceyar N, Tarlaci S, Kiylioglu N, Pehlivan M, et al. Electrodiagnostic methods for neurogenic dysphagia. Electroencephalogr Clin Neurophysiol 1998; 109: 331–340.
15. Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials 1990; 11: 116–128.
16. Ertekin C, Pehlivan M, Aydogdu I, Ertas M, Uludag B, Celebi G, et al. An electrophysiological investigation of deglutition in man. Muscle Nerve 1995; 18: 1177–1186.
17. Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol 2003; 114: 2226–2244.
18. Argon M, Secil Y, Duygun U, Aydogdu I, Kocacelebi K, Ozkilic H, et al. The value of scintigraphy in the evaluation of oropharyngeal dysphagia. Eur J Nucl Med Mol Imaging 2004; 31: 94–98.
19. Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB Jr, Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc 2004; 52: 625–634.
20. Refshauge KM, Parry S, Shirley D, Larsen D, Rivett DA, Boland R. Professional responsibility in relation to cervical spine manipulation. Aust J Physiother 2002; 48: 171–179; discussion 180–185.
21. Sran MM, Khan KM. Is spinal mobilization safe in severe secondary osteoporosis? – a case report. Man Ther 2006; 11: 344–351.
22. Sran MM, Khan KM, Zhu Q, McKay HA, Oxland TR. Failure characteristics of the thoracic spine with a posteroanterior load: investigating the safety of spinal mobilization. Spine 2004; 29: 2382–2388.