OBJECTIVE: To examine effectiveness of standardized occupational therapy and physical therapy assessments in detecting functional changes and predicting clinical improvement in patients with suspected normal pressure hydrocephalus undergoing cerebrospinal fluid drainage.
DESIGN: Cohort study.
Patients: Eighty-seven patients admitted to an inpatient neurology unit for elective cerebrospinal fluid drainage for suspected normal pressure hydrocephalus.
METHODS: Before and after a protocol of continuous cerebrospinal fluid drainage via spinal catheter, patients were administered the Functional Independence Measure (FIMTM), Timed Up and Go (TUG), Tinetti Assessment Tool of Gait and Balance, 9-hole peg test, and Cognitive Assessment of Minnesota (CAM). Following cerebrospinal fluid drainage, changes in functional performance were compared for responders to cerebrospinal fluid drainage and non-responders to cerebrospinal fluid drainage.
RESULTS: At baseline, CAM was more sensitive than the Mini Mental State Exam in predicting responders. Post-drainage: responders improved on 52% of tests while non-responders improved on only 11%. Assessments that differentiated magnitude of improvement in responders vs non-responders were: TUG (p < 0.05), Tinetti total (p < 0.001), Tinetti balance (p < 0.001), Tinetti gait (p < 0.001), FIM toilet transfer (p < 0.001), and FIM lower body dressing (p < 0.001).
CONCLUSION: Specific occupational therapy and physical therapy assessments demonstrate sensitivity to change and predictive value with patients with suspected normal pressure hydrocephalus undergoing cerebrospinal fluid drainage.
Key words: normal pressure hydrocephalus, occupational therapy, physical therapy, rehabilitation, functional status, cognition, outcome measurement, assessment.
J Rehabil Med 2008; 40: 715–720
Correspondence address: Felicia Hill-Briggs, Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Medical Institutions, 2024 E. Monument Street, Suite 2-600, Baltimore, MD 21205, USA. E-mail: fbriggs3@jhmi.edu
Submitted December 18, 2007; accepted May 5, 2008
INTRODUCTION
Idiopathic normal pressure hydrocephalus (NPH) is caused by a dysfunction in the flow of cerebrospinal fluid (CSF) resulting in an abnormal increase of CSF in the ventricles of the brain (1). Gait disturbance, urinary incontinence and cognitive decline make up the triad of symptoms, which tends to characterize patients with NPH (2). Monitoring of changes in these functional symptoms is utilized to help diagnose probable NPH, to determine effectiveness of CSF drainage and likelihood of therapeutic benefit from shunt surgery, and course of improvement following shunt surgery.
Although the rehabilitation disciplines of occupational therapy (OT) and physical therapy (PT) are part of medical teams providing evaluation and therapy for patients with NPH in acute settings, there has been limited to no research reporting objective measurement of functional and cognitive status in cohorts of patients with NPH using OT and PT assessments. The most commonly reported evaluations used to gauge changes in functional status over the course of NPH diagnosis and treatment are the neurological examination (1, 3) and neuropsychological evaluation (4–6). PT expertise in assessing gait impairment, which is one of the most common NPH symptoms (7, 8), and OT expertise in assessing changes in performance of personal and instrumental activities of daily living (ADLs), psychomotor speed, and cognition can make a valuable contribution to the identification and quantification of loss of functional ability that accompanies NPH. Perhaps most importantly, OT and PT assessments can uniquely characterize functional abilities within the context of performance of routine life activities that are germane to quality of life.
The disciplines of OT and PT have a repertoire of evaluations for neurological conditions (9, 10), but, to date, there is no body of research describing OT and PT assessment specifically in patients with NPH. Moreover, although functional status is a primary criterion in determining suitability of patients for shunt surgery, no OT and PT assessments have been studied in order to determine differential effectiveness and sensitivity of standardized assessments to change following CSF drainage.
The purpose of this study, therefore, was threefold: (i) to examine performance of selected OT and PT standardized assessments administered during the routine care of patients with suspected NPH undergoing a CSF drainage procedure; (ii) to determine the sensitivity of these OT and PT assessments to change following CSF drainage; and (iii) to determine whether the assessments were sensitive to differential functional performance between patients determined to be Responders to CSF drainage and those determined to be Non-Responders to CSF drainage. We consider this study to be the first phase in identifying evidence-based rehabilitation assessments needed for optimal effectiveness in working with patients with NPH during the various phases of NPH diagnosis and treatment.
METHODS
Patient selection
Research participants were patients with suspected NPH who were admitted to an inpatient neurology unit for elective spinal catheter insertion for controlled cerebrospinal fluid drainage between January 2003 and February 2005. To be eligible for inclusion in the study reported herein, patients were required to have: (i) gait and/or balance disturbance either in combination with impaired cognition and bladder control upon admission; (ii) documentation of ventricular enlargement (e.g. Evan’s index ≥ 0.3) (11) on computerized tomography (CT) or magnetic resonance imagining scan (MRI); and (iii) completed PT and OT assessments at the designated pre- and post-CSF drainage evaluation time-points. Patients were excluded if: (i) no or minimal gait disturbance with severe dementia was observed during initial physician evaluation; (ii) no ventricular enlargement was observed on radiological workup; (iii) the patient had previous treatment for hydrocephalus; (iv) catheter-associated meningitis occurred; (v) the catheter had to be removed due to low CSF pressure symptoms; or (vi) the subject was too impaired to participate in both evaluation time-points. The study was approved by the Institutional Review Board, and informed consent was obtained.
Procedures
Patients meeting eligibility criteria described above were admitted to the hospital for 2 days of continuous CSF pressure monitoring followed by 3 days of controlled CSF drainage. The external lumbar drainage (ELD) procedure consisted of insertion of a spinal catheter into the lumbar subarachnoid space under local anesthesia, followed by recording of CSF pressure continuously for 2 days. Abnormal CSF pressure waveforms were identified according to criteria adapted from the original description of Lundberg (12). After the 2 days of pressure monitoring, 3 days of controlled continuous CSF drainage was performed. The CSF drainage rate was controlled to approximately 10 ml/h (240 ml/day). Patients were examined clinically for their response at least once daily. An expert NPH neurologist (M.W.), in accordance with NPH consensus guidelines (13, 14), classified patients as appropriate for CSF shunting only if they had: (i) ventriculomegaly confirmed on CT or MRI; (ii) presence of 2 or more clinical features of NPH; (iii) either A- or B-waves present during artifact-free time on continuous CSF pressure monitoring; and (iv) clinical improvement in symptoms (gait, cognition, or bladder control) during a 3-day trial of controlled CSF drainage, based on clinical examination, mental status exam, and detailed symptom observation questionnaires completed by patients or their legal guardians.
OT and PT assessments were conducted independently of the neurological examinations and monitoring. The patients received initial OT and PT assessments prior to insertion of the catheter for ELD. The OT and PT standardized assessments were repeated after the ELD was removed and the neurologist was satisfied with the amount of CSF drained (as detailed above). Therapists conducting the post-CSF drainage assessments were blinded to the neurologists’ classification of patients’ responder status.
Measures
The OT and PT assessments consisted of 6 functional status measures testing domains of ADLs, mobility, and cognition.
Functional Independence Measure (FIMTM) (15). Developed through a collaboration between the American Congress of Rehabilitation Medicine and the American Academy of Physical Medicine and Rehabilitation as a uniform method for gauging disability, the FIMTM is the most widely accepted functional measure utilized by the rehabilitation community within the USA. Six FIMTM measures of ADL were included in this study: feeding, grooming, dressing-upper body, dressing-lower body, toileting, and toilet transfers. The FIMTM is a clinician rating tool with 7 classifications of patient functional status, ranging from total assistance to independence. Clinicians who provided FIMTM ratings had completed FIMTM standardized training, competency examination, and certification. The FIMTM has demonstrated reliability and validity across a variety of settings, raters, and patients, with an inter-rater reliability of 0.95 and a test-retest reliability of 0.95 (16, 17).
Timed “Up & Go” Test (TUG) (18). The TUG is a timed observation of a patient’s ability to rise from an armchair, walk 10 feet, turn, walk back, and sit down again. The TUG test is a reliable and valid test for predicting fall risk at home, quantifying functional mobility, and monitoring clinical change over time (18, 19).
Tinetti Assessment Tool of Gait and Balance (Tinetti) (20). The Tinetti is an observed performance assessment of mobility. The scale has 2 subtests: (i) Tinetti Balance, with subtests of sitting balance, sit-to-stand, immediate standing balance, standing balance, balance with eyes closed, turning 360°, and sitting down; and (ii) Tinetti Gait, with subtests of gait initiation, step length, step symmetry and continuity, path deviation, trunk stability, and walking stance. The maximum total Tinetti score is 28 points. Higher scores indicate better mobility. The Tinetti test has intra- and inter-rater reliability ranging from 0.93 to 0.99 regardless of the subject’s level of cognitive impairment, and strong discriminant validity (20, 21).
9-Hole Peg Test (Peg Test) (22). This is a timed test of fine motor speed that utilizes a peg board. The subject uses the right hand and left hand in separate, consecutive trials to place 9 dowels into 9 holes on the board and then to remove them all. The score is the amount of time, in sec, taken to perform the task using each hand. Higher scores indicate greater impairment. The test has good test-retest reliability and concurrent/convergent validity (22, 23).
Cognitive Assessment of Minnesota (CAM) (24). The CAM is designed to assess a hierarchy of cognitive skills from a rehabilitation perspective. The CAM provides an objective baseline from which to measure change and treatment outcome. Cognitive skills assessed are: attention, memory/orientation, neglect, ability to follow directions, immediate memory, temporal awareness, matching skills, object identification, visual memory, recall/recognition, auditory memory, simple money skills, simple math skills, foresight/planning, safety/judgment, concrete problem solving, and abstract reasoning. The maximum score is 80 points, with cut-off scores to identify severity of impairment. The CAM has acceptable inter-rater reliability, is sensitive to cognitive impairment in traumatic and non-traumatic brain injury or illness, and has 95% specificity to correctly classify patients with and without cognitive impairment (24).
Mini Mental State Exam (MMSE) (25, 26). The MMSE is a widely used brief screening tool for detection and quantification of severity of mental status impairment in adults. It comprises 30 items assessing domains of orientation, registration, attention and calculation, recall, and language. The maximum score is 30 points, and cut-off scores of 25 and 23 are commonly used thresholds for detection of impairment (26).
Statistical analyses
For all analyses, the sample was stratified by Responder/Non-Responder status to CSF drainage. t-tests with equal variance were applied to test differences in scores between the 2 groups at baseline and post-drainage. Paired t-tests comparing baseline and post-drainage scores were performed for the Responder and the Non-Responder groups independently. Change scores, reflecting change in test scores from baseline to post-drainage were also compared between Responders and Non-Responders using t-tests. The level of significance was set at p < 0.05.
Logistic regression models for binary variables were used to estimate odds ratios that indicate probability of being responder based on change scores. Only tests for which the change scores were significantly different between Responders and Non-Responders were used. Unadjusted and adjusted models were tested. For the adjusted model, demographic characteristics that were different between groups (i.e. gender) were entered in the regression model. All statistical analyses were performed using the STATA 8 statistical package.
RESULTS
Patient characteristics
One hundred and thirty-two patients were screened. Of these, 87 (51 men, 36 women) met eligibility criteria and were included in the study. At baseline, demographic characteristics of Responders and Non-Responders were similar for age (mean 72.3 (standard deviation (SD) 9.0) and 72.0 (SD 13.3), respectively) and race/ethnicity (92.3% vs. 97.1% white, respectively). However, the proportion of men was significantly higher in the Responder group than in the Non-Responder group (67.0% vs 45.7%, respectively, p < 0.05).
Functional status at baseline
Table I shows baseline scores for the tests of ADL, mobility, and cognitive function. Assessment of ADL function at baseline revealed no significant differences between persons who were subsequently determined to be CSF drainage Responders and those who were determined to be Non-Responders; both required supervision or set-up to perform personal care tasks prior to CSF drainage. With regard to baseline gait and balance, the Tinetti total score did not differ between the 2 groups; however, the walking stance subtest was significantly lower in Responders than Non-Responders (p < 0.01).
| Table I. Baseline scores for activities of daily living, mobility, and cognitive function tests in patients subsequently classified as responders and non-responders to cerebrospinal fluid drainage |
| Test | Responders (n = 54) Mean (SD) | Non-Responders (n = 33) Mean (SD) |
| FIMTM | | |
| Feeding | 6.9 (0.5) | 6.6 (1.1) |
| Grooming | 6.0 (1.1) | 5.9 (1.3) |
| Dressing Upper Body | 5.8 (1.2) | 5.7 (1.5) |
| Dressing Lower Body | 5.1 (1.5) | 5.0 (1.9) |
| Toilet Transfer | 5.3 (1.3) | 5.2 (1.8) |
| Toileting | 5.7 (1.5) | 5.3 (2.0) |
| Timed Up and Go Test | 45.6 (59.6) | 42.5 (49.0) |
| Tinetti Total Score | 16.1 (6.7) | 15.5 (8.3) |
| Tinetti Balance | 9.2 (4.0) | 8.9 (4.9) |
| Tinetti Gait | 6.9 (3.0) | 6.5 (3.7) |
| 9-Hole Peg Test | | |
| Right Handa | 32.4 (9.6) | 38.6 (26.5) |
| Left Handa | 38.1 (27.3) | 43.6 (30.8) |
| CAM Total Score* | 68.8 (7.0) | 62.9 (11.0) |
| MMSE | 26.1 (3.5) | 23.7 (6.3) |
| *p < 0.01 for difference in total scores between groups aFor 9-Hole Peg test, n = 52 for Responders and n = 30 for Non-Responders. FIMTM: Functional Independence Measure; CAM: Cognitive Assessment of Minnesota; MMSE: Mini Mental State Exam. |
Baseline MMSE scores were slightly higher in Responders than Non-Responders; however, this difference was not statistically significant. In contrast, baseline CAM total score differences between the 2 groups did reach statistical significance (p < 0.01) as well as clinical significance. CAM scores for Non-Responders were within the moderate to severe range of cognitive impairment, while Responders were within the mild to moderate impairment range.
Functional status following CSF drainage
Table II shows test and subtest scores following the ELD procedure. For Responders, CSF drainage resulted in statistically significant improvement on 24 out of 46 (52%) tests administered. In contrast, for Non-Responders, the procedure resulted in significant improvement on only 5 out of 46 (11%) tests. In Responders, the functional improvement was seen across domains of ADLs, mobility, and cognition. In Non-Responders, improvement was generally limited to select cognitive tasks, and the increased scores remained in a moderate to severe impairment range. CAM subtests on which Responders scored significantly higher than Non-Responders were auditory memory (p < 0.05), simple math skills (p < 0.01), simple money skills (p < 0.01), foresight/planning (p < 0.01), and abstract reasoning (p < 0.05).
| Table II. Test scores following cerebrospinal fluid drainage, by responder status |
| Test | Responders (n = 54) | Non-Responders (n = 33) |
| Post-Drainage Score | Post-Drainage Function | Post-Drainage Score | Post-Drainage Function |
| FIMTM | | | |
| Feeding | 6.88 | Stable | 6.69 | Stable |
| Grooming | 6.21* | Improved | 5.86 | Stable |
| Dressing Upper Body | 6.00* | Improved | 5.69 | Stable |
| Dressing Lower Body | 5.67*** | Improved | 5.03 | Stable |
| Toilet Transfer | 5.85*** | Improved | 5.26 | Stable |
| Toileting | 5.74 | Stable | 5.27 | Stable |
| Timed Up and Go | 29.91* | Improved | 57.28 | Stable |
| Tinetti Total Score | 19.31*** | Improved | 16.20 | Stable |
| Tinetti Balance Score | 10.92*** | Improved | 9.30 | Stable |
| Sitting Balancea | 0.97* | Improved | 0.93 | Stable |
| Arisesa | 1.33 | Stable | 1.29 | Stable |
| Attempts to Arisea | 1.77 | Stable | 1.50 | Stable |
| Immediate Standing Balancea | 1.53** | Improved | 1.07 | Stable |
| Standing Balancea | 1.37** | Improved | 1.29 | Stable |
| Nudgeda | 1.37 | Stable | 1.21 | Stable |
| Eyes Closeda | 0.53 | Stable | 0.50 | Stable |
| Turning 360°a | 1.23** | Improved | 1.00 | Stable |
| Sitting Downa | 1.23 | Stable | 1.07 | Stable |
| Tinetti Gait Score | 8.12*** | Improved | 6.61 | Stable |
| Initiation of Gaita | 0.87 | Stable | 0.64 | Stable |
| Step Length and Heighta | 3.20 | Stable | 2.50 | Stable |
| Step Symmetrya | 0.97** | Improved | 0.79 | Stable |
| Step Continuitya | 0.67 | Stable | 0.57 | Stable |
| Patha | 1.37* | Improved | 1.36 | Stable |
| Trunka | 0.83* | Improved | 0.86 | Stable |
| Walking Stancea | 0.50 | Stable | 0.29* | Improved |
| 9-Hole Peg Test | | | | |
| Right Handb | 31.17 | Stable | 41.87 | Stable |
| Left Handb | 33.42 | Stable | 46.92 | Stable |
| CAM Total Score | 70.87** | Improved | 64.21** | Improved |
| Attention | 2.79 | Stable | 2.46 | Stable |
| Memory/Orientation | 5.79 | Stable | 5.37 | Stable |
| Neglect | 2.00 | Stable | 1.97 | Stable |
| Follow Directions | 6.94 | Stable | 6.74 | Stable |
| Immediate Memory | 3.94 | Stable | 3.83 | Stable |
| Temporal Awareness | 1.98* | Improved | 1.89 | Stable |
| Matching | 3.00 | Stable | 2.91 | Stable |
| Object Identification | 3.00 | Stable | 2.94 | Stable |
| Visual Memory | 4.27 | Stable | 3.77 | Stable |
| Recall/Recognition | 4.67** | Improved | 4.04* | Improved |
| Auditory Memory | 5.42* | Improved | 5.03* | Improved |
| Simple Money Skills | 5.88* | Improved | 5.34 | Stable |
| Simple Math Skills | 5.48* | Improved | 4.60 | Stable |
| Foresight/Planning | 2.63 | Stable | 2.00 | Stable |
| Safety/Judgment | 2.83*** | Improved | 2.63* | Improved |
| Concrete Problem Solving | 7.65* | Improved | 6.66 | Stable |
| Abstract Reasoning | 2.58* | Improved | 2.03 | Stable |
| * p < 0.05, **p < 0.01 and ***p < 0.001, for Pre- and Post-drainage test score difference. an = 34 for Responders Group and n = 13 for Non-Responders Group. bn = 52 for Responders Group and n = 30 for Non-Responders Group. FIMTM: Functional Independence Measure; CAM: Cognitive Assessment of Minnesota. |
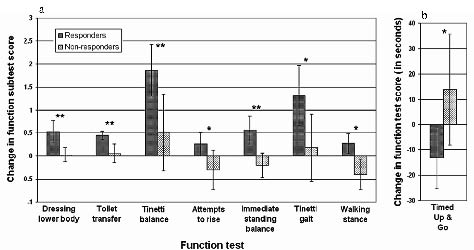
Using baseline to post-drainage change scores, eight tests differentiated Responders from Non-Responders with regard to magnitude of functional change post-drainage (Fig. 1). Change scores on these tests indicated improved ADL, gait, and balance function in Responders, while change scores indicated functional decline in gait and balance in Non-Responders.
Fig. 1. (a) Baseline to post-drainage change scores for eight tests and subtests demonstrating statistically significant differences between the Responder and Non-Responder groups. Subtests are Dressing Lower Body and Toilet Transfer (FIMTM);Tinetti Balance; Attempts to Rise and Immediate Standing Balance (Tinetti Balance subscales); Tinetti Gait; Walking Stance (Tinetti Gait subscale); and Timed Up and Go Test (b). Higher scores indicate better function on tests shown in (a). Lower scores indicate better function on Timed Up and Go (b). *p < 0.05, **p < 0.01.
Logistical regression analyses were conducted to control for gender as a possible confounder in the observed change scores between groups. As shown in Table III, in unadjusted models, with each unit increase in change score, the odds of being a Responder increased 3-fold for the FIM dressing lower body test and increased 4-fold for the FIM toilet transfer test. TUG, Tinetti total, Tinetti balance, and Tinetti gait change scores were also associated with significantly increased odds of being a Responder. These associations remained significant after controlling for gender, suggesting that each of these six tests/subtests is sensitive to detecting a magnitude of change in function following CSF drainage that differentiates Responders from Non-Responders.
| Table III. Unadjusted and gender-adjusted logistic regression models for tests predicting responder status based on test change scores from baseline to post-drainage |
| Covariates | Model I (Unadjusted) OR (95% CI) | Model II (Gender-adjusted) OR (95% CI) |
| FIMTM – Dressing Lower Body | 3.47* (1.35–8.89) | 3.56* (1.33–9.56) |
| FIMTM – Toileting | 4.74* (1.45–15.44) | 4.73* (1.46–15.33) |
| Timed Up and Go | 0.97* (0.94–1.00) | 0.97* (0.95–1.00) |
| Total Tinetti Score | 1.21** (1.05–1.39) | 1.22** (1.06–1.41) |
| Tinetti Balance | 1.34* (1.07–1.69) | 1.38** (1.09–1.75) |
| Tinetti Gait | 1.29* (1.02–1.63) | 1.30* (1.03–1.65) |
| *p < 0.05; **p < 0.01 OR: odds ratio; 95% CI: 95% confidence interval; FIMTM: Functional Independence Measure. |
DISCUSSION
To our knowledge, this is the first study of the use of standard OT and PT functional assessments in NPH, and the first study specifically designed to examine sensitivity of these assessments to changes in functional status in a trial of CSF drainage. Recently, evaluation of clinical and functional response to CSF drainage has been supported as an accurate method of selecting patients for shunt surgery for NPH (7, 13). However, no studies of this technique have utilized functional evaluations performed by occupational and physical therapists, despite their expertise in assessing ADL, mobility, and cognitive functions in the context of real life activities that contribute to quality of life. Findings from this study can contribute to compilation of a selective battery of OT and PT assessments with demonstrated sensitivity in measuring the functional changes produced by the CSF trial evaluation, first, and by the surgical intervention later.
Because improvement in gait usually precedes improvement in incontinence or cognition after shunt surgery (7, 27), test sensitivity to differential changes in gait is critical. We found that while TUG and Tinetti scores of gait and balance were similar in Responders and Non-Responders at baseline, following CSF drainage, only the Responders had significant improvement in the TUG and Tinetti total score as well as gait and balance subscores. This finding strongly supports future inclusion of Tinetti and TUG in an NPH assessment battery for patients assessed with CSF drainage.
With regard to ADLs, while baseline FIMTM ADL scores were similar in Responders and Non-Responders, only Responders had significant changes in ADL scores following CSF drainage. In particular, there was improvement in lower body dressing and toilet transfers, which corresponds with improvements in the TUG and Tinetti. Their inclusion in an NPH assessment battery is warranted and may prove to be useful for monitoring changes in ADL function as long-term outcomes of shunt surgery.
At baseline, we found the CAM to be more sensitive than the MMSE in predicting responder status, with Responders scoring 6 points higher than Non-Responders on the CAM, consistent with research showing that patients with milder dementia may show greater improvement with shunting (28). Cognitive impairment appears less likely to show short-term improvement after shunting for NPH. Nonetheless, Responders demonstrated statistically significant changes in eight of the subtests whereas Non-Responders improved significantly in only three subtests. The CAM subtests demonstrate that significant cognitive changes can be detected within a short time period (3 days of CSF drainage). Further exploration is needed in order to develop or modify existing cognitive assessments (i.e. neuropsychological, occupational therapy, speech language pathology) that are sensitive enough to measure the often subtle changes seen anecdotally, such as arousal, attention, encoding/retrieval, processing speed, and mental flexibility. It is noteworthy that, on post-hoc analysis, the difference in improvement of Responders and Non-Responders was much more significant on subtests of the CAM assessing higher cortical functions than those assessing lower functions. On CAM items resembling the MMSE, which can be characterized as lower cognitive functions (items 1 to 11), Responders showed significant improvement on 3 out of 11 (27.3%) subtests and Non-Responders on 2 out of 11(18.1%). However, on the CAM items that can be characterized as higher cognitive functions, (items 12 to 17), Responders had significant improvement on 5 out of 6 (83.3%) subtests whereas Non-Responders only had significant improvement only on 1 out of 6 (16.7%) (Fisher’s test p = 0.04). Further investigations should be performed in order to determine whether this subset of more sensitive items may serve as a more reliable and efficient cognitive assessment for use in NPH research and clinical practice. The CAM did function well as a screening tool for detecting cognitive deficits in probable NPH patients (CSF drainage Responders).
The 9-hole peg test scores did not change with CSF drainage, which may reflect either lack of change in psychomotor speed over a short time period or poor sensitivity of this measure. The Grooved Pegboard test (Lafayette Instruments, Lafayette, IN), which has demonstrated greater sensitivity to impaired fine motor speed and coordination than the 9-hole peg test (29), would be recommended for inclusion in a future battery to determine whether it is the test or the skill that has low predictive value in the context of the CSF drainage.
Limitations of the study are the following: We did not assess inter-rater reliability for tests utilizing clinical ratings of performance. However, the therapists who administered the tests were all experienced OT and PT professionals who were trained and certified to administer the tests used in this study. Moreover, the therapists who administered the tests were blinded to the neurologist’s classification of Responder vs. Non-Responder status, thereby eliminating this potential bias in test scoring or interpretation. Second, as with all functional assessments, some patients may have been too severely impaired to participate in certain parts of the tests. We have indicated where sample size was smaller on a given test or subtest, and the smaller sample size may have reduced statistical power. Third, a common limitation of any functional assessment is the possibility of floor and ceiling effects, with insensitivity in detecting and quantifying subtle differences in very severe impairment, and insensitivity in detecting very mild impairment, respectively.
Nevertheless, in this novel study, we demonstrated that specific, standard OT and PT functional tests were sensitive to change following CSF drainage during evaluation for NPH, and the magnitude of change on specific tests differentiated CSF Responders and Non-Responders. The most significant changes reflect ADL, balance, and gait. In addition, the OT cognitive assessment appeared sensitive in detecting subtle baseline differences in cognitive function between the groups, indicating potential predictive value. These results may be useful in creating a standardized functional NPH assessment battery that can be used by PT and OT who assist physicians in the evaluation of such patients.
ACKNOWLEDGEMENT
This work was supported by a research grant from Medtronic, Inc. and the Schoendorf Foundation (M.W. and D.R.).
REFERENCES
1. Graff-Radford NR. Normal pressure hydrocephalus. Neurol Clin 2007; 25: 809–832.
2. Vacca V. Diagnosis and treatment of idiopathic normal pressure hydrocephalus. J Neurosci Nurs 2007; 39: 107–111.
3. Savolainen S, Hurskainen H, Paljarvi L, Alafuzoff I, Vapalahti M. Five-year outcome of normal pressure hydrocephalus with or without a shunt: predictive value of the clinical signs, neuropsychological evaluation and infusion test. Acta Neurochir (Wien) 2002; 144: 515–523.
4. Devito EE, Pickard JD, Salmond CH, Iddon JL, Loveday C, Sahakian BJ. The neuropsychology of normal pressure hydrocephalus (NPH). Br J Neurosurg 2005; 19: 217–224.
5. Thomas G, McGirt MJ, Woodworth G, Heidler J, Rigamonti D, Hillis AE, et al. Baseline neuropsychological profile and cognitive response to cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord 2005; 20: 163–168.
6. Ogino A, Kazui H, Miyoshi N, Hashimoto M, Ohkawa S, Tokunaga H, et al. Cognitive impairment in patients with idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord 2006; 21: 113–119.
7. McGirt MJ, Woodworth G, Coon AL, Thomas G, Williams MA, Rigamonti D. Diagnosis, treatment, and analysis of long-term outcomes in idiopathic normal-pressure hydrocephalus. Neurosurgery 2005; 57: 699–705.
8. Cowan JA, McGirt MJ, Woodworth G, Rigamonti D, Williams MA. The syndrome of hydrocephalus in young and middle-aged adults (SHYMA). Neurol Res 2005; 27: 540–547.
9. Holm MB, Rogers JC. Occupational therapy assessment of adult brain function. In: Goldstein G, Beers SR, editors. Rehabilitation. New York: Plenum Press; 1998, p. 9–31.
10. Light KE, Reilly MA, Clendenin M. Physical therapy. In: Goldstein G, Beers SR, editors. Rehabilitation. New York: Plenum Press; 1998, p. 33–57.
11. Williams MA, Razumovsky AY, Hanley DF. Comparison of Pcsf monitoring and controlled CSF drainage diagnose normal pressure hydrocephalus. Acta Neurochir Suppl 1998; 71: 328–330.
12. Lundberg N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr Scand 1960; 36 Suppl 149: 1–193.
13. Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005; 57 Suppl 3: S17–S28.
14. Marmarou A, Bergsneider M, Relkin N, Klinge P, Black PM. Development of guidelines for idiopathic normal-pressure hydrocephalus: introduction. Neurosurgery 2005; 57 Suppl 3: S1–S3.
15. FIMTM Guide for the Uniform Data System for Medical Rehabilitation. Amherst, NY, Uniform Data System for Medical Rehabilitation, 1996.
16. Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil 1996; 77: 1226–1232.
17 Stineman MG, Shea JA, Jette A, Tassoni CJ, Ottenbacher KJ, Fiedler R, et al. The Functional Independence Measure: tests of scaling assumptions, structure, and reliability across 20 diverse impairment categories. Arch Phys Med Rehabil 1996; 77: 1101–1108.
18. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148.
19. Perell KL, Nelson A, Goldman RL, Luther SL, Prieto-Lewis N, Rubenstein LZ. Fall risk assessment measures: an analytic review. J Gerontol A Biol Sci Med Sci 2001; 56: M761–M766.
20. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med 1986; 80: 429–434.
21. Lin MR, Hwang HF, Hu MH, Wu HD, Wang YW, Huang FC. Psychometric comparisons of the timed up and go, one-leg stand, functional reach, and Tinetti balance measures in community-dwelling older people. J Am Geriatr Soc 2004; 52: 1343–1348.
22. Kellor M, Frost J, Silberberg N, Iversen I, Cummings R. Hand strength and dexterity. Am J Occup Ther 1971; 25: 77–83.
23. Oxford GK, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available Nine Hole Peg Test for finger dexterity. Am J Occup Ther 2003; 57: 570–573.
24. Rustard RA, Degroot TL, Junqkunz ML, Freeberg KS, Borowick LG, Wanttie AM, editors. The cognitive assessment of Minnesota: Examiners Guide. San Antonio: Harcourt Assessment, Inc.; 1993.
25. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198.
26. Folstein MF, Folstein SE, Fanjiang G, editors. Mini mental state examination clinical guide. Lutz, FL: Psychological Assessment Resources, Inc. (PAR); 2001.
27. Marmarou A, Young HF, Aygok GA, Sawauchi S, Tsuji O, Yamamoto T, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg 2005; 102: 987–997.
28. Croarkin E, Danoff J, Barnes C. Evidence-based rating of upper-extremity motor function tests used for people following a stroke. Phys Ther 2004; 84: 62–74.
29. Lezak M, Howieson DB, Loring DW, editors. Neuropsychological assessment. 4th edn. New York: Oxford University Press; 2004.