OBJECTIVE: Post-stroke shoulder pain is a common phenomenon in hemiplegia and impedes rehabilitation. The aim of this study was to identify a possible relationship between post-stroke shoulder pain, scapula resting position and shoulder motion.
METHODS: Shoulder kinematics of 27 patients after stroke (17 men) were compared with 10 healthy age-matched control subjects. Using an electromagnetic tracking device, the kinematics of both the contralateral and ipsilateral (i.e. paretic and non-paretic) arm during active and passive abduction and forward flexion were measured and expressed in Euler angles.
RESULTS: Scapular lateral rotation relative to the thorax was increased in patients with post-stroke shoulder pain compared with both patients without post-stroke shoulder pain and control subjects at rest as well as during arm abduction and forward flexion. Additionally, glenohumeral elevation was decreased in patients with post-stroke shoulder pain during passive abduction. No differences were found regarding scapula position (displacement relative to the thorax).
CONCLUSION: In patients with post-stroke shoulder pain a particular kinematical shoulder pattern was established, characterized by enhanced scapular lateral rotation and diminished glenohumeral mobility.
Key words: stroke, hemiplegia, shoulder pain, kinematics.
J Rehabil Med 2008; 40: 482–486
Correspondence address: Martijn Niessen, Research Institute MOVE, Faculty of Human Movement Sciences, VU University Amsterdam, Van der Boechorststraat 9, NL-1081 BT Amsterdam, The Netherlands. E-mail: m.niessen@fbw.vu.nl
Submitted November 15, 2007; accepted February 8, 2008
Introduction
Post-stroke shoulder pain (PSSP) is a common phenomenon in hemiplegia, with an estimated incidence of between 16% and 84% (1–5). PSSP impedes rehabilitation and may also interfere with balance, walking, transfers, performance of self-care activities, and quality of life (3). The occurrence of PSSP is probably not related to age and gender and may be related to the severity of the paresis. However, within the group of patients with substantial upper limb deficits it is stated that factors such as soft tissue damage or spasticity have a greater effect on pain than the severity of the stroke deficit per se (4). Among the various factors thought to be contributing to the occurrence of shoulder pain, some are related to the shoulder joint, such as rotator cuff injuries (6) or subluxation of the humeral head (7), whereas other factors are related to the neurological lesion, such as central post-stroke pain, lack of sensibility, unilateral neglect and spasticity (7, 8).
It has been shown that the kinematics of both shoulders are affected as result of a stroke (9, 10). To our knowledge, no study has evaluated a possible relation between the alterations in shoulder kinematics and the occurrence of PSSP. The goal of this study, therefore, was to identify a possible relationship between PSSP, shoulder-resting pose (i.e. position and orientation) and shoulder motion. We hypothesize that shoulder pain is related to a disturbed scapular and humeral resting pose and/or a deviating contribution of the scapula or humerus to the abduction and forward flexion of the arm. Then, chronic shoulder pain, whatever the initiating factor, may eventually be the consequence of a vicious circle of repetitive soft tissue damage caused by improper kinematics (11–13).
Methods
Subjects
Twenty-seven patients in the subacute phase after stroke were recruited from the inpatient ward of a rehabilitation centre (Table I). All patients had experienced their first stroke and had no history of shoulder complaints prior to the stroke. They were able to perform all the required physical, cognitive and communicative tests for this study. Prior to the measurements, current shoulder pain, muscle tone and degree of paralysis were scored, using a 0–100-mm visual analogue scale (VAS) (14, 15) (0: no pain; 100: unbearable pain), a 0–5 scale modified Ashworth Scale (16, 17) (0: no increase in muscle tone; 5: limb rigid in flexion or extension) and 0–6 scale Brunnstrom stage (18) (0: complete hemiparalysis; 6: no hemiparalysis), respectively (Table I). Measurements were performed on the contralateral side (relative to the side of the lesion, i.e. “paretic”) as well as the ipsilateral side (relative to the side of the lesion, i.e. “non-paretic”).
Ten age-matched healthy subjects (Table I) with no history of shoulder complaints were used as a control group. The study was approved by the local Institutional Review Board and all subjects signed an informed consent statement before the start of the measurements.
| Table I. Characteristics of subjects with and without post-stroke shoulder pain (PSSP) and controls |
| | Patients with PSSP | Patients without PSSP | Control subjects |
| Number of subjects (male/female) | 13 (10/3) | 14 (7/7) | 10 (4/6) |
| Age, years (mean (SD)) | 59.3 (11.1) | 57.0 (9.5) | 49.3 (7.2) |
| VAS score, mm (mean (SD)) | 49.7 (24.7) | NA | NA |
| Range Brunnstrom stage (degree of paralysis) | 1–5 | 2–5 | NA |
| Range Modified Ashworth scale (muscle tone) | 0–2 | 0–3 | NA |
| Time since stroke, weeks (mean (SD)) | 14.4 (9.3) | 13.0 (7.6) | NA |
| Side of brain lesion: left/right | 10/3 | 5/9 | NA |
| Lesion location: cortical/sub-cortical | 8/5 | 9/5 | NA |
| NA: not applicable; VAS: visual analogue scale; SD: standard deviation. |
Kinematics
An electromagnetic tracking device (MotionMonitor; Innovative Sports Training, Inc., Chicago, IL, USA) was used to quantify shoulder kinematics. This setup consists of a transmitter creating a weak magnetic field in which the position and orientation of several receivers can be followed. The manufacturer has reported an accuracy of 0.5° (root mean square (RMS)) for orientation and 1.8 mm (RMS) for position (system documentation). Measurements were performed on both arms according to the standardized protocol for motion recordings of the shoulder of the International Shoulder Group (ISG) (19). Three receivers were placed on the thorax, upper and lower arm, respectively, using Velcro straps or tape. A fourth receiver was mounted on the flat surface of the acromion using Fixomull stretch. This method has proven to be accurate (20, 21). A fifth receiver was placed on a pointer, to be used as a spatial digitizer.
With the subject sitting in a wooden chair in front of the transmitter, local co-ordinate systems (LCSs) of the thorax, scapula and upper arm were determined using the standardized protocol of the ISG. These LCSs were calculated by the MotionMonitorTM software using 15 bony landmarks, with a similar layout for all segments (X-axis pointing forward, the Y-axis pointing upward and the Z-axis pointing to the right) (19). The orientations of these LCSs were decomposed and expressed in Euler angles using the decomposition order as proposed by the ISG (19), i.e. Y, X’, Z’’ (pro/retraction, medial/lateral rotation, anterior/posterior tilt) and Y, X’, Y’’ (plane of elevation, internal/external rotation, elevation) for scapula and humerus, respectively.
Passive and active (if possible) arm elevations (to 120° maximally or until the pain threshold) were performed in the sagittal (forward flexion) and frontal plane (abduction) with both the contralateral as well as the ipsilateral arm. A target angle of 120° was chosen since this was expected to be feasible for most patients (9), and readings of the sensor on the acromion are questionable above this angle (20). The movements were standardized by aligning the elevation plane to an adjustable semi-circular wooden arch mounted along side the subject. Poses (position and orientation) of the scapula relative to the thorax and the humerus relative to the scapula at 30°, 60°, 90° and 120° of arm elevation were used for analysis. Each elevation was performed 3 times and outcomes were averaged. Additionally, maximal passive internal and external rotations of the humerus were determined in the frontal and sagittal plane for both shoulders. During these measurements, the subjects' arm was elevated approximately 60° and the elbow was in 90° of flexion. Movements of the scapula and humerus were expressed relative to the thorax. Glenohumeral poses were obtained by expressing the movements of the humerus relative to the scapula.
Statistics
Scapular and humeral poses of the shoulders of patients after stroke with and without PSSP and control subjects were compared using a General Linear Model analysis of variance (ANOVA) with repeated measures, with arm elevation as a within factor and group (control, patients with and without PSSP) as a between factor. One-way ANOVA was used to compare the poses of the scapula and humerus at rest and to evaluate maximal internal and external humeral rotations. Results were considered significant at p < 0.05. When a significant difference was found, a Tukey honestly significant difference post-hoc test was used to determine which groups were different. An a priori power analysis was conducted based on previous studies (9, 13, 22–24). The effect size was 0.7 based on an inter-individual variability of scapular rotations of 7°. With an alpha of 0.05 and a desired smallest detectable difference between the groups of 3.5°, a sample size of n = 8 per group results in a power of 0.8.
Results
As only 2 patients were able to reach the target elevation angle of 120° with their contralateral (“paretic”) arm, only the scapular and humeral poses in rest and at 30°, 60° and 90° of arm elevation were used for analysis. For patients with PSSP it appeared to be very difficult to perform any active arm elevation with their contralateral arm. With their ipsilateral (“non-paretic”) arm, all patients reached 90° during active and passive abduction and forward flexion.
Resting pose
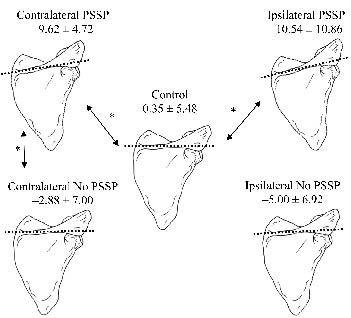
Fig. 1 shows a schematic representation of scapular medial/lateral rotation at rest of all subjects. At rest, the contralateral shoulder of patients with PSSP showed more scapular lateral rotation than both patients without PSSP and control subjects (p = 0.03 and p <0.01; Figs 1 and 2). Scapula laterorotation of patients with PSSP at the ipsilateral side was enhanced compared with control subjects but not to patients without PSSP (p = 0.01 and p = 0.25 respectively, Figs 1 and 2). No differences in scapular anterior/posterior tilt or pro/retraction were found. All differences in poses between the groups were found to be regarding scapular orientation, not position (displacement relative to the thorax).
Fig. 1. Schematic representation of scapular medial/lateral rotation in degrees (dorsal view) during rest of patients with and without post-stroke shoulder pain (PSSP), and of control subjects. *Significant differences. Positive values: medial rotation; negative values: lateral rotation.
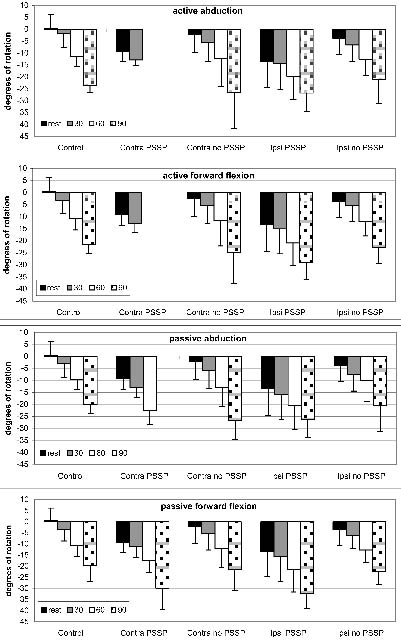
Fig. 2. Scapular lateral rotation during rest and 30°, 60° and 90° of arm abduction and forward flexion. Negative values on the Y-axis denote lateral rotation. Control: control subjects; Contra: patients’ contralateral side; Ipsi: patients’ ipsilateral side. PSSP: post-stroke shoulder pain.
Contralateral shoulder movements
During passive abduction a significant group-angle interaction effect for glenohumeral elevation was found (p = 0.04), indicating a larger increase in glenohumeral elevation from rest to 30° arm elevation for control subjects than the paretic shoulder of all patients.
Patients with PSSP showed enhanced scapular lateral rotation during active and passive abduction and forward flexion when compared with control subjects. When compared with patients without PSSP, scapular lateral rotation was only found to be enhanced during passive abduction, while no significant differences were found during active and passive forward flexion and active abduction (Table II). No differences were found between patients without PSSP and controls in shoulder kinematics during all performed movements.
A decreased glenohumeral elevation during passive abduction was found in patients with PSSP when compared with both control subjects and patients without PSSP (p = 0.03 for both).
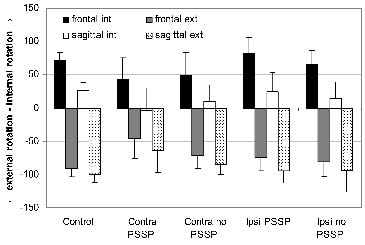
For patients with PSSP, less maximal internal and external glenohumeral rotation was found, compared with control subjects but not to patients without PSSP, during passive external arm rotation in both frontal and sagittal planes (Fig. 3, Table III).
| Table II. p-values of scapular lateral rotation |
| Side | Mode | Movement | PSSP + vs PSSP – | PSSP + vs Control | PSSP – vs Control |
| Contralateral | Active | Abduction | 0.10 | 0.01 | 0.34 |
| | | Forward flexion | 0.30 | 0.04 | 0.37 |
| | Passive | Abduction | 0.03 | < 0.00 | 0.33 |
| | | Forward flexion | 0.25 | 0.05 | 0.55 |
| Ipsilateral | Active | Abduction | 0.03 | 0.03 | 0.96 |
| | | Forward flexion | 0.03 | 0.02 | 0.95 |
| | Passive | Abduction | 0.04 | 0.03 | 0.90 |
| | | Forward flexion | 0.02 | 0.01 | 0.92 |
| PSSP: patients with (+) and without (–) post-stroke shoulder pain. |
Fig. 3. Maximal internal (int) and external (ext) rotation in degrees in the frontal and sagittal plane. Control: control subjects; Contra: patients’ contralateral side; Ipsi: patients’ ipsilateral side. PSSP: post-stroke shoulder pain.
| Table III. p-values of maximal internal and external glenohumeral rotations |
| | Plane of rotation | Movement direction | PSSP + vs PSSP – | PSSP + vs Controls | PSSP – vs Controls |
| Contralateral | Frontal | Internal | 0.82 | 0.06 | 0.16 |
| | | External | 0.02 | < 0.00 | 0.12 |
| | Sagittal | Internal | 0.36 | 0.02 | 0.28 |
| | | External | 0.08 | 0.00 | 0.22 |
| Ispilateral | Frontal | Internal | 0.08 | 0.46 | 0.66 |
| | | External | 0.68 | 0.12 | 0.42 |
| | Sagittal | Internal | 0.50 | 0.10 | 0.42 |
| | | External | 0.97 | 0.09 | 0.75 |
| PSSP: patients with (+) and without (–) post-stroke shoulder pain. |
No differences in scapular position (displacement relative to the thorax) were found between the groups during active and passive abduction and forward flexion.
Ipsilateral shoulder movements
Patients with PSSP showed more scapular lateral rotation during active as well as passive abduction and forward flexion than control subjects and patients without PSSP (Table II).
No differences were found in maximal internal or external glenohumeral rotation or in scapular position during active and passive abduction and forward flexion between the groups (Table III).
Discussion
The goal of this study was to identify a possible relationship between PSSP and deviations in shoulder kinematical patterns. This relation could indeed be established. We therefore state that shoulder pain is related to a disturbed scapular and humeral resting position and/or a deviating contribution of the scapula or humerus to the abduction and forward flexion of the arm.
The most remarkable findings in this study are the clear differences in scapular lateral rotation in patients with PSSP in both the contralateral (“paretic”) and the ipsilateral (“non-paretic”) shoulder when compared with patients without PSSP and control subjects. Price et al. (25) also found a relation between a more laterally rotated scapula (scapular lead) on the contralateral side and shoulder pain. However, they only compared the contralateral to the ipsilateral side and not to a control group. Therefore, changes in kinematics of the contralateral shoulder could have been underestimated and possible changes on the ipsilateral side were not noticed.
From Table II it seems that patients with PSSP predominantly have a left hemispheric lesion, while this seems to be inversed in patients without PSSP. However, this effect is not significant and is probably due to the small group sizes.
Based on the data from the present study, it is not possible to make comments about the origin of shoulder pain. There are several possible relations between PSSP and changes in shoulder kinematics.
First, changes in shoulder kinematics could lead to shoulder pain. Stroke patients might not be able to compensate for the gravitational pull on the scapula due to the hemiparesis. Muscles such as the m. trapezius, m. levator scapulae, m. rhomboid minor, major and serratus anterior are responsible for maintaining scapula position (26), and if the strength of these muscles is diminished, the positioning of the scapula can be affected. Since the scapula only articulates with the clavicle (at the acromioclavicular joint), the inability to compensate for the gravitational pull could cause a lateral rotation of the scapula with the acromioclavicular joint acting as a pivot, as found in this study. This, in turn, could initiate or worsen pathologies such as subluxation, impingement and capsulitis, resulting in PSSP. However, not all patients after stroke have PSSP. It might be that patients who do not suffer from PSSP have more control over their shoulder muscles and are thus better able to compensate for the mechanisms that cause PSSP. It must be noted that the alterations found in shoulder kinematics of the ipsilateral shoulder did not cause PSSP in that shoulder, which indicates that the movement alteration itself does not cause pain. However, the ipsilateral shoulder is much less affected by the stroke and therefore this shoulder is probably better able to cope with, and compensate for, mechanisms leading to PSSP.
Secondly, PSSP could cause the changes in shoulder kinematics found. If PSSP starts to develop, shoulder kinematics could be altered as pain relief. In a normal, healthy shoulder, next to stabilization of the shoulder by the rotator cuff, lateral (upward) rotation of the scapula prevents impingement by rotating the major tubercle of the humerus away from the coraco-acromial arch, thus preventing the supraspinatus outlet from being narrowed (4, 13). This mechanism can be used by patients suffering from PSSP. The fact that both shoulders of patients with PSSP show altered kinematics, with only the contralateral shoulder being painful, indicates that a central compensating mechanism for pain relief affecting both shoulders could be involved. However, this bilateral effect can also be explained by the fact that both hemispheres control both sides of the body (27–29), so a lesion in one-half of the brain also affects the other half. It has been shown before that the ipsilateral side of stroke patients is also affected after stroke (9, 30).
Thirdly, an additional factor causes both PSSP and changes in kinematics. Impingement syndrome is often considered as a cause of shoulder pain in hemiplegic stroke patients and could also result in changes in kinematics (4, 31). Adhesive capsulitis (frozen shoulder), is characterized by diminished range of shoulder motion (especially exorotation) and shoulder pain (32) and the term is used quite generally in relation to PSSP (4). The shoulders of our patients with PSSP show these characteristics but this does not mean that these patients suffer from capsulitis. It has been shown that the scapula of patients with capsulitis is oriented normally during rest, but during arm elevation, the scapula shows larger lateral rotation (33). It must be noted, however, that the patients used in that study were not stroke patients. A common complication in stroke patients is build up of bilateral muscle tension around the head and neck and the restriction of blood flow to those areas as a result. This could eventually lead to tension-type headaches and could also explain differences in scapular kinematics of the ipsilateral shoulder found in the present study (34–36).
Based on the data from the present study, it is difficult to draw conclusions about the origin of shoulder pain. A longitudinal study, in which patients are measured multiple times during the course of rehabilitation, starting as soon as possible after the stroke, could provide more insight into the process. If pain occurs before alterations in scapular kinematics are observed, then the alterations of the lateral rotation of the scapula could act as a compensatory mechanism. If the scapular lateral rotations are already altered before the pain occurs, then the effect of the alteration itself could cause PSSP. Also, the effects of pain relief by means of injections (e.g. marcaine, lidocaine, bupivacaine) on the scapular kinematics of patients with PSSP could be of great value.
The clinical implications of the observed kinematic differences in the shoulders of stroke patients with and without PSSP remain speculative. Many pathologies could lead to PSSP, some related to the shoulder joint and some related to the neurological lesion (4, 6–8). The present study shows a clear relation between PSSP and altered shoulder kinematics, but a causal relation between the 2 could not be established. However, the fact remains that patients with PSSP show altered shoulder kinematics and it could be that these kinematic alterations worsen the initial pathology or cause secondary pathologies and thus initiate a vicious circle of repetitive soft tissue damage leading to chronic PSSP.
In general we can conclude that patients with PSSP show a particular kinematical shoulder pattern, characterized by enhanced scapular lateral rotation and diminished glenohumeral mobility. The question remains as to what causes these changes and what the relation is between PSSP and the altered shoulder kinematics. It could be that PSSP causes the kinematic differences found, but it could also be the other way around. It is also possible that an additional factor could cause both PSSP and changes in shoulder kinematics.
ReferenceS
1. Gamble GE, Barberan E, Bowsher D, Tyrrell PJ. Post stroke shoulder pain: more common than previously realized. Eur J Pain 2000; 4: 313–315.
2. Gamble GE, Barberan E, Laasch H-U, Bowsher D, Tyrrell PJ, Jones AKP. Poststroke shoulder pain: a prospective study of the association and risk factors in 152 patients from a consecutive cohort of 205 patients presenting with stroke. Eur J Pain 2002; 6: 467–474.
3. Jespersen HF, Jorgensen HS, Nakayama H, Olsen TS. Shoulder pain after a stroke. Int J Rehabil Res 1995; 18: 273–276.
4. Turner-Stokes L, Jackson D. Shoulder pain after stroke: a review of the evidence base to inform the development of an integrated care pathway. Clin Rehabil 2002; 16: 276–298.
5. Lindgren I, Jonsson AC, Norrving B, Lindgren A. Shoulder pain after stroke: a prospective population-based study. Stroke 2007; 38: 343–348.
6. Yamaguchi K, Sher JS, Andersen WK, Garretson R, Uribe JW, Hechtman K, et al. Glenohumeral motion in patients with rotator cuff tears: a comparison of asymptomatic and symptomatic shoulders. J Shoulder Elbow Surg 2000; 9: 6–11.
7. Chantraine A, Baribeault A, Uebelhart D, Gremion G. Shoulder pain and dysfunction in hemiplegia: effects of functional electrical stimulation. Arch Phys Med Rehabil 1999; 80: 328–331.
8. Vuagnat H, Chantraine A. Shoulder pain in hemiplegia revisited: contribution of functional electrical stimulation and other therapies. J Rehabil Med 2003; 35: 49–54; quiz 56.
9. Meskers CG, Koppe PA, Konijnenbelt MH, Veeger DH, Janssen TW. Kinematic alterations in the ipsilateral shoulder of patients with hemiplegia due to stroke. Am J Phys Med Rehabil 2005; 84: 97–105.
10. Smith DL, Akhtar AJ, Garraway WM. Proprioception and spatial neglect after stroke. Age Ageing 1983; 12: 63–69.
11. Myers JB, Lephart SM. The role of the sensorimotor system in the athletic shoulder. J Athl Train 2000; 35: 351–363.
12. Myers JB, Lephart SM. Sensorimotor deficits contributing to glenohumeral instability. Clin Orthop 2002: 98–104.
13. Meskers CGM, van der Helm FCT, Rozing PM. The size of the supraspinatus outlet during elevation of the arm in the frontal and sagittal plane: a 3-D model study. Clin Biomech 2002; 17: 257–266.
14. Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain 1985; 22: 1–31.
15. Tiplady B, Jackson SH, Maskrey VM, Swift CG. Validity and sensitivity of visual analogue scales in young and older healthy subjects. Age Ageing 1998; 27: 63–66.
16. Gregson JM, Leathley M, Moore AP, Sharma AK, Smith TL, Watkins CL. Reliability of the Tone Assessment Scale and the modified Ashworth scale as clinical tools for assessing poststroke spasticity. Arch Phys Med Rehabil 1999; 80: 1013–1016.
17. Pandyan AD, Price CI, Barnes MP, Johnson GR. A biomechanical investigation into the validity of the modified Ashworth Scale as a measure of elbow spasticity. Clin Rehabil 2003; 17: 290–293.
18. Brunnstrom S, editor. Movement theory in hemiplegia: a neurophysiological approach. New York: Harper & Row; 1970, p. 113–122.
19. Wu G, van der Helm FC, Veeger HE, Makhsous M, Van Roy P, Anglin C, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion-Part II: shoulder, elbow, wrist and hand. J Biomech 2005; 38: 981–992.
20. Meskers CG, van de Sande MA, de Groot JH. Comparison between tripod and skin-fixed recording of scapular motion. J Biomech 2007; 40: 941–946.
21. Karduna AR, McClure PW, Michener LA, Sennett B. Dynamic measurements of three-dimensional scapular kinematics: a validation study. J Biomech Eng 2001; 123: 184–190.
22. Meskers CGM, Vermeulen HM, de Groot JH, van der Helm FCT, Rozing PM. 3D shoulder position measurements using a six-degree-of-freedom electromagnetic tracking device. Clin Biomech 1998; 13: 280–292.
23. Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther 2000; 80: 276–291.
24. Endo K, Yukata K, Yasui N. Influence of age on scapulo-thoracic orientation. Clin Biomech (Bristol, Avon) 2004; 19: 1009–1013.
25. Price CI, Franklin P, Rodgers H, Curless RH, Johnson GR. Non-invasive evaluation of shoulder problems after stroke. Lancet 1999; 353: 298.
26. Cailliet R, editor. Shoulder pain. Third edition. Pain series. Philadelphia, PA: Davis; 1993, p. 277.
27. Esparza DY, Archambault PS, Winstein CJ, Levin MF. Hemispheric specialization in the co-ordination of arm and trunk movements during pointing in patients with unilateral brain damage. Exp Brain Res 2003; 148: 488–497.
28. Sugarman H, Avni A, Nathan R, Weisel-Eichler A, Tiran J. Movement in the ipsilesional hand is segmented following unilateral brain damage. Brain Cogn 2002; 48: 579–587.
29. Urban PP, Morgenstern M, Brause K, Wicht S, Vukurevic G, Kessler S, et al. Distribution and course of cortico-respiratory projections for voluntary activation in man. A transcranial magnetic stimulation study in healthy subjects and patients with cerebral ischemia. J Neurol 2002; 249: 735–744.
30. Niessen MHM, Veeger DH, Koppe PA, Konijnenbelt MH, van Dieen J, Janssen TW. Proprioception of the shoulder after stroke. Arch Phys Med Rehabil 2008; 89: 333–338.
31. Joynt RL. The source of shoulder pain in hemiplegia. Arch Phys Med Rehabil 1992; 73: 409–413.
32. Yu D. Shoulder pain in hemiplegia. Phys Med Rehabil Clin N Am 2004; 15: 683–697.
33. Vermeulen HM, Stokdijk M, Eilers PH, Meskers CG, Rozing PM, Vliet Vlieland TP. Measurement of three dimensional shoulder movement patterns with an electromagnetic tracking device in patients with a frozen shoulder. Ann Rheum Dis 2002; 61: 115–120.
34. Tentschert S, Wimmer R, Greisenegger S, Lang W, Lalouschek W. Headache at stroke onset in 2196 patients with ischemic stroke or transient ischemic attack. Stroke 2005; 36: e1-e3.
35. Edmeads J. Headache in cerebrovascular disease. A common symptom of stroke. Postgrad Med 1987; 81: 191–193, 196–198.
36. Vestergaard K, Andersen G, Nielsen MI, Jensen TS. Headache in stroke. Stroke 1993; 24: 1621–1624.