Controversy regarding the aetiology and treatment of patients with chronic fatigue syndrome continues among the medical professions. The Cochrane Collaboration advises practitioners to implement graded exercise therapy for patients with chronic fatigue syndrome using cognitive behavioural principles. Conversely, there is evidence that exercise can exacerbate symptoms in chronic fatigue syndrome, if too-vigorous exercise/activity promotes immune dysfunction, which in turn increases symptoms. When designing and implementing an exercise programme for chronic fatigue syndrome it is important to be aware of both of these seemingly opposing viewpoints in order to deliver a programme with no detrimental effects on the pathophysiology of the condition. Using evidence from both the biological and clinical sciences, this paper explains that graded exercise therapy for people with chronic fatigue syndrome can be undertaken safely with no detrimental effects on the immune system. Exercise programmes should be designed to cater for individual physical capabilities and should take into account the fluctuating nature of symptoms. In line with cognitive behaviourally and graded exercise-based strategies, self-management for people with chronic fatigue syndrome involves encouraging patients to pace their activities and respect their physical and mental limitations, with the ultimate aim of improving their everyday functioning.
Key words: chronic fatigue, syndrome, physical therapy, graded exercise therapy, evidence based, fibromyalgia.
J Rehabil Med 2008; 40: 241–247
Correspondence address: Jo Nijs, University College Antwerp, Van Aertselaerstraat 31, BE-2170 Merksem, Belgium. E-mail: j.nijs@ha.be
Submitted August 20, 2007; accepted February 1, 2008
Introduction
Chronic fatigue syndrome (CFS) describes a disorder that consists of chronic debilitating fatigue that cannot be explained by any known medical or psychological condition (1). The symptoms of CFS are numerous and include generalized headaches, sore throat, mild fever, myalgia and sleep disturbances (1). To date, controversy regarding the aetiology and treatment of patients with CFS continues to affect the medical and allied professions.
From our current understanding it is generally accepted that, as in many other conditions, CFS represents a combination of physiological and psychological impairments. Consequently, a comprehensive approach to patients with CFS must address both the biological and the psychosocial aspects. Practicing evidence-based medicine requires that a clinician integrates the best evidence from both the clinical and pure sciences in order to provide the best possible care for an individual patient (2). Nowadays, research data from both the biological and clinical sciences can be incorporated in the clinical reasoning process and treatment of patients with CFS.
The Cochrane Collaboration advises practitioners to implement graded exercise therapy for patients with CFS, using cognitive behavioural principles (3, 4). Cognitive behavioural therapy represents a psychological and physical intervention approach aimed at assisting individuals in re-evaluating concepts related to their illness and in adopting thoughts and behaviours designed to promote recovery (5). This approach to graded exercise therapy, however, advises patients to continue exercising at the same level even when they develop symptoms in response to the exercise (6, 7). Conversely, there is some evidence of immune dysfunction in CFS, and recent experimental research shows further deregulation of the immune system in response to too-vigorous exercise, leading to an increase in fatigue and musculoskeletal pain (post-exertional malaise) (8, 9). This seemingly contradictory evidence presents clinicians with a dilemma: on the one hand it is clear from the clinical sciences that we should advise people with CFS to undertake a graded exercise programme; however, we need to avoid damaging the patient’s immune system. The important questions are: (i) does graded exercise therapy in fact damage the immune system?; and (ii) is it possible to design an appropriate exercise programme for people with CFS that will avoid exacerbating their symptoms?
The aim of this paper is to provide an integrated model for exercise therapy in patients with CFS. The present report explains that it is possible to integrate evidence from the biological and clinical sciences in order to design a programme of graded exercise therapy for people with CFS that can be undertaken safely with no detrimental effects on the immune system. The first part of the report provides an overview of the interactions between psychology, biology and exercise physiology in patients with CFS, in order to create a firm theoretical basis for designing and implementing an exercise programme for patients with CFS. The second part of the report explains how graded exercise therapy may be applied in order to account for CFS biology, psychology and the clinical evidence for exercise interventions in CFS.
Interactions between biology, psychology and exercise physiology in patients with CFS
Patients with CFS often report a fluctuating pattern to their symptoms including their physical and cognitive capabilities. The fluctuating nature of the condition is reflected in the current diagnostic criteria for CFS (1). Clinical studies of patients with CFS have provided evidence for a high variability of mental and physical fatigue over a 4-week period (10). Furthermore, it has been shown that too-vigorous exercise (8, 9, 11) or even a 30% increase in activity (13) frequently triggers a relapse, which may consequently explain at least part of the fluctuating symptom pattern commonly seen in CFS. In line with this are the findings that the lifestyle of patients with CFS is characterized by activity peaks followed by very long rest periods (14), and that a pre-morbid overactive lifestyle may play a predisposing and/or initiating role in CFS (15). Even so, patients with CFS are generally able to perform light to moderate exercise (40% of peak oxygen capacity) without exacerbating their symptoms or cognitive performance (16, 17).
The severe exacerbation of symptoms following too-vigorous exercise, as seen in patients with CFS, is not present in other disorders where fatigue is a predominant symptom, such as depression, rheumatoid arthritis, systemic lupus erythematosus, or multiple sclerosis (9, 18). This post-exertional malaise is a primary characteristic evident in up to 95% of people with CFS (19). A recent study has shown that post-exertional malaise was one of the best predictors in the differential diagnosis of CFS and major depressive disorder (20).
So why do patients with CFS experience increases in symptoms following activity or exercise peaks? It may be that the exercise is prescribed at an intensity and/or duration that exceeds an individual’s current physical capabilities. This premise is supported by studies that reported relapse after vigorous exercise, as well as after a 30% activity increase. It is possible that exercise at any intensity that exceeds a CFS patient’s physical capabilities may result in the worsening of symptoms. However previous trials examining the effect of graded exercise therapy in CFS have reported positive outcomes (7, 21, 22). This may be due to the subject selection criteria applied in these trials, where subjects included in the studies reflect a group of subjects with CFS whose overall health and fitness is more robust than other individuals with CFS. This may result in the possibility that those patients with CFS participating in exercise trials are more able to cope with the exercise levels (intensity and duration) used in these trials. CFS is a heterogeneous disorder that can be so severe as to leave people bed-bound, whilst at the other end of the continuum those with mild CFS symptoms are able to function at close to normal, acceptable levels. Ideally, therapies employed for people with CFS should be suitable over the entire range of illness severity. Alternatively, it would be legitimate to have one approach in, for example, mild-moderate CFS, and another approach in moderate-severe CFS. It is the intention of the authors to explain how one can apply the clinical evidence in support of graded exercise therapy to patients with CFS and possibly to participants who are initially not coping with the exercise levels.
A literature review on psychiatric perspectives on CFS, published in 1998, concluded that it was unclear how exercise stimuli related to relapses in severe symptoms in patients with CFS (23). Since then, however, a number of studies have provided more insight into this issue. Exercise performance and exercise- or activity-induced symptom exacerbations in patients with CFS appear to be related to immune (dys)function in CFS. Resting immune status has been studied in depth in subjects with CFS, with evidence of immune activation (24, 25) and immune deregulation (26, 27) in patients with CFS being provided. Deregulation and activation of intracellular immune variables (i.e. activity of the elastase enzyme and cleavage of the RNase L enzyme) were identified as predictors of physiological exercise parameters in patients with CFS (28, 29). Moreover, it appears that an impaired immune system, which is typically observed in patients with CFS, is further downregulated by a (sub)maximal bout of exercise. Indeed, it has been shown that patients with CFS respond to an exercise challenge with enhanced complement activation (9) and an exaggeration of resting differences in gene expression profile in peripheral blood mononuclear cells (30). Vigorous exercise, as well as inappropriate intensities of submaximal exercise, can result in increased oxidative stress and subsequent increased fatigue and musculoskeletal pain (post-exertional malaise) in patients with CFS (8).
In addition, both isometric and aerobic exercise activate endogenous opioid and adrenergic pain inhibitory mechanisms in healthy subjects, while aerobic exercise increases experimental pain ratings in patients with CFS (31). In patients with fibromyalgia, a condition that overlaps with CFS, altered central pain processing is further augmented by isometric exercise (32), and an increase in muscular vascularity in response to both dynamic and static contractions is blunted (33). This can result in diminishing blood flow to the working muscles both during and following exercise (33). The altered central pain processing brings us to the central nervous system and the recent findings of reduction in global grey matter volume in patients with CFS compared with healthy controls (34). This reduction in grey matter volume was found to be associated with reduced physical activity in the CFS group but not in the healthy subjects (34).
In summary, too vigorous exercise/activity can potentially trigger immune dysfunction in patients with CFS, which in turn increases symptoms. This highlights the importance of designing exercise programmes that cater for individual’s physical capabilities and that also account for the fluctuating nature of symptoms commonly reported by people with CFS.
Apart from the biological aspects, psychological factors have been identified as perpetuating factors for CFS (35). Kinesiophobia and subsequent avoidance behaviour, catastrophic thoughts, hypervigilance, acceptance, a poor sense of control over symptoms, and social processes can all have a negative impact on rehabilitation in those with CFS. Social processes of potential relevance to CFS are a lack of social support and solicitous behaviour. Hypervigilance refers to a strong focus on bodily sensations and is likely to imply a strong focus on post-exertional symptoms. Not all patients with CFS accept the fact that they are seriously ill and need to change their lifestyle accordingly, suggesting that these patients are unlikely to comply with self-management and exercise programmes unless acceptance is thoroughly addressed prior to commencing these interventions. These and other psychological and social processes may influence exercise performance and activity levels in individuals with CFS. The psychology of CFS has been discussed at length in the scientific literature, yet few studies have addressed the interactions between psychology and exercise or activity performance.
Avoidance behaviour towards physical activity is likely to influence exercise performance and compliance with exercise interventions in any chronic illness. It has been shown that specific activities, which were expected to result in high fatigue levels, were less frequently performed by patients with CFS and, furthermore, high fatigue expectations were related to low activity levels (36). Kinesiophobia, a specific kind of fear-avoidance behaviour, is defined as “an excessive, irrational, and debilitating fear of physical movement and activity resulting from a feeling of vulnerability to painful injury or re-injury” (37). In patients with CFS, kinesiophobia represents a common feature that was found to be of clinical importance (i.e. related to disability), but did not appear to be a determinant of exercise performance (38–40). This observation is in line with a study showing stronger voluntary efforts (i.e. stronger brain signals recorded with electroencephalogram) during motor tasks in patients with CFS compared with healthy controls (41).
Unlike kinesiophobia, pain catastrophizing has recently been identified as a major contributor to both exercise behaviour and musculoskeletal pain severity in patients with CFS (42). Pain catastrophizing concerns interpretations of pain in terms of relevance and potential danger and is therefore classified as an attribution (43). Contrary to pain catastrophizing, anxiety or somatization were not related to the inability of patients with CFS to perform a graded exercise test (44). In addition, concurrent psychiatric illnesses have been reported to not adversely affect physical functional capacity (45). Finally, cognitive impairments (e.g. poor memory, poor concentration), which are typically seen in patients with CFS, are not exacerbated by (moderate to severe) exercise (17, 46).
To date, the authors are unaware of studies examining the interactions between the fluctuating symptom pattern of people with CFS and psychological issues such as catastrophic beliefs, depressive thoughts and mood. Since it is unlikely that immune changes are the sole reason for post-exertional symptoms in those with CFS, studies examining these interactions are warranted.
Applying science to practice
There is strong evidence to support specific exercise therapies as a cornerstone in the comprehensive management of CFS (7, 21, 22, 47, 48). Evidence from individual randomized clinical trials is underscored by the conclusions of systematic literature reviews by the Cochrane Collaboration (3, 4). So why is it that 50% of British patients with CFS reported that exercise therapy made them worse? (49).When designing and implementing an exercise programme for patients with CFS, it is essential to take into account our current understanding of the specific nature of CFS. This suggests that graded exercise therapy for patients with CFS should be performed with appropriate supervision by well-trained professionals who understand the potential harm it might cause (6). Indeed, there is currently no evidence that graded exercise therapy, on average, causes harm to patients with CFS (3). However, it remains important to prevent exercise-induced exacerbations in symptoms and immune status when applying exercise therapy to patients with CFS, in particular to guarantee treatment compliance.
Initial success of exercise therapy in CFS is most like due to the realization by sufferers that exercise can be undertaken safely without the consequence of relapse. This assists patients to abandon any avoidance behaviours to which they may have previously adhered (50). Therefore, one should design graded exercise programmes that cater for individual physical capabilities and that account for the fluctuating nature of symptoms commonly reported by people with CFS. In what follows, the reader is provided with guidelines to implement such a graded exercise therapy programme.
When implementing graded exercise therapy with people with CFS, the intensity and duration of activities attempted is crucial. While symptom exacerbation has not been associated with light to moderate exercise (16, 17), attempts by patients with CFS to perform exercise bouts at intensities that exceed their physical capabilities may trigger a further downregulation of the already impaired immune system with a concomitant exacerbation of related symptoms (8, 9, 30). In addition, it is commonly noted that on days that patients with CFS feel comparatively better, they often perform many more physical tasks than normal (51), most likely in an attempt to make up for the time that they have been incapacitated. This increase in activity may result in over-exertion followed by a relapse the next day (51), which reinforces the association between exercise and the exacerbation of symptoms. Such an inappropriate activity pattern is likely to prevent positive outcomes for exercise interventions. However, this negative scenario and association can be addressed by the employment of self-management techniques together with, or prior to, a graded exercise programme.
In line with cognitive behaviourally and graded exercise-based strategies, self-management for people with CFS involves encouraging patients to pace their activities and respect their physical and mental limitations (49, 52). This self-management strategy has been termed “pacing” and involves encouraging patients to achieve an appropriate balance between activity and rest in order to avoid exacerbating symptoms. Furthermore, this energy management strategy requires patients to set realistic activity/exercise goals on a daily basis (49, 53) and to regularly monitor and manipulate exercise/activity in terms of intensity, duration and rest periods in order to avoid possible over-exertion, which can result in worsening symptoms (49, 53). Pacing takes into account the considerable fluctuations in symptom severity (49) and delayed recovery from exercise (54) that typically occurs in patients with CFS. The pacing approach is consistent with recent observations regarding the interactions between malfunctioning of the immune system, physical activity, and symptoms in patients with CFS (outlined above). In addition, some patients with CFS are reluctant to undertake psychological treatments, such as cognitive behavioural therapy, for what they believe to be a physical condition. Pacing self-management techniques encourage a behavioural change and at the same time acknowledge the physical aspects of the illness.
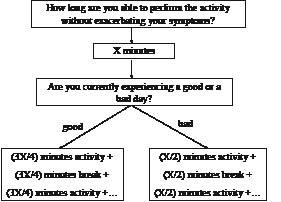
In order to pace activities (daily activities and exercise bouts) appropriately, patients with CFS need to learn to estimate their current physical capabilities prior to commencing an activity, keeping in mind the regular fluctuating nature of their symptoms. Daily activities are defined as those duties typically performed at work and around the home, including shopping, housework, gardening, etc. In the absence of kinesiophobia, the activity duration used within the programme is less than that reported by the patient, so as to account for typical overestimations made by the patient (Fig. 1). Each block of activity is interspersed with breaks, with the length of the break equating to the duration of the activity. This procedure is followed in order to account for the delayed recovery from exercise commonly demonstrated in patients with CFS. “Breaks” are defined as relative periods of rest, with the patient just relaxing or performing another type of light activity (for example, in a break between 2 sessions of ironing, the patient may perform a light mental activity such as reading). Employing the principle of pacing during a CFS patient’s daily life implicates a behavioural change. Thus, care must be taken to explain the rationale and potential benefits of the programme prior to commencement, while the patient’ expectations for care should be taken into account and subsequently utilized so to encourage adherence to the programme.
Fig. 1. Scheme for teaching a patient with chronic fatigue syndrome the pacing self-management principles (stabilization phase). X: number of minutes a patient feels to be able to perform the activity without exacerbating their symptoms. Example: a CFS patient believes she is capable of walking for 20 min without exacerbating her symptoms and is currently having a relatively good day. We advise her to walk for no longer than 15 min followed by a 15-min break. At that point the patient is instructed to reassess her health status: if her symptoms are still approximate to prior to commencing the walking exercise, then she is allowed to start a second 15-min bout of walking. On a bad day she is instructed to further decrease the walking duration to 10 min (i.e. 50% of 20 min).
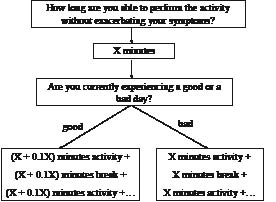
When a person with CFS is able to manage their daily activity (i.e. symptom fluctuation is reduced to a manageable level) (stabilization phase), the therapist can then start to progress activity and exercise levels (grading phase). Patients who are functioning within the limits of their individual physical capabilities do not require pacing self-management (stabilization phase) and can immediately enter the grading phase. Indeed, the heterogeneous CFS population can be divided into 3 subgroups: (i) inactive or passive patients, (ii) patients who have a fluctuating activity pattern or moderately active patients, and (iii) rather active or pervasively active patients (55). During this grading phase, the same pacing techniques are applied to grade both daily activities and exercise levels (Fig. 2). When determining an appropriate exercise level, a formal, regulated exercise regime that is gentle, graded, flexible and manageable according to each individual’s capabilities is required.
Fig. 2. Scheme for teaching a patient with chronic fatigue syndrome the pacing self-management principles (grading phase). X: number of minutes a patient feels to be able to perform the activity without exacerbating their symptoms.
Twelve weeks of paced and carefully monitored graded exercise therapy applied to 20 cases of CFS was found to result in decreased psychological stress (reduced phobic anxiety, somatization and paranoid ideation) with no evidence of any exacerbation in symptoms (56). The results of this uncontrolled study were extended in a randomized controlled clinical trial, which reported that paced and individually-tailored graded exercise was superior to relaxation and flexibility training in patients with CFS (21).
Success of the graded exercise therapy described by Wallman et al. (57) in CFS most likely related to the ability of patients with CFS to reduce or even cease their exercise depending on symptom severity (pacing), while exercise levels (intensity and duration) were only increased when the individual was deemed as having coped with the current exercise regime. Coping was determined by the individual’s averaged sense of effort scores determined on the Borg scale associated with exercise sessions performed over a 2-week period (21). Of importance, there were no drop-outs from the exercise group in this study once the programme commenced.
In order to reduce the possibility of exacerbating symptoms, it is very important that on days that a CFS patient feels comparatively well that they still adhere to their current exercise regime and do not perform any extra exercise above this level. This rule also applies to normal everyday physical tasks such as housework and shopping. As noted earlier, overdoing physical activity on days that patients with CFS feel comparatively better often results in a relapse the following day. Additionally, on a day when symptoms are worse, the patient should either reduce their exercise duration to a time that they consider manageable or, if feeling particularly unwell, abandon the exercise session altogether.
For more details regarding how to apply appropriate exercise therapy to individual cases of CFS, the reader is referred to other manuscripts reporting the graded exercise interventions in detail (6, 21, 57).
Conclusion
There is currently strong evidence to support the use of graded exercise therapy for people with CFS. Early approaches to graded exercise therapy advised patients to continue exercising at the same level when they developed symptoms in response to the exercise (6, 7). This led to exacerbation of symptoms and adverse feedback from patients and patient charities. However, graded exercise therapy for people with CFS has developed and has been influenced by studies addressing the biology and psychology of the illness. It is explained that rehabilitation specialists can apply evidence from both the biological and clinical sciences when treating patients with CFS. Graded exercise programmes for people with CFS can be safely undertaken without detrimental effects to the immune system and therefore the individual. To achieve these goals, it is important to use exercise at an intensity and duration that does not exceed an individual’s current physical capabilities.
Patients with CFS who have a fluctuating activity pattern and are moderately active, as well as those who are rather active or pervasively active (55), may benefit from a self-management programme that encompasses graded exercise therapy. This self-management programme should focus on teaching the patient to estimate their current physical capabilities prior to commencing an activity, keeping in mind the regular fluctuating nature of their symptoms. For very inactive or passive patients, a formal, regulated exercise regime that is gentle, graded, flexible and manageable according to the individual’s capabilities is required. The aim of exercise therapy is ultimately to improve everyday functioning of the individual.
Although many issues raised here are supported by evidence from clinical and biological sciences, further work is required. Firstly, studies examining whether the intensity and duration of activities attempted are aetiologically related to the exacerbation of symptoms following physical exertion, as proposed here, are warranted. Secondly, it would be interesting to determine whether the pacing self-management programme on its own rather than combined with graded exercise therapy has positive effects on the health status of moderately active or pervasively active patients with CFS. This issue will be addressed in the ongoing large PACE trial (Pacing, graded Activity on Cognitive behaviour therapy: a randomised Evaluation) in the UK (58). Thirdly, more work is required to unravel the interactions between the fluctuating symptom pattern of people with CFS and psychological issues such as catastrophic beliefs, depressive thoughts and mood.
References
1. Fukuda K, Strauss SE, Hickie I, Sharpe MC, Dobbins JG, Anthony Komaroff International Chronic Fatigue Syndrome Study Group. The Chronic Fatigue Syndrome, a comprehensive approach to its definition and study. Ann Intern Med 1994; 121: 953–959.
2. Sackett DL, Rosenberg WMC, Gray MJA, Haynes RB, Richardson WS. Evidence based medicine: what is it and what isn’t it. BMJ 1996; 312: 71–72.
3. Edmonds M, McGuire H, Price J. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev 2004; 3: CD003200.pub2. DOI: 10.1002/14651858.CD003200.pub2.
4. Price JR, Couper J. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst Rev 1998; 4:CD001027.
5. Chalder A, Deale A, Wessely S, Marks I. Cognitive behaviour therapy for chronic fatigue syndrome. Am J Med 1995; 98: 419–420.
6. Clark LV, White PD. The role of deconditioning and therapeutic exercise in chronic fatigue syndrome (CFS). J Mental Health 2005; 14: 237–252.
7. Fulcher KY, White PD. Randomised controlled trial of graded exercise in patients with the chronic fatigue syndrome. BMJ 1997; 314: 1647–1652.
8. Jammes Y, Steinberg JG, Mambrini O, Brégeon F, Delliaux S. Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J Intern Med 2005; 257: 299–310.
9. Sorensen B, Streib JE, Strand M, Make B, Giclas PC, Fleshner M, Jones JF. Complement activation in a model of chronic fatigue syndrome. J Allergy Clin Immunol 2003; 12: 397–403.
10. Wallman KE, Morton AR, Goodman C, Grove R. Reliability of physiological, psychological, and cognitive variables in chronic fatigue syndrome. Res Sports Med 2005; 13: 231–241.
11. Lapp CW. Exercise limits in chronic fatigue syndrome. Am J Med 1997; 103: 83–84.
12. Bazelmans E, Blijenberg G, Voeten MJM, van der Meer JWM, Folgering H. Impact of a maximal exercise test on symptoms and activity in chronic fatigue syndrome. J Psychosom Res 2005; 59: 201–208.
13. Black CD, O’Connor PJ, McCully KK. Increased daily physical activity and fatigue symptoms in chronic fatigue syndrome. Dynamic Med 2005; 4: 3.
14. van der Werf SP, Prins JB, Vercoulen JHMM, van der Meer JWM, Bleijenberg G. Identifying physical activity patterns in chronic fatigue syndrome using actigraph assessment. J Psychosom Res 2000; 49: 373–379.
15. Van Houdenhove B, Neerinckx E, Onghena P, Lysens R, Vertommen H. Premorbid “overactive” lifestyle in chronic fatigue syndrome and fibromyalgia. An etiological factor or proof of good citizenship? J Psychosom Res 2001; 51: 571–576.
16. Clapp LL, Richardson MT, Smith JF, Wang M, Clapp AJ, Pieroni RE. Acute effects of thirty minutes of light-intensity, intermittent exercise on patients with chronic fatigue syndrome. Phys Ther 1999; 79: 749–756.
17. Cook DB, Nagelkirk PR, Peckerman A, Poluri A, Mores J, Natelson BH. Exercise and cognitive performance in chronic fatigue syndrome. Med Sci Sports Exerc 2005; 37: 1460–1467.
18. Ohashi K, Yamamoto Y, Natelson BH. Activity rhythm degrades after strenuous exercise in chronic fatigue syndrome. Physiol Behav 2002; 77: 39–44.
19. Knoop H, Bleijenberg G, Gielissen MFM, van der Meer JWM, White PD. Is a full recovery possible after cognitive behavioural therapy in chronic fatigue syndrome? Psychother Psychosom 2007; 76: 171–176.
20. Hawk C, Jason LA, Torres-Harding S. Differential diagnosis of chronic fatigue syndrome and major depressive disorder. Int J Behav Med 2006; 13: 244–251.
21. Wallman KE, Morton AR, Goodman C, Grove R, Guilfoyle AM. Randomised controlled trial of graded exercise in chronic fatigue syndrome. Med J Austral 2004; 180: 444–448.
22. Powell P, Bentall RP, Nye FJ, Edwards RHT. Randomised controlled trial of patient education to encourage graded exercise in chronic fatigue syndrome. BMJ 2001; 322: 387–390
23. Jain SS, DeLisa JA. Chronic fatigue syndrome. A literature review from a psychiatric perspective. Am J Phys Med Rehabil 1998; 77: 160–167.
24. Klimas N, Salvato F, Morgan R, Fletcher MA. Immunological abnormalities in chronic fatigue syndrome. J Clin Microbiol 1990; 28: 403–1410.
25. Nijs J, De Becker P, De Meirleir K, Demanet C, Vincken W, Schuermans D, McGregor N. Associations between immune cell parameters and bronchial hyperresponsiveness in patients with chronic fatigue syndrome. Chest 2003; 123: 998–1007.
26. Whiteside TL, Frideberg D. Natural killer cells and natural killer cell activity in chronic fatigue syndrome. Am J Med 1998; 105: 27S–34S.
27. Nijs J, De Meirleir K. Impairments of the 2-5A synthetase/RNase L pathway in chronic fatigue syndrome (review). In Vivo 2005; 19: 1013–1022.
28. Snell CR, Vanness JM, Strayer DR, Stevens SR. Physical performance and prediction of 2-5A Synthetase/RNase L antiviral pathway activity in patients with chronic fatigue syndrome. In Vivo 2002; 16: 107–110.
29. Nijs J, Meeus M, McGregor NR, Meeusen R, De Schutter G, Van Hoof E, De Meirleir K. Chronic fatigue syndrome: exercise performance related to immune dysfunction. Med Sci Sports Exerc 2005; 37: 1647–1654.
30. Whistler T, Jones JF, Unger ER, Vernon SD. Exercise responsive genes measured in peripheral blood of women with chronic fatigue syndrome and matched control subjects. BMC Physiol 2005; 5: 5.
31. Whiteside A, Hansen S, Chaudhuri A. Exercise lowers pain threshold in chronic fatigue syndrome. Pain 2004; 109: 497–499.
32. Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain 2005; 118: 176–184.
33. Elvin A, Siösteen A-K, Nilsson A, Kosek E. Decreased muscle blood flow in fibromyalgia patients during standardised muscle exercise: a contrast media enhanced colour doppler study. Eur J Pain 2006; 10: 137–144.
34. de Lange FP, Kalkman JS, Bleijenberg G, Hagoort P, van der Meer JWM, Toni I. Gray matter volume reduction in the chronic fatigue syndrome. Neuroimage 2005; 26: 777–781.
35. Prins JB, van der Meer JWM, Bleijenberg G. Chronic fatigue syndrome. Lancet 2006; 367: 346–355.
36. Vercoulen JHMM, Bazelmans E, Swanink CMA, Fennis JFM, Galama JMD, Jongen PJH, et al. Physical activity in chronic fatigue syndrome: assessment and its role in fatigue. J Psychiat Res 1997; 31: 661–673.
37. Kori SH, Miller RP, Todd DD. Kinesiophobia: a new view of chronic pain behavior. Pain Management 1990; Jan/Feb: 35–43.
38. Silver A, Haeney M, Vijayadurai P, Wilks D, Pattrick M, Main CJ. The role of fear of physical movement and activity in chronic fatigue syndrome. J Psychosom Res 2002; 52: 485–493.
39. Nijs J, Vanherberghen K, Duquet W, De Meirleir K. Chronic fatigue syndrome: lack of association between pain-related fear of movement and exercise capacity and disability. Phys Ther 2004; 84: 696–705.
40. Nijs J, De Meirleir K, Duquet W. Kinesiophobia in chronic fatigue syndrome: assessment and associations with disability. Arch Phys Med Rehabil 2004; 85: 1586–1592.
41. Siemionow V, Fang Y, Calabrese L, Sahgal V, Yue GH. Antered central nervous system signal during motor performance in chronic fatigue syndrome. Clin Neurophysiol 2004; 115: 2372–2381.
42. Nijs J, Van de Putte K, Louckx F, Truijen S, De Meirleir K. Exercise performance and chronic pain in chronic fatigue syndrome: The role of pain catastrophizing. Pain Med 2008, in press.
43. Spinhoven P, ter Kuile M, Kole-Snijders AMJ, Hutten Mansfeld M, den Ouden D-J, Vlaeyen JWS. Catastrophizing and internal pain control as mediators of outcome in the multidisciplinary treatment of low back pain. Eur J Pain 2004; 8: 211–219.
44. Fischler B, Dendale P, Michiels V, Cluydts R, Kaufman L, De Meirleir K. Physical fatigability and exercise capacity in chronic fatigue syndrome: association with disability, somatization and psychopathology. J Psychosom Res 1997; 42: 369–378.
45. Tiersky LA, Matheis RJ, Deluca J, Lange G, Natelson BH. Functional status, neuropsychological functioning, and mood in chronic fatigue syndrome (CFS). Relationship to psychiatric disorder. J Nerv Ment Dis 2003; 191: 324–331.
46. Claypoole K, Mahurin R, Fischer ME, Goldberg J, Schamling KB, Schoene RB, et al. Cognitive compromise following exercise in monozygotic twins discordant for chronic fatigue syndrome: fact or artifact? Appl Neuropsychol 2001; 8: 31–40.
47. Prins JB, Bleijenberg G, Bazelmans E, Elving LD, de Boo TM, Severens JL, et al. Cognitive behaviour therapy for chronic fatigue syndrome: a multicenter randomised controlled trial. Lancet 2001; 357: 841–847.
48. Deale A, Chalder T, Marks I, Wessely S. Cognitive behavior therapy for chronic fatigue syndrome: a randomized controlled trial. Am J Psychiat 1997; 154: 408–414.
49. Shephard C. Pacing and exercise in chronic fatigue syndrome. Physiotherapy 2001; 87: 395–396.
50. Deale A, Chalder T, Wessely S. Illness beliefs and treatment outcome in chronic fatigue syndrome. J Psychosom Res 1998; 45: 77–83.
51. Deale A, Davis A. Chronic fatigue syndrome: evaluation and management. J Neuropsychiatry. 1994; 6: 189–194.
52. Pardaens K, Haagdorens L, Van Wambeke P, Van den Broeck A, Van Houdenhove B. How relevant are exercise capacity measures for evaluating treatment effects in chronic fatigue syndrome? Results from a prospective, multidisciplinary outcome study. Clin Rehabil 2006; 20: 56–66.
53. CFS/ME Working Group. Report to the Chief Medical Officer of an independent working group. London: Department of Health; 2001. www.doh.gov.uk/cmo/cfsmereport/index.htm
54. Paul L, Wood L, Behan WMH, Maclaren WM. Demonstration of delayed recovery from fatiguing exercise in chronic fatigue syndrome. Eur J Neurol 1999; 6: 63–69.
55. Van der Werf SP, Prins JB, Vercoulen JHMM, van der Meer JWM, Bleijenberg G. Identifying physical activity patterns in chronic fatigue syndrome using actigraphic assessment. J Psychosom Res 2000; 49: 373–379.
56. Coutts R, Wetherby R, Davie A. The use of a symptom “self-report” inventory to evaluate the acceptability and efficacy of a walking program for patients suffering with chronic fatigue syndrome. J Psychosom Res 2001; 51: 425–429.
57. Wallman KE, Morton AR, Goodman C, Grove R. Exercise prescription for individuals with chronic fatigue syndrome. Med J Austr 2005; 183: 142–143.
58. White PD, Sharpe MC, Chalder T, DeCesare JC, Walwyn R and the PACE trial group. Protocol for the PACE trial: A randomised controlled trial of adaptive pacing, cognitive behaviour therapy, and graded exercise as supplements to standardised specialist medical care versus standardised specialist medical care alone for patients with the chronic fatigue syndrome/myalgic encephalomyelitis or encephalopthy. BMC Neurol 2007; 7: 6.