OBJECTIVES: To investigate the applicability of the Swedish Occupational Fatigue Inventory and its ability to identify different dimensions of fatigue in people with multiple sclerosis with varying degrees of disease severity, and the correlation of each of its 5 dimensions with the Fatigue Severity Scale.
DESIGN: An observational, prospective study.
SUBJECTS: Two hundred and nineteen outpatients: 59.5% had mild, 17% moderate and 23.5% severe disease severity; 83% received immunomodulatory treatment.
METHODS: Both questionnaires were administered at inclusion, and at 12 and 24 months. Analyses of internal consistency, item-total correlation, factor analysis and tests of correlations were performed.
RESULTS: The instrument was completed by 97% of subjects. Internal consistency was satisfactory in the dimensions Lack of energy, Lack of motivation and Sleepiness, but not in Physical exertion and Physical discomfort. Factor analysis revealed that all but 3 items (2 in Physical exertion, 1 in Physical discomfort) loaded satisfactorily in 5 dimensions. Correlations between the dimensions and the Fatigue Severity Scale were low, except for a moderate correlation found for Lack of energy.
CONCLUSION: The dimensions Lack of energy, Lack of motivation and Sleepiness appear applicable for use in people with multiple sclerosis. Further development of the physical dimensions and studies on the instrument’s capacity to measure changes are needed.
Key words: fatigue, ICF, measurement, multiple sclerosis, outcome assessment, reliability, validity.
J Rehabil Med 2008; 40: 737–743
Correspondence address: Sverker Johansson, Division of Neurology R54, Karolinska University Hospital, Huddinge, SE-141 86 Stockholm, Sweden. E-mail sverker.johansson@ki.se
Submitted June 28, 2007; accepted April 7, 2008
INTRODUCTION
Fatigue in multiple sclerosis (MS) is commonly defined as a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities (1). In most studies of people with MS fatigue is reported by the majority (2, 3). Fatigue negatively influences the social role performance (4) and quality of life of people with MS (5) and is a major barrier for “taking care of health” (6). The causes of fatigue are not well understood. Primary fatigue may be the result of processes within the central nervous system and/or of immune and/or neuroendocrine dysregulation (7, 8). Fatigue may also arise secondarily as a result of, for example, disability in mood (3, 9, 10), impaired sleep (5, 10, 11), physical deconditioning (8, 12) and medications (12, 13). Among the mechanisms suggested to play a role in MS-related fatigue are also personal factors (4, 14–16). Thus, there is little agreement regarding the aetiology of fatigue in people with MS; furthermore, there is no consensus on how it should best be assessed or treated.
The International Classification of Functioning, Disability and Health (ICF) (17) defines 2 sets of components: (i) functioning, encompassing body functions/body structures and activities and participation; and (ii) contextual factors, encompassing environmental and personal factors. According to the ICF, the different components involved in an individual’s functioning interact dynamically; thus, if the full health experience is to be described, it is important to collect data on various components independently and then to explore associations and causal links between them.
Instruments frequently used for assessing fatigue in people with MS include the Fatigue Severity Scale (FSS) (18), the Fatigue Impact Scale (FIS) (19) and the Modified Fatigue Impact Scale (MFIS) (1). The main emphases of these scales are, for the FSS, the perceptions of people with MS regarding the impact of fatigue on daily functioning; and, for the FIS and the MFIS, the impact of fatigue on quality of life through its effect on activities. These emphases suggest that these scales might be measuring aspects of the ICF component activities and participation. The Swedish Occupational Fatigue Inventory (SOFI) was developed to contribute to the understanding of the concept of fatigue by investigating the subjective qualities of fatigue in people in different occupations (20–23). The SOFI consists of 20 items belonging to the dimensions Lack of energy, Physical exertion, Physical discomfort, Lack of motivation and Sleepiness (23). In the context of the ICF, all dimensions may be seen as belonging to the component body functions. Thus, they might measure aspects of fatigue that are not covered in commonly used scales such as the FSS.
In summary, the negative impact of fatigue on daily activities in people with MS highlights the need to identify components modifiable by interventions. Knowledge of which dimensions of fatigue are present in people with MS is lacking. Thus, an instrument that assesses fatigue within, for example, body functions, and that can discriminate between different dimensions of fatigue is needed and should be tested in people with MS. The aims of this study were to investigate:
• the applicability of the SOFI, in a group of people with MS with varying degrees of disease severity;
• the ability of the SOFI to identify various dimensions of fatigue in people with MS; and
• correlation of each dimension of the SOFI with the FSS.
METHODS
Participants and procedures
This study was performed using data from an observational, prospective study of functioning, disability and resource utilization of health-related services in people with MS who were followed every 6 months for 2 years. Those eligible were all people with MS diagnosed according to the Poser criteria (24) who, from 1 February 2002 to 12 June 2002, were scheduled for an outpatient appointment with either of 2 of the senior neurologists at the MS Centre of the Department of Neurology of Karolinska University Hospital, Huddinge, in Stockholm, Sweden. Of the 255 eligible people with MS, 219 were included; of those 200 (91%) completed the study at 24 months. Detailed description of the study has been reported elsewhere (25). Data were collected in connection with regular visits by the people with MS to her/his senior neurologist, who determined disease course and assigned disease severity scores using the Expanded Disability Status Scale (EDSS) (26); scores were then categorized as EDSS normal (0), EDSS mild (1.0–3.5), EDSS moderate (4.0–5.5) or EDSS severe (6.0–9.5) in accordance with the Swedish MS Registry (27). The remaining data were collected by one of 5 research physiotherapists, when possible by the same investigator at the same time of day for all points of evaluation. Data collected at inclusion and at 12 and 24 months were used in the present study. Clinical and contextual characteristics of the sample at inclusion are presented in Table I. The study was approved by the ethics committee of Karolinska Institutet in Stockholm.
| Table I. Clinical and contextual characteristics of the sample (n = 219) |
| Characteristics | | |
| Women, n (%) | | 149 (68) |
| Age, years, mean (SD, range) | | 47 (12, 20–75) |
| Disease severity, n (%) |
| | EDSS mild, 1–3.5* | 130 (59.5) |
| | EDSS moderate, 4–5.5 | 37 (17) |
| | EDSS severe, 6–9.5 | 52 (23.5) |
| Years since diagnosis, mean (SD, range) | | 14 (10, 0–44) |
| Disease course, n (%) |
| | Relapsing remitting | 127 (58) |
| | Secondary progressive | 83 (38) |
| | Primary progressive | 9 (4) |
| Immunomodulatory treatment, n (%) | | 182 (83) |
| Pharmacological fatigue treatment, n (%) | | 23 (10) |
| *One person categorized as EDSS normal is presented within EDSS mild. EDSS: Expanded Disability Status Scale used for categorization of disease severity; SD: standard deviation. |
Instruments used to assess fatigue
The SOFI was originally developed to measure subjective dimensions of work-related fatigue in people from 16 occupational settings who rated 95 verbal expressions on a Likert scale (20). The instrument consists of 20 items, in which feelings of being tired are graded from 0 (not had such feelings at all) to 6 (had such feelings to a very high degree) (23). Factor analysis shows that the items have loadings distributed across 5 latent factors that can be interpreted as Lack of energy (items: worn out, spent, drained, overworked), Physical exertion (items: palpitations, sweaty, out of breath, breathing heavily), Physical discomfort (items: tense muscles, numbness, stiff joints, aching), Lack of motivation (items: lack of concern, passive, indifferent, uninterested) and Sleepiness (items: falling asleep, drowsy, yawning, sleepy). Lack of energy is defined as a general latent factor, and the other 4 factors are assumed to represent unique differences in various states of fatigue (23). The applicability of the SOFI has been studied in patients with cancer undergoing radiotherapy treatment (28). Using the ICF (17) as a point of departure, all items can be regarded as belonging to the component body functions (b): b126; b130; b134; b265; b270; b280; b450; b455; b460; b710; b735; b780; b830.
The FSS (18) has been used frequently in studies of people with chronic conditions (18, 29) and in the general population (30). High internal consistency and test-retest reliability have been shown (29), as well as strong correlation with other fatigue scales (18, 29, 31). The mean score on the 9 FSS items is used as the FSS score (range 1–7), which can be categorized as non-fatigue (FSS ≤ 4.0), borderline fatigue (4.0 < FSS < 5.0) or fatigue (FSS ≥ 5.0) (2, 3, 16, 31). In the present study the FSS was completed at inclusion by 216 people with MS (99%), of whom 50% had fatigue, 17% borderline fatigue and 33% non-fatigue. The proportion of people with MS with fatigue, by EDSS category, was 43% with mild, 81% with moderate and 46% with severe disease severity. Using the ICF (17) as a point of departure, the items of the FSS can be considered as belonging to components of both body functions (b) and activity and participation (d): b130; b455; d175; d2; d4; d5; d6; d7; d8; d9. Six of the 9 items are predominantly related to the impact of fatigue on daily activities, while 3 items are primarily related to body functions.
In the present study the people with MS were asked to rate the statements in the SOFI and the FSS in relation to their experience during the past 6 months.
Statistical analysis
Descriptive statistics were used to present fatigue in the sample, with regard to the SOFI and the FSS. Compliance, the proportion of people with MS able to complete the SOFI, was analysed at the different points of data collection. The internal consistency of each dimension of the SOFI was calculated with Cronbach’s alpha at each point of data collection. Alpha values of 0.70–0.80 were regarded as satisfactory (32). In case of alpha values lower than 0.70, analyses were performed to explore if the internal consistency could be improved by removing 1 or 2 of the items of the dimension at a time while determining the alpha values for each possible combination. Correlations between each item and the total score of all other items of the same dimension were calculated with item-total correlation (ITC) for each point of data collection. ITCs higher than 0.30 were considered satisfactory (33). Internal consistency and ITCs were analysed with regard to the whole sample and to disease severity at inclusion, categorized as mild or moderate/severe disease severity.
Explorative factor analyses were performed on the data collected at inclusion to determine presence of underlying dimensions in the SOFI and factor loadings of the items included in the SOFI for people with MS. As the SOFI items are rated on an ordinal scale, factor analyses using polychoric correlations (34) and promax rotation (35) were performed. Factors with eigenvalues higher than 1 in the extraction phase were selected. Items with factor loadings higher than 0.50 and loading distinctly on one factor only were considered satisfactory. Correlations between the different dimensions arrived at in the final factor analysis of the SOFI were assessed by the Spearman’s rank correlation coefficient with the a priori hypothesis that the correlations would be at most low. In addition, correlations between the dimensions of the SOFI and the FSS were assessed by the Spearman’s rank correlation coefficient on data from people with MS who completed the SOFI both at inclusion and at 12 and 24 months with the a priori hypothesis that the correlations would be at most low. Coefficients from 0 to 0.25 were considered as “little if any correlation”; 0.26–0.49 as “low correlation”; 0.50–0.69 as “moderate correlation”; 0.70–0.89 as “high correlation”; and 0.90–1.0 as “very high correlation” (36).
The SPSS, version 14.0 (SPSS Inc., Chicago, Illinois, USA), was used for the statistical analyses, except in the factor analyses, for which SAS® System 9.1 (SAS Institute Inc., Cary, North Carolina, USA) was used.
RESULTS
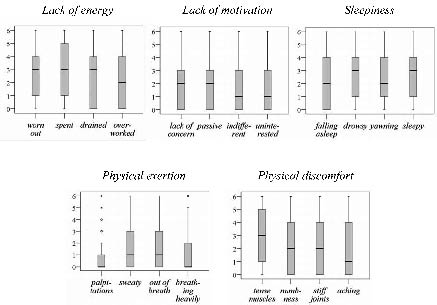
At inclusion and at 12 and 24 months, 213, 204 and 198 people with MS completed the SOFI: of these 128, 125 and 123 people with MS were categorized as having mild disease severity and 85, 79 and 75 people with MS as having moderate/severe disease at the different points of data collection. A total of 195 people with MS completed the SOFI at all evaluations. The proportion of people with MS who completed the SOFI at the points of data collection varied from 97% to 99%. Reasons for not completing the SOFI were inability due to MS, personal stress, exhaustion and refusal. The whole range (0–6) was used in all items of the SOFI and floor effects were found. In 2 items (palpitations, breathing heavily) more than 50% of the sample, and in 10 items (lack of concern, falling asleep, sweaty, passive, stiff joints, indifferent, out of breath, drained, aching, uninterested) more than 25%, scored zero at each point of data collection. In 2 items (numbness and overworked) more than 25% scored zero at least once. Box-plots of the distribution of the items within each dimension at inclusion are shown in Fig. 1.
Fig. 1. Box-plots of items within each dimension of the Swedish Occupational Fatigue Inventory, illustrating the distribution at inclusion (n = 213). The y-axis shows ratings describing feelings of being tired from 0 (not had such feelings at all) to 6 (had such feelings to a very high degree). The box length is the interquartile range, the bold line in the box is the median value, the bars describe the 1st and 4th quartiles, and the * describe extremes (values more than 3 box lengths from the upper or lower edge of the box).
In Table II mean scores, standard deviations, median scores, interquartile ranges and ranges for each dimension of the SOFI, based on data from 195 people with MS who completed all 20 items of the SOFI at inclusion, and at 12 and 24 months, are presented. When the dimensions were ranked by their median score, Lack of energy had the highest ratings at all points of data collection, while Physical exertion had the lowest.
| Table II. Mean scores, standard deviations (SD), median scores, interquartile ranges (IQR) and ranges in the 5 dimensions of the Swedish Occupational Fatigue Inventory (20 items), n = 195 |
| Dimension | Inclusion Mean (SD) Median (IQR) [Range] | 12 months Mean (SD) Median (IQR) [Range] | 24 months Mean (SD) Median (IQR) [Range] |
| Lack of energy | 2.7 (1.7) 2.8 (1.3–4.3) [0–6] | 2.5 (1.7) 2.5 (1.0–3.8) [0–6] | 2.5 (1.7) 2.3 (1.0–3.8) [0–6] |
| Physical exertion | 1.3 (1.22) 1.0 (0.3–2.3) [0–5.3] | 1.2 (1.16) 1.0 (0.3–2.0) [0–5.0] | 1.3 (1.28) 0.8 (0.3–2.0) [0–5.3] |
| Physical discomfort | 2.4 (1.5) 2.3 (1.0–3.5) [0–6] | 2.3 (1.4) 2.3 (1.0–3.3) [0–6] | 2.3 (1.5) 2.3 (1.0–3.3) [0–6] |
| Lack of motivation | 1.9 (1.5) 1.8 (0.5–2.8) [0–6] | 1.7 (1.5) 1.3 (0.5–2.8) [0–6] | 1.6 (1.5) 1.3 (0.3–2.8) [0–6] |
| Sleepiness | 2.6 (1.5) 2.5 (1.3–3.8) [0–5.5] | 2.3 (1.4) 2.3 (1.0–3.3) [0–5.8] | 2.3 (1.6) 2.0 (1.0–3.5) [0–6] |
At each point of data collection, internal consistency in the whole sample was satisfactory in: Lack of energy (0.87–0.91), Lack of motivation (0.88–0.92) and Sleepiness (0.81–0.86). The ITCs of these dimensions were also satisfactory: Lack of energy (0.70–0.84), Lack of motivation (0.67–0.88) and Sleepiness (0.55–0.76). The internal consistency did not reach a satisfactory level at all points of data collection in either Physical exertion (0.69–0.79) or Physical discomfort (0.68–0.75). Some ITCs were too low in Physical exertion (0.23–0.70), but all were satisfactory in Physical discomfort (0.33–0.61). Items with the lowest ITCs were palpitations and numbness. Further analyses revealed that the most satisfactory internal consistency in Physical exertion was achieved with palpitations and sweaty removed (0.81–0.84), and in Physical discomfort with numbness removed (0.70–0.75).
The internal consistency in mild disease and in moderate/severe disease was at each point of data collection satisfactory in: Lack of energy (0.88–0.92; 0.85–0.91), Lack of motivation (0.89–0.92; 0.86–0.91) and Sleepiness (0.86–0.86; 0.71–0.87). Also the ITCs were satisfactory: Lack of energy (0.70–0.85; 0.68–0.85), Lack of motivation (0.68–0.88; 0.61–0.88) and Sleepiness (0.62–0.77; 0.36–0.78). In Physical exertion, the internal consistency was satisfactory in mild disease (0.70–0.79), but not in moderate/severe disease (0.63–0.81); ITCs in mild disease were satisfactory (0.32–0.70), but were unsatisfactory in moderate/severe disease (0.06–0.75). In Physical discomfort, the internal consistency in mild disease was satisfactory (0.70–0.76), but not in moderate/severe disease (0.65–0.75): ITCs were satisfactory in mild disease (0.38–0.65), but not in moderate/severe disease (0.28–0.65). Items with the lowest ITCs in both categories were palpitations and numbness. In both mild and moderate/severe disease, the most satisfactory internal consistency in Physical exertion was achieved with palpitations and sweaty removed (0.76–0.84; 0.84–0.86). In Physical discomfort the most satisfactory internal consistency was achieved with numbness removed; in mild disease (0.70–0.76) and in moderate/severe disease (0.67–0.75).
The factor analysis identified 5 factors with initial eigenvalues of 0.42, 0.10, 0.07, 0.06 and 0.05, respectively, in total 0.70. After promax rotation, 17 items had loadings higher than 0.50 distinctly located in 5 factors (Table III). One item, palpitations, loaded close to 0.5 in 2 factors (0.57 and 0.43), thus implying unsatisfactorily, indistinct belonging. Numbness and sweaty, did not load satisfactorily in any factor. The explained variance was in the rotated model 0.08 for Lack of energy, 0.07 for Physical exertion, 0.09 for Physical discomfort, 0.11 for Lack of motivation and 0.08 for Sleepiness – in total 0.43. Additional analyses revealed that the most satisfactory loadings in 5 factors were achieved when palpitations, numbness and sweaty were removed (Table IV), the explained variance of the remaining 17 items was in total 0.47. The correlations between the 5 dimensions based on these 17 items were low, except for moderate correlations found between Lack of energy and both Lack of motivation (0.60) and Sleepiness (0.62), as well as between Lack of motivation and Sleepiness (0.61).Except for moderate correlations between Lack of energy and the FSS (0.53–0.61) at the 3 points of data collection, the correlations were low between the dimensions of the SOFI and the FSS: Physical exertion (0.32–0.42), Physical discomfort (0.36–0.49), Lack of motivation (0.40–0.52) and Sleepiness (0.43–0.52).
| Table III. Factor loadings of the Swedish Occupational Fatigue Inventory (20 items) (n = 213). Loadings higher than 0.50 are presented in bold. |
| | Factor 1 (Lack of energy) | Factor 2 (Physical exertion) | Factor 3 (Physical discomfort) | Factor 4 (Lack of motivation) | Factor 5 (Sleepiness) |
| Palpitations | 0.57 | 0.43 | –0.07 | –0.16 | –0.19 |
| Lack of concern | –0.03 | –0.02 | –0.06 | 0.90 | 0.05 |
| Worn out | 0.86 | –0.04 | 0.03 | 0.12 | –0.03 |
| Tense muscles | 0.06 | –0.03 | 0.86 | –0.05 | 0.01 |
| Falling asleep | –0.16 | 0.12 | 0.00 | 0.04 | 0.78 |
| Numbness | 0.37 | –0.04 | 0.47 | –0.24 | 0.09 |
| Sweaty | 0.08 | 0.49 | 0.18 | –0.07 | 0.27 |
| Spent | 0.54 | –0.04 | 0.07 | 0.12 | 0.29 |
| Drowsy | –0.02 | 0.06 | 0.12 | 0.28 | 0.60 |
| Passive | 0.01 | –0.05 | 0.04 | 0.81 | 0.07 |
| Stiff joints | –0.11 | 0.01 | 0.87 | 0.13 | –0.10 |
| Indifferent | 0.03 | 0.01 | 0.08 | 0.88 | –0.03 |
| Out of breath | 0.06 | 0.84 | –0.12 | 0.08 | 0.07 |
| Yawning | 0.10 | 0.02 | –0.07 | –0.06 | 0.78 |
| Drained | 0.56 | 0.02 | 0.04 | 0.24 | 0.17 |
| Sleepy | 0.07 | –0.09 | –0.06 | –0.04 | 0.93 |
| Overworked | 0.72 | 0.03 | 0.00 | 0.14 | 0.03 |
| Aching | 0.04 | 0.10 | 0.75 | 0.03 | –0.02 |
| Breathing heavily | –0.05 | 0.84 | 0.16 | 0.04 | –0.02 |
| Uninterested | 0.13 | 0.08 | –0.09 | 0.87 | –0.10 |
| Table IV. Factor loadings of the Swedish Occupational Fatigue Inventory (17 items) (n = 213). Loadings higher than 0.50 are presented in bold. |
| | Factor 1 (Lack of energy) | Factor 2 (Physical exertion) | Factor 3 (Physical discomfort) | Factor 4 (Lack of motivation) | Factor 5 (Sleepiness) |
| Lack of concern | –0.01 | –0.08 | –0.03 | 0.93 | 0.04 |
| Worn out | 0.94 | –0.02 | 0.00 | 0.03 | –0.07 |
| Tense muscles | 0.10 | –0.06 | 0.88 | –0.10 | 0.03 |
| Falling asleep | –0.18 | 0.20 | –0.03 | 0.04 | 0.79 |
| Spent | 0.65 | –0.05 | 0.09 | 0.03 | 0.25 |
| Drowsy | 0.02 | 0.03 | 0.14 | 0.28 | 0.58 |
| Passive | –0.00 | –0.11 | 0.10 | 0.81 | 0.09 |
| Stiff joints | –0.09 | 0.03 | 0.87 | 0.08 | –0.06 |
| Indifferent | 0.01 | 0.05 | 0.04 | 0.88 | –0.02 |
| Out of breath | 0.04 | 0.89 | 0.08 | 0.04 | 0.09 |
| Yawning | 0.09 | –0.04 | –0.02 | –0.04 | 0.78 |
| Drained | 0.66 | 0.09 | –0.01 | 0.14 | 0.12 |
| Sleepy | 0.13 | –0.08 | –0.05 | –0.06 | 0.89 |
| Overworked | 0.89 | 0.06 | –0.02 | 0.00 | –0.05 |
| Aching | 0.03 | 0.10 | 0.74 | 0.02 | 0.01 |
| Breathing heavily | 0.02 | 0.91 | 0.16 | –0.06 | –0.04 |
| Uninterested | 0.13 | 0.13 | –0.11 | 0.85 | –0.10 |
DISCUSSION
The present study analysed the applicability of the SOFI to people with MS and found that although the compliance with the instrument was good it was not acceptable for use in people with MS in the original 20-item version. Floor effects were found. Internal consistency and ITCs were satisfactory in the dimensions Lack of energy, Lack of motivation and Sleepiness, but not in Physical exertion and Physical discomfort. The internal consistency and ITCs were lower in people with MS with moderate/severe disease compared with people with MS with mild disease, although these differences were small. Internal consistency of the 2 physical dimensions was improved by the removal of 3 items. The 5 dimensions of the SOFI reported in healthy people were also found to be present in people with MS. Four out of 5 dimensions of the SOFI had low correlations with the FSS, implying that the instruments, at least partly, measure different constructs of fatigue. The fact that data collected at 3 time-points provided similar outcomes strengthens the results.
Ratings were found across each item’s entire range, but most items had floor effects. When the SOFI was studied in patients with cancer, the ratings were also on the lower end of the scale in all dimensions, but all dimensions exhibited variation in levels during radiotherapy treatment (28). Furthermore, when the SOFI was studied in different occupations, the various settings were associated with different levels in the dimensions (23). The results of both the present study and previous studies suggest that further development of the SOFI is needed to decrease the floor effects, e.g. by improving the functioning of rating scale categories. The highest mean and median scores on the SOFI at each point of data collection were found in Lack of energy, Physical discomfort and Sleepiness, while Lack of motivation and Physical exertion had lower ratings. However, since the properties of Physical discomfort and Physical exertion were found to be unsatisfactory, we cannot propose that the mean and median scores in these dimensions are appropriate descriptions of physical dimensions of fatigue in people with MS.
Internal consistency and ITCs were satisfactory in Lack of energy, Lack of motivation and Sleepiness. Unsatisfactory internal consistency and ITCs were found in Physical exertion and Physical discomfort, especially in people with MS with moderate/severe disease, which calls into question the validity of these dimensions. Although the internal consistency in these dimensions were lower and unsatisfactory in moderate/severe disease compared with mild disease, the differences were small. In previous studies of the SOFI in other populations: various occupations (20, 23), patients with cancer (28), and in the context of either physical (21) or mental work (22), these dimensions (in particular Physical exertion) also had lower internal consistency compared with the other dimensions. In one study (22), low variance in the ratings was suggested as a reason for the low internal consistency. In the present study the internal consistency rose satisfactorily after the removal of palpitations and sweaty from Physical exertion and numbness from Physical discomfort. With regard to moderate/severe disease the removal of numbness did improve the internal consistency of Physical discomfort but not to a satisfactory level. The factor analyses revealed that the same items (palpitations, sweaty and numbness) also had unsatisfactory factor loadings. With these items excluded, a second factor analysis of the remaining items confirmed the presence of the 5 underlying dimensions that have been described in other populations, as well as higher explained variance. It is possible that these 3 items are not associated with physical exertion or physical discomfort. Furthermore, palpitations, sweaty and numbness in people with MS may not coincide with an experience of being fatigued and should be replaced. Other appropriate items reflecting physical aspects of fatigue as perceived by people with MS regardless of degree of disease severity ought to be considered and tested for validity. Such items should include known experiences of physical functioning among people with MS, e.g. reduced endurance, physical deconditioning or low physical capacity. Further studies of the SOFI are warranted in order to develop its psychometric properties, such as functioning of rating scale categories, unidimensionality and hierarchy, which preferably could be performed with Rasch analysis (37).
Lack of energy, Lack of motivation and Sleepiness all correlated moderately. Yet, each dimension had satisfactorily high internal consistency and distinct factor loadings, implying that they measure different dimensions that are valid among people with MS. Recent studies have shown that people with MS are almost twice as likely to have reduced sleep quality compared with healthy people (5), and that sleep impairment and fatigue are associated (5, 10, 11). In the ICF, both sleep function and motivation are categorized in the chapter “Global mental functions” of the component body functions, a chapter which also encompasses energy and drive functions (17). The ICF categorization indicates that Lack of energy, Lack of motivation and Sleepiness are associated mental aspects of fatigue, and the use of this model might be one way to explain why the dimensions were moderately correlated with one another.
When the dimensions of the SOFI were correlated with the FSS, an instrument considered to describe the severity of fatigue, Lack of energy had the strongest, albeit a moderate, correlation. This finding is in concordance with that of Åhsberg (23), who proposes that Lack of energy should be defined as a dimension describing more general characteristics of fatigue, containing its unique characters but also the common variance of other dimensions. Lack of energy might describe more general features of fatigue also in people with MS. Correlations between the other 4 dimensions and the FSS were low, supporting our a priori hypothesis that the SOFI and the FSS measure different constructs of fatigue.
A majority of people with MS included in the present study was categorized with mild disease severity, but even in the mild category more than 40% experienced fatigue, as assessed with the FSS. The proportion of fatigued people with MS with severe disease was approximately the same, but in those people with MS categorized with moderate disease severity the proportion of fatigue was even higher, 81%. These results underline the frequent presence of fatigue irrespective of grade of MS severity and, furthermore, the importance of developing instruments able to capture aspects of fatigue that are specific regardless of degree of disease severity.
The people with MS in this study attended an outpatient MS specialist clinic, and the results are likely to be applicable to people with MS in similar contexts. The alpha values considered as satisfactory in this study, 0.70–0.80, are values recommended for use in research contexts; however, for a clinical application higher alpha values (0.90–0.95) are needed (32). As Lack of energy and Lack of motivation reached the desired level at various points of data collection, particularly in people with MS with mild disease severity, these dimensions might be useful in the clinic. The results should be interpreted keeping in mind that a large proportion of the sample received immunomodulatory treatment. However, a previous analysis of this sample has shown that such treatment was not a predictor of either an increase or a decrease in fatigue over time, as measured with the FSS (16). Furthermore, the aim was to study dimensions of fatigue and how they can be expressed with the SOFI in people with MS; since the capacity of the SOFI to measure changes in fatigue over time was not studied, further studies are required for full validation of the instrument.
In conclusion, for full applicability of the SOFI to people with MS with varying degrees of disease severity the instrument needs further development. Lack of energy and the mental dimensions of the SOFI, Lack of motivation and Sleepiness, are valid for the identification and assessment of such dimensions of fatigue in MS. However, the physical dimensions of the SOFI need further development. In addition, it should be kept in mind that the SOFI needs to be studied further regarding its capacity to measure changes in fatigue over time. The SOFI might be a useful addition for the assessment of fatigue in people with MS, since it appears to measure a different construct of fatigue than other frequently used scales.
ACKNOWLEDGEMENTS
The authors wish to express their gratitude to the participating people with MS; to the staff at the MS Centre of Karolinska University Hospital, Huddinge; to Elizabeth Åhsberg of the Institute for Evidence-Based Social Work Practice at the National Board of Health and Welfare; to Thomas Masterman of the Department of Clinical Neuroscience of Karolinska Institutet; to Professor Hans Link; and to Elisabeth Berg and Jakob Bergström, statisticians at the Department of Learning, Informatics, Management and Ethics of Karolinska Institutet.
Data collection was supported by an unrestricted grant from Biogen Idec. The study was funded by grants from the Centre for Health Care Sciences; from the Health Care Sciences Postgraduate School of Karolinska Institutet; from the Swedish Association of Persons with Neurological Disabilities; and from the Swedish Research Council.
REFERENCES
1. Fatigue Guidelines Development Panel of the Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and multiple sclerosis: evidence-based management strategies for fatigue in multiple sclerosis. Washington DC: Paralyzed Veterans of America; 1998.
2. Lerdal A, Celius EG, Moum T. Fatigue and its association with sociodemographic variables among multiple sclerosis patients. Mult Scler 2003; 9: 509–514.
3. Bakshi R, Shaikh ZA, Miletich RS, Czarnecki D, Dmochowski J, Henschel K, et al. Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Mult Scler 2000; 6: 181–185.
4. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil 1996; 77: 165–170.
5. Lobentanz IS, Asenbaum S, Vass K, Sauter C, Klosch G, Kollegger H, et al. Factors influencing quality of life in multiple sclerosis patients: disability, depressive mood, fatigue and sleep quality. Acta Neurol Scand 2004; 110: 6–13.
6. Becker H, Stuifbergen A. What makes it so hard? Barriers to health promotion experienced by people with multiple sclerosis and polio. Fam Community Health 2004; 27: 75–85.
7. Bakshi R. Fatigue associated with multiple sclerosis: diagnosis, impact and management. Mult Scler 2003; 9: 219–227.
8. Kos D, Kerckhofs E, Nagels G, D’hooghe MB, Ilsbroukx S. Origin of fatigue in multiple sclerosis: review of literature. Neurorehabil Neural Repair 2008; 22: 91–100.
9. Chwastiak LA, Gibbons LE, Ehde DM, Sullivan M, Bowen JD, Bombardier CH, et al. Fatigue and psychiatric illness in a large community sample of persons with multiple sclerosis. J Psychosom Res 2005; 59: 291–298.
10. Strober LB, Arnett PA. An examination of four models predicting fatigue in multiple sclerosis. Arch Clin Neuropsychol 2005; 20: 631–646.
11. Stanton BR, Barnes F, Silber E. Sleep and fatigue in multiple sclerosis. Mult Scler 2006; 12: 481–486.
12. MacAllister WS, Krupp LB. Multiple sclerosis-related fatigue. Phys Med Rehabil Clin N Am 2005; 16: 483–502.
13. Gottberg K, Gardulf A, Fredrikson S. Interferon-beta treatment for patients with multiple sclerosis: the patients’ perceptions of the side-effects. Mult Scler 2000; 6: 349–354.
14. van der Werf SP, Evers A, Jongen PJ, Bleijenberg G. The role of helplessness as a mediator between neurological disability, emotional instability, experienced fatigue and depression in patients with multiple sclerosis. Mult Scler 2003; 9: 89–94.
15. Merkelbach S, Konig J, Sittinger H. Personality traits in multiple sclerosis (MS) patients with and without fatigue experience. Acta Neurol Scand 2003; 107: 195–201.
16. Johansson S, Ytterberg C, Widén Holmqvist L, von Koch L. A longitudinal study of variations in and predictors of fatigue in multiple sclerosis. J Neurol Neurosurg Psychiatry 2008; 79: 454–457.
17. The International Classification of Functioning, Disability and Health. Geneva: World Health Organization; 2001. Available from: www.who.int/classification/icf [accessed 22 March 2008].
18. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123.
19. Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci 1994; 21: 9–14.
20. Åhsberg E, Gamberale F, Kjellberg A. Perceived quality of fatigue during different occupational tasks. Development of a questionnaire. Int J Indust Ergonomics 1997; 20: 121–135.
21. Åhsberg E, Gamberale F. Perceived fatigue during physical work: an experimental evaluation of a fatigue inventory. Int J Indust Ergonomics 1998; 21: 117–131.
22. Åhsberg E, Gamberale F, Gustafsson K. Perceived fatigue after mental work: an experimental evaluation of a fatigue inventory. Ergonomics 2000; 43: 252–268.
23. Åhsberg E. Dimensions of fatigue in different working populations. Scand J Psychol 2000; 41: 231–241.
24. Poser C, Paty D, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983; 13: 227–231.
25. Johansson S, Ytterberg C, Claesson IM, Lindberg J, Hillert J, Andersson M, et al. High concurrent presence of disability in multiple sclerosis: associations with perceived health. J Neurol 2007; 254:767–773.
26. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452.
27. Swedish MS-registry. Available at: http://www.msreg.net [accessed 20 March 2008].
28. Åhsberg E, Fürst CJ. Dimensions of fatigue during radiotherapy – an application of the Swedish Occupational Fatigue Inventory (SOFI) on cancer patients. Acta Oncol 2001; 40: 37–43.
29. Kleinman L, Zodet MW, Hakim Z, Aledort J, Barker C, Chan K, et al. Psychometric evaluation of the fatigue severity scale for use in chronic hepatitis C. Qual Life Res 2000; 9: 499–508.
30. Lerdal A, Wahl A, Rustoen T, Hanestad BR, Moum T. Fatigue in the general population: a translation and test of the psychometric properties of the Norwegian version of the fatigue severity scale. Scand J Public Health 2005; 33: 123–130.
31. Tellez N, Rio J, Tintore M, Nos C, Galan I, Montalban X. Does the Modified Impact Scale offer a more comprehensive assessment of fatigue in MS? Mult Scler 2005; 11: 198–202.
32. Bland JM, Altman DG. Cronbach’s alpha. BMJ 1997; 314: 572.
33. Nunnally, JC, Bernstein IH, editors. Psychometric theory, 3rd edn. New York: McGraw-Hill; 1994.
34. Olsson U. Maximum likelihood estimation of the polychoric correlation coefficient. Psychometrika 1979; 44; 443–460.
35. Thompson B, editor. Exploratory and confirmatory factor analysis: understanding concepts and applications. Washington, DC; American Psychological Association; c2004.
36. Altman DG, editor. Practical statistics for medical research. London: Chapman & Hall; 1995.
37. Bond TG, Fox CM, editors. Applying the Rasch model. Fundamental measurement in the human sciences. Mahawah, NJ: Erlbaum Publishers; 2001.