OBJECTIVE: To estimate the extent to which the impairments associated with lymphoedema (volume increase, local oedema and sensory alteration) are linked to arm dysfunction and sub-optimal health-related quality of life.
Patients and methods: A cross-sectional study, embedded within a pilot for an epidemiologic study, was undertaken involving women who had undergone surgery for unilateral stage I or II breast cancer. Two questionnaires (a lymphoedema screening questionnaire and the Disabilities of Arm, Shoulder and Hand questionnaire) were mailed and 72 of 204 responders reported having one or more symptoms of lymphoedema (prevalence 35%). A total of 50 women with symptoms attended for further testing.
RESULTS: Women with self-reported symptoms of lymphoedema had a significantly higher score on the Disabilities of Arm, Shoulder and Hand questionnaire (mean difference 23.4, 95% confidence interval 19.3–27.5), indicating activity limitation and participation restriction. Pain was the only impairment directly correlated with activity limitation, participation restriction and sub-optimal health-related quality of life.
CONCLUSION: These findings have implications for treatment, and the outcome measures used for the assessment of lymphoedema. Treatments focusing on decreasing arm volume without addressing issues of pain may not result in improvements in activity, participation, or health-related quality of life.
Key words: lymphoedema, quality of life, pain measurement.
J Rehabil Med 2008; 40: 651–658
Correspondence address: Diana Dawes, Division of Clinical Epidemiology, Royal Victoria Hospital R4.27, 687 Pine Ave West, Montreal, Qc. H3A 1A1, Canada. E-mail: diana.dawes@clinepi.mcgill.ca
Submitted February 4, 2008; accepted April 2, 2008
INTRODUCTION
Breast cancer is the most commonly diagnosed malignancy among Canadian women, with over 22,200 new cases per year (1). Approximately one in 100 women have had a diagnosis of breast cancer some time in the past 15 years, thus there are an estimated 162,600 breast-cancer survivors in Canada (1).
Unfortunately some survivors experience long-term sequelae that include physical impairments, psychological distress and sub-optimal health-related quality of life (HRQL) (2, 3). Lymphoedema is one of the predominant physical sequelae and has an impact on physical function, psychological distress and HRQL (4).
Lymphoedema following breast cancer surgery is caused by mechanical lesion of the lymphatic system; protein accumulates in the tissues causing fibrosclerosis. Metabolic processes in the interstitium are disturbed by the oedema, and inflammatory processes are facilitated. Lymphoedema is considered to be chronic when present for longer than 3 months (5). The prevalence of chronic lymphoedema is difficult to assess due to the varying definitions, populations and methods of measurement. The reported prevalence varies from 0% to 16.8% with sentinel lymph node biopsy and from 7.1% to 56% with axillary lymph node dissection (ALND) (6–8).
Treatment for chronic lymphoedema has focused on reducing limb size by use of pharmacological and physical therapeutic methods (9, 10). A variety of physical therapeutic interventions are used to reduce oedema, including elevation, massage, exercise and the application of external pressure, and some of these therapies are used in combination (“complex physiotherapy”). Badger et al. (11) showed in a randomized controlled trial (n = 83) that compression bandaging followed by the application of a compression garment achieved greater and more sustained reduction in volume of the affected limb than compression garment alone. The mean percentage reduction at 24 weeks was 31% for compression and garment compared with 15.8% for garment alone, yielding a between-group difference of 15.2% (95% confidence interval (CI) 6.2–24.2). Hence, it is important to detect lymphoedema because there are effective treatments, but it is also important to go beyond limb size and evaluate all the impairments, activity limitations and participation restrictions that women with breast cancer experience in order that the intervention will have the intended impact on function and quality of life.
The functional problems faced by women with breast cancer have been classified by Brach et al. (12), using the World Health Organization’s (WHO) International Classification of Functioning, Disability and Health (ICF) (12). Specifically, women report reduced strength in elbow flexors, shoulder abductors and grip, increased pain and oedema (13, 14). Women post-ALND report significantly more limitations in activities of daily living because of shoulder impairments than women post-sentinel lymph node biopsy (14). Lymphoedema has been found to be independently associated with decreased quality of life scores (15). The impairments associated with lymphoedema lead to functional limitations that can be targeted successfully by specific interventions (16).
To improve the efficacy of strategies or interventions to reduce the occurrence of lymphoedema and minimize its impact on function and HRQL, it is important to understand how lymphoedema relates to function and HRQL. The objective of this study was to estimate the extent to which the impairments associated with lymphoedema are linked to activity limitations, participation restrictions and sub-optimal HRQL.
METHODS
This study was a sub-study of a pilot for an epidemiologic study to test the feasibility of developing a clinically-based prevalence study of lymphoedema and arm dysfunction among women operated on for stage I or II breast cancer. Through this pilot it was possible to add the measures needed to test relationships among variables related to lymphoedema and arm disability. The epidemiologic pilot study used a postal survey which included: (i) a questionnaire on arm function, the Disabilities of Arm Shoulder and Hand (DASH), and (ii) 5 questions, informed by the work of Kwan et al. (17), about signs and symptoms of lymphoedema (Appendix I). For this study, we had the opportunity to invite women who responded positively to any one of the signs and symptoms to participate in a comprehensive physical assessment in our clinic.
Participants
The study population for the epidemiologic pilot comprised a subset of women treated surgically for stage I or II breast cancer at the McGill University Health Centre, Montreal, Canada from January 1992 to January 2002. The focus of the pilot study was to identify feasible sampling strategies, particularly for persons who were difficult to trace. For this study, the available sample was women who responded to the first wave of questionnaires.
The study was approved by the Research Ethics Board (Surgery) of the McGill University Health Centre. Subjects provided written informed consent to participate in this study.
Measures used for the comprehensive assessment (Phase II)
The WHO ICF framework was used to inform the measurement strategy for this study (18). To measure the impairments associated with lymphoedema, the following devices were used and arm dominance was noted:
• Volumetric measurements by water displacement: the volume difference between the arms was calculated to the nearest 10 ml (19).
• Bioelectrical Impedance (lymphometer): this instrument measures impedance and phase, and calculates resistance and reactance. These measurements were used to calculate the difference in volume between the affected and unaffected limbs (see www.impedimed.com).
• Tape measurements: using a spring-loaded tape measure, 3 circumferential measurements were taken of both arms. Measurements were taken to the nearest mm (19).
• Short-Form McGill Pain Questionnaire: the questionnaire was designed to provide quantitative measures of pain, with higher scores indicating more pain (20).
The following measures were used to measure activity limitations, including tests of speed and accuracy of movement, and participation restrictions:
• Box and Block: measures gross unilateral manual dexterity. The requirement is to move the maximum number of small blocks from one compartment of a box across to another identical compartment within 1 min. The test-retest reliability is high (intra-class correlation (ICC) 0.89–0.97) and validity has been demonstrated with significant correlations (21).
• Nine-hole Peg Test: measures upper extremity function. The subject inserts 9 dowels into a board and then removes them while being timed. The test has very high inter-rater reliability (0.97–0.99), test-retest is moderately high (right r = 0.69, left r = 0.43), and is correlated to another test of manual dexterity (right r = –0.61, left r = –0.53) (22).
• Grip strength is an important requisite for good hand function. The Jamar dynamometer (Simmonds Preston Inc., Bolingbrook, USA) was used to measure grip strength (kg), 3 attempts were made with each hand, and the average of the 3 scores used. The test has very high inter-rater reliability (ICC < 0.97) and test-retest reliability (> 0.80) (23).
• Disabilities of Arm Shoulder and Hand Outcome Measure (DASH) Questionnaire: a 30-item, self-report questionnaire designed to measure physical function and symptoms in persons with any or multiple musculoskeletal disorders of the upper limb (www.dash.iwh.on.ca). It covers 3 domains (physical function, symptoms, social/role function). Scores are transformed to a 0–100 scale, where 0 reflects good function and 100 reflects considerable disability. Internal consistency and test-retest reliability exceeds 0.95 (24).
• Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36): a 36-item survey including 8 multi-item scales measuring physical functioning, role limitations due to physical health problems, pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health. The scores on all subscales range from 0 to 100 (higher scores indicate better health status). Reliability, both test-retest and internal consistency, has been demonstrated extensively, and content, criterion and construct validity have also been demonstrated (25).
• European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC) Questionnaire: has been designed for use in a wide range of cancer populations, and is supplemented by a breast specific module. It includes 8 multi-item scales measuring physical functioning, role functioning, emotional functioning, cognitive functioning, social functioning, fatigue, nausea, pain, as well as dyspnoea, insomnia, appetite loss, constipation, diarrhoea, and financial difficulties. Global health is measured by a 2-item scale. The core questionnaire has high internal consistency, good inter-scale correlation, and discriminative validity (26). The supplementary breast module shows high internal consistency of most scales and good known-group discriminative ability (27).
To place in context the extent of disability experienced by women with lymphoedema, we provide normative data as well as data for representative cancer survivors. The Box and Block, Nine-hole Peg and grip strength normative data is from a sample of 310 male and 328 female adults, ages 20–94 years, from the 7-county Milwaukee area (21, 22, 28). SF-36 norms are from a cohort of 9423 randomly selected Canadian men and women aged 25 years or more living in the community (29). The values for the EORTC breast cancer module (BR23) come from data collected from 158 American women 3-months post breast cancer surgery (27).
Statistical methods
Characteristics of those not recruited into the study and participants were compared using logistic regression. For each variable under study, odds ratios (OR) and 95% CI are reported. For continuous variables, age, time since diagnosis and number of nodes examined, between-group differences and 95% CI in the means were estimated. The extent of arm disability for women with or without symptoms of lymphoedema was compared by difference in means and 95% CI. The relationships between screening items and volume and disability were estimated using linear regression. Differences in scores on various tests between women include in this study sample and normative or reference values were tested using t-tests.
To delineate the relationships between impairments, activity limitations, participation restrictions and sub-optimal health-related quality of life, a path analysis was completed using Mplus software. Path analysis is an extension of a multiple regression model and is used to test the fit of the correlation matrix against 2 or more causal models that are being compared. The theoretical model used to inform this path analysis was that proposed by Wilson & Cleary (30), a model of HRQL that integrates biological and psychological aspects of health. There are 5 different levels in this model, namely, physiological factors, symptom status, functional health, general health perceptions, and overall quality of life. Their model has been widely applied to different populations, including patients living with cancer.
A regression is performed for each variable in the model; variables identified as “dependent” on others are modelled with respect to those variables hypothesized as being “causally” related. The regression weights predicted by the model are compared with the observed correlation matrix for the variables, and a goodness-of-fit statistic is calculated. The best-fitting model is selected based on goodness of fit tests including χ2 tests associated with a probability ≥ 0.05 and a Comparative Fit Index (CFI) > 0.9 (31). Single indicator variables are depicted as squares (circles are for multiple indicator latent variables); single arrows indicate potentially “causal” relationships and double-headed arrows indicate correlation with no directionality assumed.
RESULTS
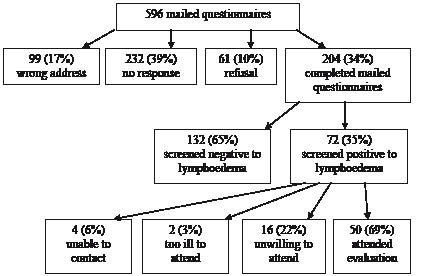
In response to the 596 questionnaires mailed, 204 women completed and returned questionnaires, giving an unprompted response rate of 34%. In all, 72 women (35%) indicated that they had one or more of the signs and symptoms of lymphoedema and 50 women (69%) attended for the comprehensive assessment (Fig. 1).
Fig. 1. Flow chart of patients invited to participate in study.
The characteristics of the women who were invited to participate in the study are shown in Table I. Non-participants were, on average, 4 years older than participants, but there was little difference by “time since diagnosis”. Of interest was that 38% of women did not have ALND. Participants were more likely to have characteristics predisposing them to lymphoedema (extensive breast surgery, numerous nodes examined). Also given in Table I is the OR for participation according to different levels of variables under study. For example, women 80 years of age or older were significantly less likely to participate than women aged 50–64 years (OR 0.32; 95% CI 0.17–0.6).
| Table I. Characteristics of 596 women who had undergone breast cancer surgery according to their response to an invitation to participate in the study |
| Population characteristics | Non-participants n =92 (66%) | Participants n = 204 (34%) | Odds ratio (95% CI) | Difference in means (95% CI) |
| Age, years (mean (SD)) | 65.3 (14.0) | 61.0 (11.8) | | 4.28 (2.02–6.54) |
| < 50 | 56 (14) | 42 (21) | 1.20 (0.74–1.93) | |
| 50–64 | 147 (37) | 92 (45) | Ref | |
| 65–79 | 119 (30) | 56 (27) | 0.75 (0.5–1.13) | |
| ≥ 80 | 70 (18) | 14 (7) | 0.32 (0.17–0.6) | |
| Time since diagnosis, years (mean (SD)) | 4.8 (3.7) | 3.6 (3.1) | | 1.18 (0.58–1.78) |
| < 2 years | 135 (34) | 98 (42) | 1.49 (0.95–2.33) | |
| ≥ 2 to < 4 years | 88 (22) | 43 (33) | Ref | |
| ≥ 4 to < 6 years | 34 (9) | 23 (40) | 1.38 (0.73–2.63) | |
| > 6 years | 135 (34) | 40 (23) | 1.13 (0.66–1.92) | |
| Axillary surgery; yes/no | 241 (61) | 163 (80) | Ref | |
| Lymph node dissection | 226 (94) | 145 (89) | | |
| Sentinel lymph node biopsy | 15 (6) | 18 (11) | | |
| No surgery | 151 (38) | 41 (20) | 0.40 (0.27–0.59) | |
| Number of nodes examined |
| 0 | 264 (67) | 116 (57) | Ref | |
| 1–8 | 50 (13) | 33 (16) | 1.50 (0.92–2.45) | |
| 9–14 | 74 (19) | 53 (26) | 1.63 (1.08–2.47) | |
| 15–52 | 0 | 1 (0.5) | | |
| Breast surgery/mastectomy performed |
| Lumpectomy | 57 (14) | 38 (19) | 1.27 (0.77–2.09) | |
| Partial | 143 (36) | 59 (29) | 0.78 (0.52–1.19) | |
| Segmental | 135 (34) | 71 (35) | Ref | |
| Total | 26 (7) | 12 (6) | 0.88 (0.42–1.84) | |
| Modified/radical | 31 (8) | 22 (11) | 1.35 (0.73–2.50) | |
| Missing | 0 | 2 (1) | | |
| Received radiotherapy | 186 (47) | 96 (47) | 0.99 (0.71–1.40) | |
| Received chemotherapy | 91 (23) | 47 (23) | 0.99 (0.66–1.48) | |
| All data are numbers and (column %) unless otherwise stated. Percentages do not always add up to 100% due to rounding. Odds ratio of participating in the study for each level of variable compared to the referent category. CI: confidence interval; SD: standard deviation. |
Of the 204 women who replied to the mailed questionnaire, 72 (35%) indicated the presence of one or more symptoms of lymphoedema. These women were more likely than those without symptoms to have had axillary lymph node dissection (87% vs 62%), but there was no significant difference between these 2 groups in age, number of nodes examined, type of breast surgery or use of adjuvant radiotherapy or chemotherapy.
Table II shows how women without symptoms of lymphoedema (n = 132), women with symptoms (n = 72) and those attending for the physical assessment (n = 50) differed by age, by number of symptoms, and by arm disability as measured by the DASH. For the DASH, only 183 (of 204) women answered sufficient questions to derive a score, there was no significant difference between the symptomatic women who did or did not attend for physical assessment; however, there was a substantial difference in DASH score for asymptomatic women (difference in means: 23; 95% CI 19–27).
Table III presents the results of measurements of arm volume for affected and unaffected arms. Tape measurement indicated a slight difference between the 2 limbs not exceeding the clinically meaningful difference of 2 cm. Measurements by water displacement indicated that the affected arm was greater in size than the unaffected arm by an average difference of 154 ml. The differences in arm volume ranged from –160 ml to +740 ml with 16 of the 50 women having a difference of more than 200 ml and 5 women having a difference of more than 400 ml.
| Table III. Comparison between the affected and non-affected arm for women with self-reported lymphoedema post breast cancer surgery on measures of size and volume |
| | Affected arm (SD†) | Non-affected arm (SD) | Mean difference (SD) | 95% CI |
| Tape measurements (cm) |
| Metacarpo-phalangeal | 18.6 (1) | 18.5 (1) | 0.1 (0.6) | –0.071 to 0.271 |
| Forearm | 24.4 (2.5) | 23.3 (2.3) | 1.1 (1.7)* | 0.617–1.58 |
| Upper arm | 31.5 (3.9) | 30.6 (3.8) | 0.9 (1.5)* | 0.474–1.33 |
| Water Displacement (ml) | 2280 (403) | 2126 (392) | 154 (178)* | 103–205 |
| Bioelectrical impedance (ml)† | | 81 (169) | 33–129 |
| *p < 0.0001 derived from a 2-sample t-test. †Measurement not given for individual arms by bioelectrical impedance. SD: standard deviation; CI: confidence interval. |
The relationships between the responses to the signs and symptoms questionnaire and measurements of arm volume and disability were investigated. Table IV shows that, for symptomatic women, as the number of positive items increased, so did the arm volume, with an estimated effect of 66 ml (p < 0.0001) per additional positive item. Also shown is the distribution of lymphoedema by numbers of symptoms: 14 of 16 women with 3 or more symptoms had limb volume difference ≥ 200 ml, yielding a sensitivity of 87.5% (specificity 62%). For disability, there was a large difference between those without and those with symptoms (see Table II), but for symptomatic women, having more symptoms did not affect the DASH score. There was no association between volume of oedema and the DASH scores (correlation –0.05).
| Table II. Comparison between 204 women who responded to the signs and symptoms and DASH questionnaires |
| Characteristics | Women without symptoms of lymphoedema n = 132 | Women with symptoms of lymphoedema n = 72 | Women attending for physical assessment n = 50 |
| Age, years, (mean (SD)) | 62.1 (12) | 58.9 (11.2) | 59 (10) |
| Number of symptoms§ (mean (SD)) | 0 | 2.7 (1.4) | 2.8 (1.4) |
| DASH score | (n = 120) † | (n = 63) † | (n = 50) |
| Mean score (SD) | 5.6 (8.3) | 28.6 (20.9) | 23.9 (17.9) |
| Minimum/maximum | 0/42 | 0/79 | 0/78 |
| 25/50/75% | 0/2.5/6.6 | 11.2/26.8/42 | 9.2/20.8/34.2 |
| §Number of symptoms of lymphoedema is number of questions replied positively to in signs and symptoms questionnaire. Possible score 0–5. Most frequent symptom = noticed arms are different sizes. DASH: Disabilities of Arm Shoulder and Hand Questionnaire Score, possible score 0–100, with lower scores indicating less disability. Minimally clinically important difference = 3.9–16.6. †Not all DASH questionnaires were completed sufficiently for analysis. SD: standard deviation. |
| Table IV. Associations between responses to the signs and symptoms questionnaire, arm volume and the DASH score |
| | Arm volume (ml) Mean (SD) n = 50 | DASH score Mean (SD) n = 72 |
| Number of symptoms, n = 72 | | |
| 1 (n = 16) | 71 (103) | 19.2 (14.4) |
| 2 (n = 21) | 59 (83) | 23.4 (21.2) |
| 3 (n = 10) | 217 (141) | 21.3 (13.9) |
| 4 (n = 15) | 176 (146) | 30.6 (20.3) |
| 5 (n = 10) | 355 (235) | 24.4 (10.5) |
| Regression coefficient† (95% CI) | 66.4 (65.5–67.4) | 1.39 (0.9–1.79) |
| < 200 ml | | |
| 1 or 2 symptoms | 21 | |
| 3–5 symptoms | 13 | |
| ≥ 200 ml | | |
| 1 or 2 symptoms | 2 | |
| 3–5 symptoms | 14 | |
| †The regression coefficient from the linear regression model is interpreted as for every 1 unit change of x yields a change in y equal to the value of the regression coefficients (e.g. one additional symptom yields a change of 66.4 ml volume). DASH: Disabilities of Arm Shoulder and Hand Questionnaire Score; 72 women with symptoms completed the DASH but only 50 women attended for physical assessment including measurement of arm volume; SD: standard deviation; CI: confidence interval. |
The characteristics of the 50 women who attended for further assessment including health questionnaires and anthropometric measurements are shown in Table V. Women with a volume difference between arms of < 200 ml (n = 34) and ≥ 200 ml (n = 16) are contrasted because differences of greater than 200 ml between the affected and non-affected arms are indicative of lymphoedema (32). Also presented are the values for all women combined and normative values, where available. The 2 volume groups were similar on age, body mass index (BMI), co-morbidity and DASH scores; they were also similar on grip strength, but the total sample of women differed significantly from normative values. The most common co-morbidities found were osteoarthritis (48%) and back pain (54%).
| Table V. Characteristics of women with self-reported lymphoedema who attended for evaluation post breast cancer surgery |
| | Women with < 200 ml difference between arm volumes n = 34 Mean (SD) | Women with ≥ 200 ml difference between arm volumes n = 16 Mean (SD) | All women attending n = 50 Mean (SD) | Normal value (SD) |
| Age (years) | 57.2 (10) | 62.4 (11) | 59.0 (10) | |
| Body mass index | 26.5 (4.1) | 27.6 (5.4) | 26.8 (4.5) | |
| Co-morbidity† | 2.4 (1.7) | 2.8 (1.6) | 2.5 (2) | |
| DASH | 23.5 (18.7) | 24.6 (16.5) | 23.9 (17.9) | |
| Grip strength (kg) | | | | |
| Mean difference between arms | 1.6 (4) | 1.7 (3.6) | 1.7* (3.8) | 1.1 (2.7) |
| Box & Block | | | | |
| Affected arm (n) | 54.5 (10.5) | 54.7 (8.3) | 54.6* (9.8) | 74 (6.6) |
| Nine-Hole Peg | | | | |
| Affected arm (sec) | 21 (3.1) | 22.5 (5.3) | 21.5* (4) | 18.7 (3.1) |
| SF36 | | | | |
| Physical Functioning Index | 74 (22) | 64.1 (30.2) | 70.8* (25.0) | 83.5 |
| Role Physical | 58.1 (42.1) | 54.7 (40) | 57.0* (41.0) | 78.7 |
| Role Emotional | 67.6 (44.6) | 75 (37.5) | 70.0* (42.2) | 81.2 |
| General Health Perceptions | 67.3 (23.4) | 56.7 (24.9) | 63.9* (24.2) | 76.4 |
| Social Functioning | 72.4 (24.4) | 76.6 (25.8) | 73.7* (24.6) | 84.3 |
| Pain | 63.6 (25.8) | 59.8 (24.1) | 62.4* (25.1) | 73.3 |
| Vitality | 56.6 (23.7) | 55.3 (21.9) | 56.2* (22.9) | 62.9 |
| Mental Health Index | 73.3 (17) | 70.5 (17) | 72.4 (16.9) | 76.1 |
| Physical Component Scale | 44.4 (10) | 40.3 (12.5) | 43.1* (10.9) | 49.7 |
| Mental Component Scale | 48.4 (10.3) | 50.3 (11.7) | 49.0 (10.6) | 50.9 |
| McGill Pain Questionnaire | 27.9 (9.7) | 30.2 (10.8) | 29 (10) | |
| EQ5D – VAS of Health | 76 (17) | 73 (19) | 75 (17) | 82 (14) |
| EORTC QLQ-C30 (0–100) |
| Global Health (0–100) | 69.1 (24.7) | 69.3 (23.5) | 69.2 (24.1) | |
| Physical Functioning (0–100) | 83.1 (14.4) | 75.4 (24.5) | 80.7 (18.4) | |
| Role Functioning (0–100) | 82.8 (26.7) | 74 (24.3) | 80.0 (26.1) | |
| Emotional Functioning (0–100) | 74.3 (22.4) | 73.4 (24.9) | 74.0 (23.0) | |
| Cognitive Functioning (0–100) | 75.5 (25) | 88.5 (11.7) | 79.7 (22.4) | |
| Social Functioning (0–100) | 82.8 (28.9) | 86.4 (20.4) | 84.0 (26.3) | |
| Fatigue (100–0) | 37.9 (28.4) | 37.5 (24.6) | 37.8 (27.0) | |
| Nausea/vomiting (100–0) | 2.9 (8.7) | 3.1 (6.7) | 3.0 (8.1) | |
| Pain (100–0) | 33.3 (31.2) | 32.3 (25.4) | 33.0 (29.3) | |
| Dyspnoea (100–0) | 26.5 (32.6) | 27 (30.3) | 26.7 (31.6) | |
| Insomnia (100–0) | 33.3 (34.8) | 45.8 (29.5) | 37.3 (33.4) | |
| Appetite Loss (100–0) | 12.7 (26) | 10.4 (20.1) | 12.0 (24.1) | |
| Financial Problems (100–0) | 10.8 (22.8) | 12.5 (20.6) | 11.3 (21.9) | |
| EORTC BR23 (0–100) |
| Body Image | 75.2 (26.1) | 85.9 (17.7) | 78.7* (24.1) | 63.0 |
| Sexual Functioning | 25.9 (21.4) | 28.9 (24.8) | 26.9 (22.3) | 23.9 |
| Sexual Enjoyment | 50 (31.5) | 53.3 (23.3) | 51.1 (28.7) | 47.1 |
| Future Perspective | 62.6 (33.1) | 68.9 (23.5) | 64.6* (30.3) | 45.1 |
| Breast Symptoms | 22.8 (24.5) | 22.4 (17.4) | 22.6 (22.3) | 21.7 |
| Arm Symptoms | 30.1 (25.1) | 41 (21) | 33.6* (24.2) | 22.2 |
| †Co-morbidity = number of co-morbidities described. Most common co-morbidities were osteoarthritis (48%) and back pain (54%). *Statistically significantly different from norm p < 0.05, derived from t-test. SF36: Medical Outcome Short Form-36 Health Survey Subscales scored out of 100, a higher score indicates better health; EORTC QLQ: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire with breast module (BR23) Subscales scored out of 100. For the functional scales, a higher score indicates better function. For the symptom scales, a higher score indicates more symptoms; SD: standard deviation; DASH: Disabilities of Arm Shoulder and Hand Questionaire Score; VAS: visual analogue scale; EQ5D: self perceived health status (0–100). |
For the Box and Block Test and Nine-hole Peg Test, the values for the affected arm are presented. These values did not differ significantly by volume group but did differ significantly and substantially from norms. The values on the subscales of the SF-36 were all significantly lower than norms for Canadian women (29) with the exception of Mental Health Index and, consequently, the Mental Component Scale, but not significantly different between volume groups. Of note is that women with volume differences < 200 ml scored, on average, 10 points higher than women with volume differences of ≥ 200 ml, a value which is clinically meaningful, but this study did not have sufficient power to detect this difference.
For pain, measured by the McGill Pain Questionnaire, there were no differences between groups. Pain was also measured using the SF-36 and the study sample of women had significantly more limitation due to pain than did the normative sample.
Women from the different volume groups did not differ significantly on subscales of the EORTC QLQ-C30 or on the Arm Module.
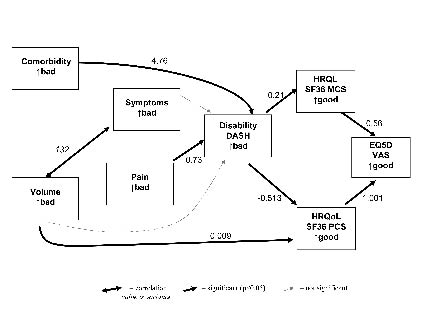
The results of the path analysis are shown in Fig. 2. This model showed acceptable fit (χ2 probability = 0.22, CFI = 0.98). The dark lines indicate statistically significant paths; the light lines indicate variables that were required for explanation but were themselves not statistically significant. The significant paths were between co-morbidity and disability, pain and disability, volume and physical function aspect of HRQL, and disability and HRQL. There was no significant path between volume and disability. There is a strong correlation between the number of symptoms of lymphoedema and the volume difference between arms (double-headed arrow).
Fig. 2. Path model of the relationships between impairments, disability and health-related quality of life. Values shown are regression coefficients. Goodness-of-fit statistic: χ2 = 13.7 df = 13 CFI = 0.994. DASH: Disabilities of Arm Shoulder and Hand questionnaire; PCS: Physical Component Scale; MCS: Mental Component Scale; HRQL: Health Related Quality of Life; SF-36: Short Form-36 Health Survey; EQ5D: self perceived health status (0–100) ;VAS: visual analogue scale.
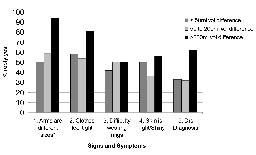
Fig. 3 shows the proportion of women responding positively to each of the symptom questions according to the size of the volume difference between arms. The most sensitive question to detect clinical lymphoedema (> 200ml difference between arms) was the question noticing a difference in size between the arms (sensitivity 0.94), however, the specificity was low (0.41). Questions 3 and 4 had sensitivity of less than 60%. As shown in Table IV, the sensitivity of 3 or more symptoms was 87% and the specificity was 61%.
Fig. 3. Relationship between symptoms of lymphoedema and difference in arm volume (n = 50). Regression coefficient = 66. Sensitivity* = 0.94. Specificity = 0.41.
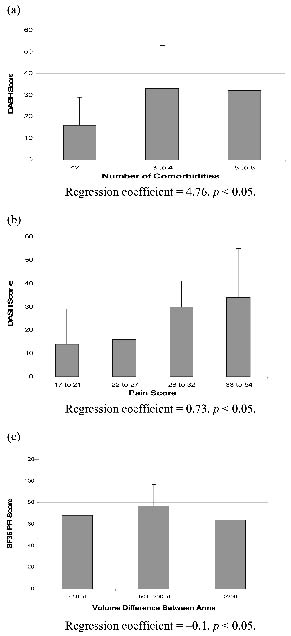
Fig. 4 depicts the magnitude of the relationships shown in key sub-paths. Fig. 4a shows the strong relationship between co-morbidity and disability, such that for every additional co-morbidity, the DASH score was greater by 4.76 units. Fig. 4b shows the relationship between quartiles on the McGill pain score and the DASH. Fig. 4c shows that women with > 200 ml differences in volume between arms had a significantly poorer physical function as measured by the SF-36 such that for every increase of 100 ml in arm volume there was a decrease in physical function of 10 units (path co-efficient –0.1).
Fig 4. (a) Relationship between number of co-morbidities and DASH score. (b) Relationship between McGill Pain Score and DASH Score. (c) Relationship between Difference in Arm Volume and SF36 Physical Component Scale. DASH: Disabilities of Arm Shoulder and Hand questionnaire; SF-36: Short Form-36 Health Survey.
DISCUSSION
Participants in the epidemiologic pilot (n = 204) had many features representative of the base-population in terms of time since diagnosis, type of breast surgery and type of adjuvant therapy, but were more likely to have had ALND, a predisposing factor for lymphoedema (see Table I). This sub-study should provide valid estimates of associations between variables because 69% of symptomatic women agreed to attend for assessment and those who declined were very similar to those agreeing. Inviting only those women with symptoms of lymphoedema was justified as, in comparison with women without symptoms of lymphoedema, symptomatic women had a substantially greater degree of arm disability (mean DASH difference: 23.4; 95% CI 19.3–27.5), exceeding the range of values reported in the literature as indicating a clinically important difference (CID: 3.9 and 16.6 (33)). Arm swelling was not the principal contributor to arm disability post breast cancer surgery, but pain and co-morbidity were predominant factors. Surprisingly, arm volume was not associated with arm disability.
The numbers of symptoms reported as present were associated with volume but not disability. This suggests that it is the perceived presence of swelling that is associated with disability rather than the degree of swelling and, within this range of oedema, pain is a stronger determinant of disability than swelling.
A limitation of this study was that the screening questionnaire we used had not previously been tested for validity, although the content of the items was drawn from questions used in other studies (17). The data generated from this study contributes preliminary evidence for the validity of this screening questionnaire (see Fig. 3 and Table IV). Endorsing 3 or more items had a sensitivity of 87.5% and a specificity of 62%. There was also support for concurrent validity because of the strong relationship between symptoms and arm disability as measured by the DASH (see Table IV).
There was a significant relationship between pain and activity limitation and participation restriction. No positive relationship was shown between any of the anthropometric measurements and activity limitation or participation restriction. This may be due to insufficient sample size, or it may be that the perception of having oedema or not having oedema causes the limitation not the observed severity of the lymphoedema.
Women with self-reported lymphoedema had a sub-optimal HRQL; they did not achieve normative values on the SF-36 or the EORTC. Sub-optimal HRQL was also found in other studies of women with lymphoedema (4, 15).
A strong relationship was found between pain (McGill pain questionnaire) and decreased HRQL (SF-36, EORTC-QLQ30 and BR23). One of the SF-36 and EORTC subscales is pain, so that subscale would be expected to correlate with the McGill pain questionnaire, but other subscales also had high correlations. A limitation of this study was that women were not asked to discriminate between pain arising from the breast surgery and pain or discomfort arising from carrying a heavy, lymphoedematous limb.
The path analysis demonstrated relationships between the impairments of pain and co-morbidity with activity limitation/participation restriction (DASH) and HRQL, but no relationship between volume and activity limitation/participation restriction (DASH). It also added to the information by demonstrating a relationship between volume and one aspect of HRQL, physical function.
Treatment is usually aimed at reducing the size and volume of oedema, and oedema reduction is measured to assess the outcome of treatment. The findings from this study suggest that, for the majority of women, treatment should be aimed at decreasing pain, activity limitations and participation restrictions, and that the success of treatment should be measured by the women’s ability to participate. Treatment aimed at reducing arm volume is likely to be more relevant to those with very large arm volumes. The importance of co-morbidity indicates that treatment should focus not only on the sequelae of surgery, but also consider concurrent health conditions and exacerbation of existing musculoskeletal co-morbidities.
Further research needs to be undertaken with a sufficiently large sample size to fully understand the role of pain and lymphoedema. As treatment of lymphoedema is aimed at reducing volume it should be ascertained whether a certain volume decrease correlates with a decrease in activity limitation, participation restriction, or HRQL, and if there is a clinically significant level of volume reduction.
Women with self-reported lymphoedema do report activity limitations, participation restrictions and a sub-optimal HRQL. The greater the pain an individual is experiencing the greater are their activity limitation and participation restrictions and the lower is their quality of life.
We were unable to demonstrate a positive relationship between any other individual measurement of impairment and activity limitation, participation restriction or health-related quality of life. It may be that psychological factors play a large part in the reporting of lymphoedema, not impairment, that thinking one has lymphoedema and experiencing pain reduces quality of life and increases activity limitation and participation restriction.
With continuing research into treatments for lymphoedema, the use of measurements of pain, activity limitation, participation restriction, and health-related quality of life would add meaningful information to supplement that obtained from traditional volume measurements. Decreasing arm volume alone may not decrease a person’s activity limitations, participation restrictions or improve their quality of life.
REFERENCES
1. National Cancer Institute of Canada. Canadian Cancer Statistics 2007 [cited 2007 Apr 11]. Available from: www.cancer.ca
2. Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients. J Natl Cancer Inst 2001; 93: 96–111.
3. Spiegel D. Psychosocial aspects of breast cancer treatment. Semin Oncol 1997; 24 Suppl 1: S1.
4. Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema. Am J Surg 1999; 177: 184–187.
5. Weisslander H, Schuchhardt C, editors. Lymphedema: diagnosis and therapy. Viavital: Viavital Verlag ed.; 2001.
6. Schrenk P, Rieger R, Shamiyeh A, Wayand W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer 2000; 88: 608–614.
7. Schunemann H, Willich N. Lymphodeme nacht Mammakarzinom [Lymphedema after breast carcinoma. A study of 5868 cases]. Dtsch Med Wochenschr 1997; 122: 536–541 (in German).
8. Francis WP, Abghari P, Du W, Rymal C, Suna M, Kosir MA. Improving surgical outcomes: standardizing the reporting of incidence and severity of acute lymphedema after sentinel lymph node biopsy and axillary lymph node dissection. Am J Surg 2006; 192: 636–639.
9. Moseley AL, Carati CJ, Piller NB. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann Oncol 2007; 18: 639–646.
10. Badger C, Preston N, Seers K, Mortimer P. Benzo-pyrones for reducing and controlling lymphoedema of the limbs. Cochrane Database Syst Rev 2004: CD003140.
11. Badger CM, Peacock JL, Mortimer PS. A randomized, controlled, parallel-group clinical trial comparing multilayer bandaging followed by hosiery versus hosiery alone in the treatment of patients with lymphedema of the limb. Cancer 2000 Jun 15; 88: 2832–2837.
12. Brach M, Cieza A, Stucki G, Fussl M, Cole A, Ellerin B, et al. ICF Core Sets for breast cancer. J Rehabil Med 2004; 36 Suppl 44: 121–127.
13. Rietman JS, Dijkstra PU, Debreczeni R, Geertzen JH, Robinson DP, De Vries J. Impairments, disabilities and health related quality of life after treatment for breast cancer: a follow-up study 2.7 years after surgery. Disabil Rehabil 2004; 26: 78–84.
14. Rietman JS, Dijkstra PU, Geertzen JH, Baas P, De Vries J, Dolsma WV, et al. Treatment-related upper limb morbidity 1 year after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast cancer. Ann Surg Oncol 2004; 11: 1018–1024.
15. Beaulac SM, Mcnair LA, Scott TE, LaMorte WW, Kavanah MT. Lymphedema and quality of life in survivors of early-stage breast cancer. Arch Surg 2002; 137: 1253–1257.
16. Lauridsen MC, Christiansen P, Hessov I. The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: a randomized study. Acta Oncol 2005; 44: 449–457.
17. Kwan W, Jackson J, Weir LM, Dingee C, McGregor G, Olivotto IA. Chronic arm morbidity after curative breast cancer treatment: prevalence and impact on quality of life. J Clin Oncol 2002; 20: 4242–4248.
18. World Health Organization. International Classification of Functioning, Disability and Health. Second revision edn. Geneva: World Health Organization; 2001.
19. Sander AP, Hajer NM, Hemenway K, Miller AC. Upper-extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther 2002; 82: 1201–1212.
20. Melzack R. The short-form McGill Pain Questionnaire. Pain 1987; 30: 191–197.
21. Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther 1985; 39: 386–391.
22. Mathiowetz V. Adult Norms for The Nine Hole Peg Test of Finger Dexterity. Occupat Ther J Res 1985; 25.
23. Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg [Am] 1984; 9: 222–226.
24. Finch E, Brooks D, Stratford PW, Mayo NE, editors. Physical rehabilitation outcome measures. 2nd edn. Hamilton: BC Decker Inc.; 2002.
25. McHorney CA, Ware JE, Lu JFR, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994; 32: 40–66.
26. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376.
27. Sprangers MA, Groenvold M, Arraras JI, Franklin J, Te VA, Muller M, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol 1996; 14: 2756–2768.
28. Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil 1985; 66: 69–74.
29. Hopman WM, Towheed T, Anastassiades T, Tenenhouse A, Poliquin S, Berger C, et al. Canadian normative data for the SF-36 health survey. Can Med Assoc J 2000; 163: 265–271.
30. Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 1995; 273: 59–65.
31. Kline RB, editor. Principles and practice of structural equation modeling. New York: The Guilford Press; 1998.
32. Knobf MK. Primary breast cancer: physical consequences and rehabilitation. Semin Oncol Nurs 1985; 1: 214–224.
33. Beaton DE. A Comparison across methodology approaches. 10th Annual Conference of the International Society for Quality of Life: Kluwer Academic; 2003.