Objective and study design: A parallel group study to investigate the effectiveness of a smoking cessation programme performed during routine rehabilitation practice for outpatients.
Patients and methods: The study participants comprised an intervention group of 102 consecutive smokers who underwent a smoking cessation programme in a rehabilitation centre and a control group of 101 consecutive smokers who were referred to a smoking cessation centre in a pulmonary hospital. All participants underwent physical examination, pulmonary function tests and received identical behavioural and/or pharmacological treatment. In addition, the intervention group underwent rehabilitation practice 3 times a week for 3 months.
RESULTS: The continuous abstinence rate at 12 months, which was validated by an expired air carbon monoxide concentration of 10 parts per million or less and a household interview, was 68% in the intervention group and 32% in the control group. Multivariable analysis showed that rehabilitation was significantly associated with smoking cessation after adjusting for years of smoking, number of cigarettes smoked, gender and treatment (odds ratio = 4.34, p < 0.001).
CONCLUSION: This study suggests that smoking cessation programmes during routine rehabilitation may be highly effective in helping smoking withdrawal and should be a strongly recommended component of rehabilitation practice.
Key words: smoking cessation, rehabilitation, bupropion, nicotine replacement therapy.
J Rehabil Med 2008; 40: 672–677
Correspondence address: Gregorino Paone, S. Camillo-Forlanini Hospital, Via Portuense 332, IT-00149 Rome, Italy. E-mail: rpaone1023@yahoo.com
Submitted December 11, 2007; accepted April 7, 2008
INTRODUCTION
Tobacco smoking is the most preventable cause of death in developed countries (1–2). From 1990 to 1999 more than 20 million individuals died due to tobacco-related diseases and half of regular smokers die prematurely because of heart diseases, lung cancer or other neoplasm, chronic obstructive pulmonary disease (COPD) and strokes (3–5).
Smoking cessation is an imperative for current smokers and its beneficial effects are remarkable, significantly decreasing the progression of most of tobacco-related diseases (3, 5–7).
The most utilized strategies to stop smoking are based on a combination of behavioural intervention and pharmacological treatment, and bupropion or nicotine replacement therapy (NRT) alone or in combination, are largely recognized as effective in helping individuals in smoking abstinence (8–13).
We organized a smoking cessation programme in a rehabilitation centre and designed a parallel group study to test the hypothesis that a smoking cessation programme including behavioural and/or pharmacological therapies would be highly effective in stopping smoking and in preventing relapse when carried out in the context of an outpatient rehabilitation programme.
METHODS
Patients
A population of current smokers with and without COPD was recruited to an outpatient smoking cessation programme. Inclusion criteria were: subjects aged over 18 years, who had smoked an average of 10 cigarettes or more per day for at least 2 years and were motivated to stop smoking. Exclusion criteria were: individuals with serious medical disorders (e.g. cancers, drug or alcohol addiction, major depression).
The study was approved by the local ethics committee and informed signed consent was obtained from all participants.
Study design
A parallel group study was performed. As an intervention group, current smokers who were willing to stop smoking were recruited among individuals attending a rehabilitation programme (pulmonary, cardiac and post-traumatic) in a rehabilitation centre. As a control group subjects who were willing to stop smoking and who were referred to a smoking cessation centre in a respiratory hospital in Rome were examined.
The sample size was calculated on the ability to detect a difference between intervention and control groups given a projected abstinence rate of 50% in the intervention group and 30% in the control group. Approximately 100 subjects were needed for each group, to have a two-sided alpha level of 0.05 and a power of 0.8. To ensure an adequate sample, 120 subjects were enrolled in each group.
Enrolment phase
Demographic data and personal medical histories of all participants were collected during the first counselling session, before entering the smoking cessation programme. A complete smoking history, including smoking habits, number of cigarettes smoked per day and previous attempts to quit smoking, was recorded. Subjects underwent a physical examination and pulmonary function tests (PFT). Spirometric measurements were performed by experienced personnel following the American Thoracic Society and European Respiratory Society recommendations and the reference values were those of the European Community for Coal and Steel approved by the European Respiratory Society (14, 15).
Diagnoses of chronic obstructive pulmonary disease were obtained according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (16).
All individuals completed the Fagerstrom Test for Nicotine Dependence (FTND). According to the literature, FTND scores were considered as mild (0–3), moderate (4–7) and high dependence (8–10) (17, 18).
Intervention
Individuals from both groups underwent an identical smoking cessation intervention. All participants received face-to-face behavioural counselling with or without a specific pharmacological treatment. Behavioural counselling sessions were performed by trained physicians and experienced nurses on a weekly basis during a 3-month period. In each individualized session subjects were provided with complete information about smoking side-effects and the benefits of tobacco withdrawal. For each individual, smoking triggering conditions were identified and specific recommendations were given to prevent them. The mean duration of each meeting was 1 h. Family members were asked to assist smokers.
Pharmacological treatment, dosage and delivery systems were based on daily cigarette consumption and on nicotine dependence following literature recommendations (10, 12, 19). Individual telephone or personal support was also offered to participants in both groups between the sessions.
In order to control for possible confounders, the intervention was conducted by physicians and nurses from the same smoking cessation team.
In addition to treatments, the intervention group participants underwent rehabilitation procedures (respiratory, cardiac or post-traumatic) 3 times per week for a 3-month period.
Pharmacological side-effects of treatment were also analysed in all participants.
Follow-up
Current smoking status was investigated weekly during behavioural counselling. After the 3-month treatment phase, participants were evaluated monthly in order to provide a 12-month analysis period. Self-assessed smoking cessation was validated by measuring carbon monoxide (CO) concentration in expired air and was confirmed by telephone interviews with household members during the 3–12 month period. Sustained quitters were defined as participants whose self-assessed continuous smoking cessation, at each evaluation, was validated by carbon monoxide concentration in exhaled air ≤ 10 ppm and by confirmation from a household member.
Current smokers were considered individuals who admitted smoking and/or whose exhaled CO levels were > 10 ppm (20).
Individuals who did not attend follow-up visits or evaluations were considered as treatment failures and as assumed smokers.
Statistical analysis
Standard statistical methods were used to compare baseline characteristics and outcome between the intervention and control group and for univariable analysis of association of patients’ characteristics with smoking cessation. In particular, the Mann-Whitney U test was used for continuous variables and the χ2 test for categorical variables. To assess the association between type of intervention, patients’ characteristics and smoking cessation univariable odds ratio (OR) were first calculated and then a multivariable logistic regression analysis was performed. All variables evaluated in the univariable analysis were considered in the multivariable analysis and the final logistic models were constructed by forward conditional selection. We first constructed a model in which rehabilitation was entered as a dichotomous variable, and then a second model in which rehabilitation type was entered as a multiple categorical variable.
The analysis was performed by using SPSS (Statistical Package for the Social Sciences, Chicago, IL, USA) V13.0 for Windows.
RESULTS
Baseline characteristics
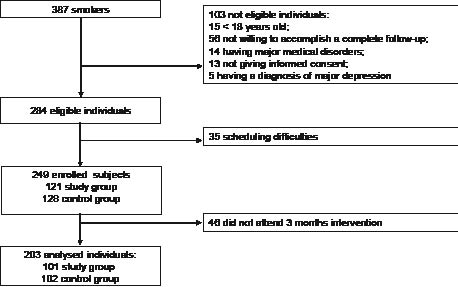
A total of 387 smokers were referred to the Don Gnocchi and Forlanini smoking cessation centres from October 2003 to January 2005. Of these, 103 patients did not satisfy the inclusion criteria. Among the 284 eligible individuals, 35 subjects were excluded due to scheduling difficulties, and 249 smokers were enrolled in the study (121 in the intervention group and 128 in the control group). Among these, 46 subjects did not attend the 3-month intervention; therefore 203 individuals were analysed (102 in the intervention group and 101 in the control group) (Fig. 1). Baseline characteristics of the 2 groups are summarized in Table I. There were no significant differences between them; all participants had similar smoking histories and the percentage of patients with COPD was equally distributed between intervention and control groups. No significant differences in pharmacological treatments were observed between the 2 groups (Table II).
Fig. 1. Flowchart of consecutive unselected smokers referred to the smoking cessation centres.
| Table I. Comparison between intervention and control group baseline characteristics. Data are presented as mean values and standard deviation of each variable (in parentheses). |
| Variables | Intervention group n = 102 | Control group n = 101 |
| Gender, male/female | 47/55 | 42/59 |
| Age, years | 53.7 (14.4) | 54.7 (12.7) |
| Age started smoking, years | 20.8 (8) | 21.5 (8.7) |
| Cigarettes/day | 21.5 (10) | 23.7 (11.8) |
| Cigarette pack years | 35.8 (24) | 39 (26) |
| FTND | 6.3 (1.6) | 6.6 (1.8) |
| FEV1, % of predicted | 72 (21) | 78 (26) |
| FEV1/FVC, % | 72 (14) | 72.9 (12) |
| COPD/HS | 41/61 | 40/61 |
| All values are non-significant. FEV1: forced expired volume in 1 sec; FVC: forced vital capacity; FEV1/FVC = FEV1 expressed as a percentage of FVC; COPD: chronic obstructive pulmonary disease; HS: healthy smokers; FTND: Fagerstrom Test for Nicotine Dependence. |
| Table II. Comparison between pharmacological protocols |
| Pharmacological treatment | Intervention group n = 102 n (%) | Control group n = 101 n (%) |
| Bupropion | 45 (44) | 40 (39.6) |
| Nicotine patch (30 mg) | 13 (12.7) | 18 (17.8) |
| Nicotine patch (15 mg) | 7 (6.8) | 6 (5.9) |
| Nicotine patch (15 mg) and inhaler as needed (0–12). | 6 (5.8) | 11 (10.9) |
| Sublingual tablets as needed (0–12) or inhaler as needed (0–12) | 10 (10) | 4 (3.9) |
| Bupropion and sublingual tablets or inhaler | 4 (3.9) | 5 (4.9) |
| No pharmacological therapy | 17 (16.6) | 17 (16.8) |
| All values are non-significant. |
Abstinence rates
At each follow-up visit, participants were questioned about their smoking status. Self-assessed smoking abstinence was validated (weekly during the treatment and monthly during the 3–12 months period) by measurement of exhaled CO levels and by interviewing a household member. Subjects with self-reported cessation, low CO levels and smoking cessation confirmed by a household member were classified as sustained quitters. Two enrolled individuals died during follow-up and were excluded from further analysis. Nineteen subjects from the intervention group and 16 from the control group did not undergo the 12-month visit and were considered as assumed smokers. After 12 months, comparison between individuals who stopped smoking revealed a significantly higher number of quitters in the intervention group compared with the control group (69/102, 67.6% vs 32/99, 32.3%; p < 0.0001). The results are summarized in Table III.
| Table III. Comparison between intervention and control group smoking status after 12 months |
| Self-reported smoking status | Category | Study group n = 102 (%) | Control group n = 99 (%) |
| Quitters Smokers Missing at follow-up | Sustained quitters Current smokers Smoking assumed | n = 69 (67.6) n = 14 (13.7) n = 19 (18.6) | n = 32 (32.3) n = 51 (51.5)* n = 16 (16.2) |
| p < 0.0001 |
Five individuals from the intervention group and 7 from the control group, who were quitters after 3 months, relapsed and were redefined as current smokers at the 12-month re-evaluation.
Predictors of smoking quitting: univariable analysis
Table IV shows factors associated with success in smoking cessation at 12 months.
| Table IV. Association of patient’s characteristics and type of intervention with smoking cessation: univariable and multivariable analysis |
| Variables | Univariable analysis | Multivariable analysis |
| Model 1 | Model 2 |
| Quitted smoking/total (%) | Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | Odds ratio (95% CI) | p |
| Gender |
| Male | 47/89 (52.8) | Ref | | | | | |
| Female | 54/112 (48.2) | 0.83 (0.46–1.51) | 0.51 | | | | |
| Age |
| Per each 5 year increase | ---- | 0.90 (0.81–0.99) | 0.041 | | | | |
| Age started smoking |
| Per each 5 year increase | ---- | 0.84 (0.74–0.96) | 0.012 | | | | |
| Years smoking |
| Per each 5 year increase | ---- | 0.86 (0.77–0.96) | 0.01 | | | | |
| Cigarettes/day |
| Per each 5 cigarette increase | ---- | 1.06 (0.89–1.25) | 0.50 | | | | |
| Cigarette pack years |
| Per each 5 pack-year increase | ---- | 0,90 (0.84–0.95) | 0.001 | 0.89 (0.84–0.96) | 0.001 | 0.89 (0.83–0.96) | 0.001 |
| FTND |
| Per each point increase | ---- | 0.82 (0.69–0.93) | 0.014 | | | | |
| COPD |
| No | 61/122 (50.0) | Ref | | | | | |
| Yes | 40/79 (50.6) | 1.03 (0.56–1.88) | 0.93 | | | | |
| Therapy |
| No medication | 14/33 (42.4) | | | | | | |
| Bupropion | 44/85 (51.8) | 1.46 (0.60–3.55) | 0.36 | | | | |
| Nicotine replacement | 40/74 (54.1) | 1.60 (0.65–3.98) | 0.26 | | | | |
| Bupropion + Nicotine replacement | 3/9 (33.3) | 0.68 (0.09 –3.91) | 0.62 | | | | |
| Rehabilitation (any) |
| No | 32/100 (32) | Ref | | Ref | | | |
| Yes | 69/101 (68.3) | 4.00 (2.11–6.70) | < 0.001 | 4.34 (2.39–8.09) | < 0.001 | | |
| Rehabilitation type |
| No rehabilitation | 32/100 (32) | Ref | | | | Ref | |
| Respiratory | 43/61 (70.5) | 5.67 (2.71–13.1) | < 0.001 | | | 5.38 (2.60–11.11) | < 0.001 |
| Cardiac | 14/22 (63.6) | 3.66 (1.27 –10.76) | 0.006 | | | 3.50 (1.29–9.44) | 0.014 |
| Post-traumatic | 12/18 (66.6) | 3.59 (1.17–11.30) | 0.01 | | | 3.19 (1.13–9.04) | 0.029 |
| COPD: chronic obstructive pulmonary disease; CI: confidence interval; FTND: Fagerstrom Test for Nicotine Dependence. |
Abstinence at 12 months was associated with younger age, starting to smoke at a younger age, fewer years of smoking, lower number of cigarette pack years, lower FTND and undergoing rehabilitation therapy. Rehabilitation was also significantly associated with increased probability of smoking cessation when patients undergoing different rehabilitation procedures (respiratory, cardiac, post-traumatic) were compared with those not undergoing rehabilitation. Gender, number of cigarettes smoked per day, the presence of COPD and the pharmacological treatment utilized to quit smoking did not affect abstinence rates.
Predictors of smoking cessation: multivariable analysis
To evaluate the association between rehabilitation and smoking cessation, while controlling for patients’ characteristics, a logistic regression analysis was performed. Variables to be included in the final logistic models were selected among those considered in the univariable analysis by forward selection. In a first model, rehabilitation, included as a dichotomous variable, remained significantly associated with smoking cessation (OR = 4.34, p < 0.001), as was the number of cigarette pack years (OR = 0.89 per each increase of 5, p = 0.001). In a second model, each different rehabilitation type also remained associated with smoking cessation (OR = 5.38, p < 0.001 for respiratory rehabilitation; OR = 3.50, p = 0.014 for cardiac rehabilitation; OR = 3.19, p = 0.029 for post-traumatic rehabilitation).
DISCUSSION
This study investigated the impact of a smoking cessation programme, performed inside a routine rehabilitation centre, upon stopping smoking. While most studies on smoking cessation have been organized in research settings, few reports have investigated the effectiveness of smoking cessation programmes in routine clinical practice. Although the highly supportive setting of rehabilitation should be very helpful in enabling smoking cessation, to our knowledge there are no published studies investigating its effectiveness.
In our study, after one year, 69 individuals in the intervention group (68%) succeeded in quitting smoking, 14 maintained their smoking habits (14%) and 19 were lost after enrolment and considered as assumed smokers (19%). Our data showed a significant improvement compared with the control group (32%, 52% and 16%, respectively).
The factors associated with successful smoking withdrawal were analysed using univariable analysis and the following significant predictors were identified: younger age; fewer years of smoking, starting to smoke at a younger age; lower FTND score, lower number of cigarette pack years, and undergoing rehabilitation.
After multivariable analysis only lower cigarette pack years and rehabilitation remained significantly associated with tobacco abstinence, with rehabilitation being the most powerful predictor.
The hypothesis that a specific rehabilitative practice could significantly increase the likelihood of abstinence was also evaluated. All the procedures performed (respiratory, cardiac, and post-traumatic) were associated with tobacco abstinence. This observation may be consistent with the hypothesis that the pathological conditions that led to the different rehabilitation programmes, being a further strong psychological motivation factor for stop-smoking, could have contributed to the outcomes observed. Therefore a possible explanation for the difference observed could be that the intervention group individuals were highly health motivated (this subset of subjects was recruited among individuals who underwent rehabilitation 3 times per week for 3 months). Nevertheless we cannot rule out that a rehabilitation setting per se may be helpful in smoking withdrawal. It may be possible that the outpatient rehabilitation setting, with the high frequency of appointments, the high control rate, the reinforced self-esteem and the improved well-being, may have also contributed to the favourable outcome observed.
This study had some limitations: first, due to ethical reasons we did not randomize participants and it was not possible to include in the control group patients with serious medical disorders; however, we believe that our results remain relevant in assessing the effectiveness of rehabilitation-based smoking cessation programmes. Secondly, since our study was performed in only 2 centres, we believe that the data obtained may suggest the opportunity of further prospective multi-centre studies to confirm our data. Thirdly, since the counselling interventions were delivered by different physicians, we cannot rule out any evaluation bias although, to limit this factor, equally trained personnel belonging to the same smoking cessation team were employed during the entire study. Fourthly, after the enrolment phase, we lost 19 individuals from the intervention group and 27 from the control group, thus we cannot rule out selection bias. To limit this possibility we also calculated the success rate considering this subset of individuals as drop-outs and including them as treatment failures according to the intention-to-treat analysis. However, the percentage of sustained quitters was significantly higher in the intervention group (57%) compared with the control group (25%, p < 0.0001) and exceeded previously reported success rates (8, 9, 11, 16, 20–25).
Smoking-related diseases have enormous social and economic implications and the most valuable approach to prevent them is smoking cessation.
The efficacy of a smoking cessation programme depends on a number of factors and, among these, the setting is crucial. A large number of people continue to smoke despite increasing information about the risks of tobacco use, while only a few people stop smoking following the simple advice from their physician and measures need to be taken to improve smoking cessation interventions in primary care (26).
The success rate is much higher in hospital settings and since hospitals are smoke-free areas, hospitalization is an enforced no smoking period for smokers (although most inpatient smokers receive inadequate smoking care during hospitalization) (27).
Nowadays, due to growing medical expenses, the healthcare policy is to make efforts to shorten the length of hospitalization encouraging people to utilize outpatient rehabilitative procedures and therefore giving rehabilitation an important role in delivering smoking cessation interventions.
In this context pulmonary rehabilitation has proven to be highly effective in tackling the consequences of COPD as well as the behavioural and educational deficiencies observed in many patients. Some rehabilitation programmes have excluded current smokers, pointing out that individuals who smoked were less likely to participate in rehabilitation compared with individuals who had stopped smoking (28, 29).
Although the inclusion of current smokers in respiratory rehabilitation programmes remains under debate, nowadays many rehabilitation programmes accept current smokers, but only a few offer smoking cessation intervention as part of rehabilitation itself (30–32).
Many efforts have been made to help smoking cessation, and countries that have established smoke-free legislation have obtained impressive results in terms of the percentage of people who have stopped smoking and decreased cigarette consumption (33, 34).
In conclusion, we believe that our data, with the high number of individuals who stopped smoking after 12 months and the increasing number of individuals referred to rehabilitative procedures, support the hypothesis that considering smoking cessation programmes as a mandatory component of rehabilitation may be highly effective in increasing smoking cessation rate and could be an additional strategy to reduce smoking habits.
ACKNOWLEDGEMENTS
We would like to thank Cecilia Midulla, MD, (Department of Experimental Medicine, University of Rome, “La Sapienza”) for critical review of the manuscript and Dr Daniel Clarke for text revision.
None of the authors have any financial or personal relationship with other parties that could bring into question the impartiality or accuracy of their work.
REFERENCES
1. Jorenby DE. Smoking cessation strategies for the 21st century. Circulation 2001; 104: 51–52.
2. Anderson JE, Jorenby DE, Scott WJ, Fiore MC. Treating tobacco use and dependence: an evidence-based clinical practice guideline for tobacco cessation. Chest 2002; 121: 932–941.
3. Rivara FP, Ebel BE, Garrison MM, Christakis DA, Wiehe SE, Levy DT. Prevention of smoking-related deaths in the United States. Am J Prev Med 2004; 27: 118–125.
4. Fernandez E, Schiaffino A, Borras JM. Epidemiology of smoking in Europe. Salud Publica Mex 2002; 44 Suppl 1: S11–S19.
5. Hsu HC, Pwu RF. Too late to quit? Effect of smoking and smoking cessation on morbidity and mortality among the elderly in a longitudinal study. Kaohsiung J Med Sci 2004; 20: 484–491.
6. Twardella D, Kupper-Nybelen J, Rothenbacher D, Hahmann H, Wunsten B, Brenner H. Short–term benefit of smoking cessation in patients with coronary heart disease: estimates based on self-reported smoking data and serum cotinine measurements. Eur Heart J 2004; 25: 2101–2108.
7. Edwards R. The problem of tobacco smoking. BMJ 2004; 328: 217–219.
8. Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. A randomized, controlled trial. Ann Intern Med 2001; 135: 423–433.
9. Dale LC, Glover ED, Sachs DP, Schroeder DR, Offord KP, Croghan IT, Hurt RD. Bupropion for smoking cessation. Predictors of successful outcome. Chest 2001; 119: 1357–1364.
10. Roddy E. Bupropion and other non-nicotine pharmacotherapies. BMJ 2004; 328: 509–511.
11. Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999; 340: 685–691.
12. Molyneux A. Nicotine replacement therapy. BMJ 2004; 328: 454–456.
13. Rigotti NA. Treatment of tobacco use and dependence. N Engl J Med 2002; 346: 506–512.
14. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task force: standardization of lung function testing. In Brusasco V, Crapo R, Viegi G, editors. Standardization of spirometry. Eur Respir J 2005; 26: 319–338.
15. Quanjier PH, Tammeling GJ, Cotes JE, Pederson OF, Peslin R, Yerault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Test, European Community for Steal and Coal. Official Statement of the European Respiratory Society. Eur Resp J Suppl 1993; 6: 5–40.
16. GOLD Committees, Global Initiative for Chronic Obstructive Lung Disease: global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Workshop Report [updated 2006]. Available from http://www.goldcopd.com
17. Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict. Behav 1978; 3: 235–241.
18. Fung PR, Snape-Jenkinson SL, Godfrey MT, Love KW, Zimmerman PV, Yang IA, et al. Effectiveness of hospital-based smoking cessation. Chest 2005; 128: 216–223.
19. Zuccaro P, Caraffa G, Corti FM, Davoli M, Enea D, Fogliani V, et al, editors. Smoking cessation guidelines. Rome: Italian National Institute of Health Editions; 2003, p. 1–77.
20. Górecka D, Bednarek M, Nowinski A, Puscinska E, Goljan-Geremek A, Zielinski J. Diagnosis of airflow limitation combined with smoking cessation advice increases stop-smoking rate. Chest 2003; 123: 1916–1923.
21. Wood-Baker R. Outcome of a smoking cessation programme run in a routine hospital setting. Intern Med J 2002; 32: 24–28.
22. Cox LS, Patten CA, Niaura RS, Decker PA, Rigotti N, Sachs DP, et al. Efficacy of bupropion for relapse prevention in smokers with and without a past history of major depression. J Gen Intern Med 2004; 19: 828–834.
23. Ferry L, Johnston JA. Efficacy and safety of bupropion SR for smoking cessation: data from clinical trials and five years of post-marketing experience. Int J Clin Pract 2003; 57: 224–230.
24. Hurt RD, Wolter TD, Rigotti N, Hays JT, Niaura R, Durcan MJ, et al. Bupropion for pharmacologic relapse prevention to smoking: predictors of outcome. Addict Behav 2002; 27: 493–507.
25. Willemse B, Lesman-Leegte I, Timens W, Postma D, ten Hacken N. High Cessation rates of cigarette smoking in subjects with and without COPD. Chest 2005; 128: 3685–3687.
26. Wilson A, Hippisley-Cox J, Coupland C Coleman T, Britton J, Barrett S. Smoking cessation treatment in primary care. Prospective cohort study. Tobacco Control 2005; 14: 242–246.
27. Freund M, Campbell E, Paul C, Sakrouge R, Wiggers J. Smoking care provision in smoke-free hospitals in Australia. Prev Med 2005; 41: 151–158.
28. Young P, Dewse M, Fergusson W, Kolbe J. Respiratory rehabilitation in chronic obstructive pulmonary disease: predictors of non-adherence. Eur Respir J 1999; 13: 855–859.
29. Connor MC, O’Driscoll MF, Ms Donnell TJ. Should patients with chronic obstructive pulmonary disease (COPD) who expresses a desire to stop smoking be enrolled in pulmonary rehabilitation? Eur Respir J 1999; Suppl 14: 263s.
30. Karrer W. Pulmonary rehabilitation in Switzerland. Swiss Med Wkly 2005; 135: 71–75.
31. Brooks D, Lacasse Y, Goldstein RS. Pulmonary rehabilitation programs in Canada. Can Respir J 1999; 6: 55–63.
32. Lacasse Y, Maltais F, Goldstein RS. Smoking cessation in pulmonary rehabilitation: goal or prerequisite? J Cardiopulm Rehabil 2002; 22: 148–153.
33. Wilson N, Thomson G. New smoke-free environments legislation stimulates calls to a national Quitline. Tobacco Control 2005; 14: 287–288.
34. Gallus S, Zuccaro P, Colombo P, Apolone G, Pacifici R, Garattini S, et al. Effects of new smoking regulations in Italy. Ann Oncol 2006; 17: 346–347.