OBJECTIVE: To investigate the time-courses of lung function and respiratory muscle pressure generating capacity after spinal cord injury.
DESIGN: Multi-centre, prospective cohort study.
SUBJECTS: One hundred and nine subjects with recent, motor complete spinal cord injury.
METHODS: Lung function and respiratory muscle pressure generating capacity were measured at first mobilization, at discharge from inpatient rehabilitation and one year after discharge. Lung function was measured in all 109 subjects, and 55 of these performed additional measurements of respiratory muscle pressure generating capacity. Trajectories of respiratory muscle function for different lesion level groups were assessed by multi-variate multi-level regression models.
RESULTS: Forced vital capacity, forced expiratory volume in 1 sec and maximal inspiratory muscle pressure generating capacity significantly increased during and after inpatient rehabilitation. Forced inspiratory volume in 1 sec, peak inspiratory flow, peak expiratory flow and maximal expiratory muscle pressure generating capacity increased only during inpatient rehabilitation, but not thereafter. Increasing lesion level had a negative effect on all measured lung function parameters, as well as on maximal inspiratory and expiratory muscle pressure generating capacity.
CONCLUSION: Respiratory function improved during inpatient rehabilitation, but only forced vital capacity, forced expiratory volume in 1 sec and maximal inspiratory muscle pressure generating capacity further improved thereafter. In particular, expiratory muscle function and subjects with tetraplegia should be screened and trained regularly.
Key words: respiration, spinal cord injuries, longitudinal survey, rehabilitation.
J Rehabil Med 2008; 40: 269–276
Correspondence address: Gabi Mueller, Swiss Paraplegic Research, CH-6207 Nottwil, Switzerland. E-mail: gabi. mueller@paranet.ch
Submitted April 27, 2007; accepted November 8, 2007
Introduction
Pulmonary complications occur in 50–67% of persons with a spinal cord injury (SCI) (1), with pneumonia being the most common cause of death in individuals with tetraplegia (2). A recent prospective mortality study showed that the respiratory system was responsible for the cause of death in 28% of cases during the first year after injury and in 22% thereafter (3). In cross-sectional studies it has been shown that with increasing lesion level lung function decreases (4) and respiratory tract infections increase (5).
In individuals with SCI, the pulmonary system is often affected due to lesion-dependent losses of respiratory muscle innervations (6). While persons with paraplegia lack proper innervation of the abdominal muscles and, depending on the lesion level, (parts of) the intercostal muscles, persons with tetraplegia lack most of the expiratory and even some of the auxiliary inspiratory muscles (7). The loss of respiratory muscle innervations mainly decreases cough capacity and therefore secretion clearance is reduced, especially in subjects with high-level tetraplegia (8). Therefore, respiratory muscle pressure generating capacity may correlate directly with respiratory tract infections; however, studies to prove this are lacking.
Although by the early 1970s Fugl-Meyer (9) had already assessed respiratory function in subjects with SCI, not much is known about longitudinal changes in respiratory function in SCI. Sinderby et al. (10) compared diaphragmatic function in subjects with tetraplegia early (1–3 years) and 10 or more years post-injury. They found no significant changes in vital capacity and trans-diaphragmatic pressure from early to more than 10 years post-injury. Some small studies that evaluated time-courses of lung function early after SCI found strong improvements during the first 6 months after injury, with smaller improvements thereafter (11–14). It is possible that the impact of spinal shock, which usually disappears within the first 4–6 months after injury, stabilizes pulmonary function and may therefore be responsible for this finding (15). All longitudinal studies show data that either exclusively includes subjects with tetraplegia (11, 13, 14, 16) or that assesses only a small number of subjects (12, 14, 16).
As described above, lung function and respiratory muscle pressure generating capacity seem to change over time and depend on the level of the lesion (9, 10). Nevertheless, the influence of lesion level and time on lung function and respiratory muscle pressure generating capacity is not yet known in a longitudinal perspective.
A better understanding of lesion-dependence and trajectories of lung function and respiratory muscle impairments in persons with recent SCI would allow better adjustment of therapeutic interventions to prevent complications and, it is hoped, further decrease mortality rate due to respiratory tract infections.
Therefore, the purpose of this study was to describe trajectories of lung function and respiratory muscle pressure generating capacity in a large group of subjects with recent SCI during and 1 year after inpatient rehabilitation, with respect to lesion level and personal factors. Gender, age, height, body weight and smoking were evaluated as personal factors, as they may influence respiratory function in addition to lesion characteristics and time.
Methods
This study was part of the Dutch research program “Physical strain, work capacity and mechanisms of restoration of mobility in the rehabilitation of persons with SCI” (17).
Subjects
Persons with recent SCI from 8 SCI rehabilitation centres in the Netherlands participated on a voluntary basis in this study between August 2000 and July 2003. Subjects were measured at the start of active rehabilitation (t1), at the end of inpatient rehabilitation (t2) and one year after discharge (t3).
Inclusion criteria for the current study were: an acute, motor complete SCI (according to American Spinal Injury Association A or B) and aged between 18 and 65 years. Potential participants were excluded if they had 1 or more of the following diseases: instable chronic obstructive pulmonary disease (COPD), severe atelectasis, lung emphysema with oxygen dependency or a history of pneumothorax. Subjects were also excluded if they had a progressive disease, psychiatric disorder or insufficient knowledge of the Dutch language to understand the purpose of the study and the testing methods. A final total of 109 subjects were included in the present study and performed lung function measurement. Of these, 55 subjects completed additional measurements of respiratory muscle pressure generating capacity. In order to investigate the time-courses of lung function and respiratory muscle pressure generating capacity, only data for those subjects who could perform the tests more than once were included in the analyses (i.e. 109 subjects for lung function and 55 subjects for respiratory muscle pressure generating capacity).
Participants’ characteristics of the lung function group and the respiratory muscle pressure generating capacity subgroup are presented in Table I for the different lesion groups separately. There were no significant differences in personal characteristics between those subjects who performed only lung function measurements (n = 54) and those who performed respiratory muscle pressure generating capacity and lung function measurements (n = 55) (all p-values between 0.919 and 0.166).
| Table I. Participants’ characteristics of the whole group (n = 109); (lung function testing) and of the sub-group (n = 55) (testing of respiratory muscle pressure generating capacity) |
| | Gender (men/women) n | Age (years) (mean (SD)) | Height (m) (mean (SD)) | Weight (kg) (mean (SD)) | BMI (kg/m2) (mean (SD)) | Smoker before injury (%) | Current smoker (%) |
| Lung function measurements |
| HT | 14/6 | 36 (14) | 1.75 (0.08) | 71 (16) | 23.3 (5.2) | 30 | 5 |
| LT | 15/4 | 33 (10) | 1.79 (0.11) | 69 (11) | 21.6 (3.3) | 79 | 26 |
| HP | 23/7 | 43 (16) | 1.79 (0.09) | 77 (13) | 24.0 (3.1) | 33 | 17 |
| LP | 29/11 | 38 (13) | 1.78 (0.09) | 70 (12) | 21.9 (3.5) | 40 | 30 |
| All subjects with SCI | 81/28 | 38 (14) | 1.78 (0.09) | 72 (13) | 22.6 (3.8) | 47 | 23 |
| Measurements of respiratory muscle pressure generating capacity |
| Subjects with tetraplegia | 20/6 | 33 (11) | 1.79 (0.10) | 70 (15) | 22.0 (4.4) | 54 | 15 |
| Subjects with paraplegia | 21/8 | 39 (14) | 1.77 (0.09) | 72 (12) | 22.8 (3.3) | 48 | 38 |
| All subjects with SCI | 41/14 | 36 (13) | 1.78 (0.09) | 71 (13) | 22.4 (3.8) | 51 | 27 |
| HT: subjects with high-level tetraplegia (C3–C5); LT: subjects with low-level tetraplegia (C6–C8); HP: subjects with high-level paraplegia (T1–T6); LP: subjects with low-level paraplegia (T7–T12); BMI: body mass index; SCI: spinal cord injury; SD: standard deviation. |
Protocol
All subjects gave their written informed consent after being informed about the testing procedure. The study was approved by the medical ethics committee. On the test day, subjects were asked to consume a light meal only, to refrain from smoking, drinking coffee and alcohol at least 2 hours prior to testing, and to empty their bladder directly before testing.
Lung function measurements
Lung function measurements were made using a cardio-pulmonary and respiratory testing device (Oxycon Delta, Jaeger, Hoechberg, Germany), which was calibrated before each test. Lung function measurements were made according to a standardized protocol (18). The following parameters were measured: forced vital capacity (FVC), forced expiratory volume in 1 sec (FEV1), forced inspiratory volume in 1 sec (FIV1), peak expiratory flow (PEF) and peak inspiratory flow (PIF). Subjects had to breathe through a mouthpiece while wearing a nose clip. Each measurement was performed until 3 reproducible measurements within at least ± 5% were registered. The highest measured value of each parameter was used for further analysis. In order to compare values of the present study and to gain more insight on the respiratory impairment of subjects with paraplegia and tetraplegia, we calculated gender-, height- and age-corrected 100% predicted values for able-bodied subjects (ABS) using the regression equations of Quanier et al. (19).
Measurement of respiratory muscle pressure generating capacity
Maximal inspiratory and expiratory muscle pressure generating capacity (Pimax, Pemax) measured at the mouth, were performed with a calibrated, respiratory threshold meter (Instrumental Department, Radboud University Nijmegen, The Netherlands) connected to a personal computer. Pimax and Pemax were measured from residual volume and total lung capacity, respectively. Subjects had to breathe through a mouthpiece with a clip on their nose. To prevent measurement of muscle force of the cheeks, subjects had to sit with their elbows on a table and their hands on the cheeks. A small air leak in the mouthpiece prevented from glottis closure. The highest pressure that could be maintained for 1 sec was determined by the computer program. Each measurement was performed until 3 reproducible measurements within at least ± 5% were registered. A rest period of at least 1 min between each effort was kept. The best values for Pimax and Pemax were used for analysis. Measurement of maximal inspiratory and expiratory pressures (Pimax and Pemax) at the mouth are widely used and accepted as measures of respiratory muscle pressure generating capacity (20). However, in subjects with SCI changes in abdominal compliance e.g. due to spasticity, may influence respiratory muscle pressure generating capacity (21).
After a resting period of 10 min, a further test was performed in order to determine inspiratory threshold muscle endurance time and pressure. This test was conducted with an inspiratory threshold meter (Threshold IMT, Respironics, Herrsching, Germany). All subjects started at an inspiratory pressure of 0.7 kPa. This pressure had to be kept up for 1 min with a paced inspiration time of 3 sec and an expiration time of 4 sec. When subjects were able to complete 1 min, inspiratory pressure was immediately increased by 15% of the individual Pimax and subjects completed the second minute. This procedure was continued until subjects were no longer able to sustain the actual pressure, i.e. when target pressure could no longer be reached throughout the inspiration. If the maximal pressure of the device (4.1 kPa) was achieved, they had to sustain this pressure as long as possible with a maximum of 3 min. The time of the whole test (tendu) and pressure at test break-off (Pendu) were used for further analysis.
Statistical analysis
Descriptive statistics (means and standard deviations (SD)) for group characteristics were calculated for each parameter. Subject characteristics of the subjects who performed only lung function measurements (n = 54) and the respiratory muscle pressure generating capacity sub-group (n = 55) were compared using unpaired t-tests. Significance was set at p < 0.05. t-tests were performed with SPSS (Version 13.0; SPSS Inc, Chicago, IL, USA).
For analysis of the longitudinal data, a multi-level modelling program (Centre for Multilevel Modelling, Institute of Education, London, UK) (22, 23) was used. Multi-level regression analysis is suitable for longitudinal datasets since: (i) it allows dependency of repeated measures within the same person; (ii) it accounts for the hierarchy of the longitudinal data used in our study where the repeated measurements (level 1) are nested within the subjects (level 2) that are nested within rehabilitation centres (level 3); and (iii) the number of observations per person may vary (22). Outcome variables were FVC, FEV1, FIV1, PEF, PIF, Pimax, Pemax, Pendu and tendu. Multi-level analysis was used to assess: (i) the course of lung function and respiratory muscle pressure generating capacity outcome measures during and 1-year after inpatient rehabilitation. Time was modelled using 2 dichotomous dummy variables with t2 as reference, in order to calculate the change during (∆t1t2) and after (∆t2t3) inpatient rehabilitation; (ii) to assess differences in trajectories for lung function data among subjects with different lesion levels. Therefore, 4 groups were defined: HT = subjects with high-level tetraplegia (C3–C5), LT = subjects with low-level tetraplegia (C6–C8), HP = subjects with high-level paraplegia (T1–T6) and LP = subjects with low-level paraplegia (T7–T12). To calculate differences among groups, 3 dummies were used and LT was determined as reference group. To assess trajectories of respiratory muscle pressure generating capacity, only 2 groups (paraplegic and tetraplegic) were built in order to not lose statistical power, since only 55 subjects performed these measurements.
In a next step interactions between time- and group-dummies were added to the above described basic model. Interaction terms were only added to the final model if at least one of them was significant (p < 0.05).
To investigate a possible influence of personal factors as known from ABS reference equations (19, 24), these factors were added one by one to the model: gender (male = 1, female = 0), age (years), height (m), weight (kg), former smoker, i.e. before injury (0 = non-smoker, 1 = former smoker) and current smoker (0 = non-smoker, 1 = current smoker) and evaluated whether they showed a significant (p < 0.10) influence on the outcome variables. After adding all significant (p < 0.10) personal factors to the basic model of time and group-dummies, a backward elimination technique was used until only determinants remained with a p-value < 0.05. Based on the final model, estimates for lung function and respiratory muscle pressure generating capacity were calculated for the 4 lesion groups (lung function) and for subjects with paraplegia and tetraplegia (respiratory muscle pressure generating capacity), respectively.
Results
There was no heterogeneity across the 8 centres in any of the tested respiratory function parameters, i.e. that one centre systematically found higher or lower values for one parameter. Specific estimates for trajectories of these parameters were calculated for men only, and with subject’s mean age (38 years) and height (1.78 m) (Figs 1 and 2). Presented models can be used to calculate estimated values for subject X at a certain time-point after injury. For example, calculation of predicted FVC for a male subject with high-level tetraplegia, 38 years old and 1.78 metres tall, 1 year after inpatient rehabilitation would be as follows:
FVC = β of constant + β of Δt2–t3 + β of gender+ (age [years] × β of age) + (height [metres] × β of age)
Presented in numbers this is:
FVC = –3.62 + 0.179 – 1.070 + 0.692 + (38 × –0.014) + (1.78 × 4.12) = 2.98 litres.
Longitudinal changes
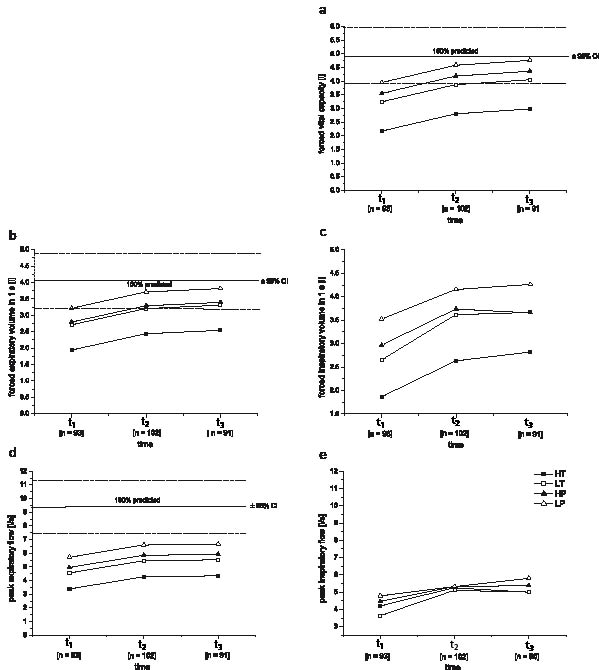
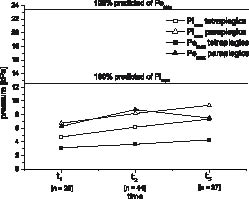
FVC and FEV1 increased in all 4 groups until 1 year after discharge from inpatient rehabilitation (Figs 1a and b). At discharge from inpatient rehabilitation FVC and FEV1 were no more significantly lower than ABS reference values, i.e. within ABS 95% confidence intervals, for all groups except HT (Figs. 1a, 1b). In contrast, FIV1, PEF and PIF generally seemed to remain constant during the first year after discharge from inpatient rehabilitation (Table II, Figs. 1c–1e). None of the 4 groups reached 100% ABS predicted values for PEF throughout the analysed time-course. For FIV1 and PIF significant differences in time-courses between groups were found. During inpatient rehabilitation, FIV1 increased significantly less in subjects with paraplegia (HP and LP) compared with subjects with tetraplegia (HT and LT) and PIF of the LP group increased significantly less than the other 3 groups (Table II). Calculated estimates of Pemax and Pimax for subjects with para- and tetraplegia all remain below 100% of ABS age- and gender-corrected reference values until 1 year after discharge from inpatient rehabilitation (Fig. 2). Pimax showed significant increases during and after inpatient rehabilitation, while Pemax showed significant increases only in subjects with paraplegia during inpatient rehabilitation (Fig. 2). Pendu significantly increased during, but not after inpatient rehabilitation. Estimates for tendu did not change over time (Table III).
| Table II. Regression coefficients (βi values) and standard errors for the final multi-level regression model describing effects of time, lesion and personal characteristics on the different lung function parameters (n = 109) |
| | FVC (l) | FEV1(l) | FIV1 (l) | PEF (l/s) | PIF (l/s) |
| β | (SE) | β | (SE) | β | (SE) | β | (SE) | β | (SE) |
| Constant | –3.620 | (1.549) | –2.485 | (1.338) | –3.173 | (1.502) | –2.612 | (2.644) | –0.756 | (2.554) |
| Δt2–t1 | –0.632 | (0.080)* | –0.498 | (0.062)* | –0.971 | (0.162)* | –0.891 | (0.127)* | –1.472 | (0.290)* |
| Δt2–t3 | 0.179 | (0.057)* | 0.103 | (0.046)* | 0.046 | (0.119) | 0.073 | (0.118) | –0.096 | (0.278) |
| ΔHT–LT | –1.070 | (0.235)* | –0.768 | (0.206)* | –0.785 | (0.245)* | –1.173 | (0.376)* | –0.898 | (0.438)* |
| ΔLT–HP | 0.311 | (0.226) | 0.085 | (0.187) | 0.119 | (0.241) | 0.399 | (0.418) | 0.155 | (0.413) |
| ΔLT–LP | 0.708 | (0.206)* | 0.505 | (0.171)* | 0.536 | (0.221)* | 1.152 | (0.395)* | 0.217 | (0.378) |
| Gender | 0.692 | (0.192)* | 0.513 | (0.167)* | 0.472 | (0.183)* | 0.844 | (0.303)* | 0.920 | (0.317)* |
| Age | –0.014 | (0.005)* | –0.021 | (0.005)* | –0.012 | (0.005)* | n.s. | –0.019 | (0.009)* |
| Height | 4.120 | (0.908)* | 3.355 | (0.784)* | 3.694 | (0.880)* | 4.054 | (1.531)* | 3.178 | (1.495)* |
| Δt1–t2×HTLT | n.s. | n.s. | 0.200 | (0.219) | n.s. | 0.420 | (0.396) |
| Δt1–t2×HPLT | n.s. | n.s. | 0.399 | (0.204)* | n.s. | 0.614 | (0.369) |
| Δt1–t2×LPLT | n.s. | n.s. | 0.538 | (0.194)* | n.s. | 0.940 | (0.349)* |
| Δt2–t3×HTLT | n.s. | n.s. | 0.139 | (0.173) | n.s. | –0.162 | (0.403) |
| Δt2–t3×HPLT | n.s. | n.s. | 0.074 | (0.157) | n.s. | 0.158 | (0.367) |
| Δt2–t3×LPLT | n.s. | n.s. | 0.263 | (0.143) | n.s. | 0.574 | (0.335) |
| *Significant influencing factor; n.s.: not significant. FVC: forced vital capacity; FEV1: forced expiratory volume in 1 second; FIV1: forced inspiratory volume in 1 second; PEF: peak expiratory flow; PIF: peak inspiratory flow; β: regression coefficient for each independent variable; SE: standard error of this regression coefficient; Δt1–t2 / Δt2–t3: time dummies with t2 as reference; HT: high-level tetraplegic; LT: low-level tetraplegic; HP: high-level paraplegic; LP: low-level paraplegic; ΔHT–LT / ΔLT–HP / ΔLT–LP: group dummies with LT as reference; gender: 0 = women; 1 = men; age = years; height = metres; Δt1–t2×HTLT / Δt1–t2×HPLT / Δt1–t2×LPLT / Δt2–t3×HTLT / Δt2–t3×HPLT / Δt2–t3×LPLT: interaction terms time × group. |
| Table III. Regression coefficients (βi values) and standard errors for the final multi-level regression models describing effects of time-courses and personal characteristics on different parameters of respiratory muscle pressure generating capacity (n = 55) |
| | Pimax (kPa) β (SE) | Pemax (kPa) β (SE) | Pendu (kPa) β (SE) | tendu (min) β (SE) |
| Constant | 3.982 | (0.748) | 2.079 | (0.935) | 2.993 | (0.213) | 4.240 | (0.381) |
| Δt2–t1 | –1.444 | (0.387)* | –0.508 | (0.759) | –0.689 | (0.200)* | –0.853 | (0.504) |
| Δt2–t3 | 1.177 | (0.538)* | 0.645 | (0.716) | 0.090 | (0.198) | –0.553 | (0.415) |
| Lesion | 2.015 | (0.641)* | 5.078 | (0.988)* | 0.538 | (0.261)* | 0.409 | (0.370) |
| Gender | 2.183 | (0.702)* | 1.583 | (0.753)* | n.s. | n.s. |
| Δt1–t2 × lesion | n.s. | –1.945 | (0.920)* | n.s. | n.s. |
| Δt2–t3 × lesion | n.s. | –1.874 | (0.916)* | n.s. | n.s. |
| *Significant influencing factor; n.s.: not significant Pimax: maximal inspiratory muscle pressure generating capacity; Pemax: maximal expiratory muscle pressure generating capacity; Pendu: maximal pressure of inspiratory threshold endurance test; tendu: time of inspiratory threshold endurance test; β: regression coefficient for each independent variable; SE: standard error of this regression coefficient; t1: start of active rehabilitation, t2: end of inpatient rehabilitation; t3: 1 year after discharge from inpatient rehabilitation; Δt1–t2 / Δt2–t3: time dummies with t2 as reference; lesion: 0 = tetraplegic, 1 = paraplegic; gender: 0 = women; 1 = men; Δt1–t2 × lesion, Δt2–t3 × lesion: interaction terms time × lesion. |
Influence of lesion level
HT showed significantly lower FVC, FEV1, FIV1 and PEF values than LT, while these values were significantly higher for LP than LT (Figs 1a–d). There were no significant differences between LT and HP in any of the tested lung function parameters (Table II). Pemax, Pimax and Pendu were lower in subjects with tetraplegia compared with subjects with paraplegia. There were significant differences in time-courses of Pemax between subjects with paraplegia and subjects with tetraplegia, while Pemax of subjects with tetraplegia did not change over time, Pemax of subjects with paraplegia increased during inpatient rehabilitation, but decreased thereafter (Fig. 2). Estimates for tendu showed no significant differences between groups (Table III).
Influence of personal factors
Personal factors, such as gender, age and height, had significant influences on most lung function parameters, with the exception of age having no influence on PEF, i.e. PEF was the only parameter that seems not to decrease with age. Body mass and smoking had no significant effect on any of the measured parameters (Table II). Calculated age- and height-corrected ABS 100% predicted (95% confidence interval (CI) of reference values) for men were 4.93 (3.93–5.93) l FVC, 4.06 (3.17–4.90) l FEV1 and 9.44 (7.45–11.43) l/s PEF (Figs 1a, 1b, 1d). Pimax and Pemax were influenced only by gender, which resulted in higher estimates for men than for women, while Pendu and tendu were not influenced by any of the tested personal factors (Table III).
Discussion
The most important finding of this study is that inspiratory and, especially, expiratory muscle pressure generating capacity is affected to a great extent after a SCI. Results clearly show that respiratory function improves with time during inpatient rehabilitation. Expiratory muscle pressure generating capacity also increases during inpatient rehabilitation, but it decreases during the first year after inpatient rehabilitation. Especially in subjects with tetraplegia, respiratory muscle pressure generating capacity should be screened and trained regularly after inpatient rehabilitation.
Longitudinal changes
This study showed that FVC, FEV1 and Pimax increased until 1 year after inpatient rehabilitation while FIV1, PEF and PIF remained constant or even decreased (Pemax) after inpatient rehabilitation. Interestingly, positive associations of Pimax to FVC and FEV1 were found in different studies (10, 25), which supports our findings of similar trajectories of these 3 parameters.
There are several factors that change over time after an acute SCI, such as changes in muscle tone, spasticity, a lower chest wall and a higher abdominal compliance (26). Furthermore, factors such as postural changes, trunk stabilization and changes in physical activity levels are known to influence respiratory function and may therefore also influence trajectories of respiratory function (27–29). Increasing spasticity and chest wall stiffness occur over time in subjects with SCI (26). Together with possible decreases in physical activity levels after inpatient rehabilitation, this may have avoided further increases in FIV1, PEF, PIF and Pemax between t2 and t3. The higher abdominal compliance, especially in subjects with tetraplegia and high-level paraplegia, changes functional residual capacity, since the diaphragm is less pushed up due to the lack of tone in the abdominal muscles (30). This reduces inspiratory capacity and therefore FVC, FIV1, PIF and Pimax, but also pulmonary recoil pressure of the diaphragm, which helps to improve expiratory muscle function, i.e. FEV1, PEF, and Pemax (31). Furthermore, it is known that, besides its function as a respiratory muscle, the diaphragm also acts as a trunk extensor and therefore supports trunk stabilization in subjects with tetraplegia (29). Therefore, a standardized measurement position is of vital importance.
Subjects with paraplegia and tetraplegia showed significant improvements in Pendu only during inpatient rehabilitation. This may result from improvements in general physical fitness due to physical activities performed during inpatient rehabilitation. Unfortunately there is no data available about the exact amount of physical exercise training during inpatient rehabilitation.
Influence of lesion level
As expected and already shown in earlier studies (32, 33), we also found significant influences of lesion level on lung function and respiratory muscle pressure generating capacity in subjects with recent SCI; the higher the lesion level, the lower the respiratory function (Figs 1 and 2). Therefore, separate regression equations for subjects with SCI, and especially for different lesion level groups, as presented in this paper, seem to be justified.
Fig. 1. Calculated estimates from the final multi-level regression models for time-courses of lung function parameters (t1: start of active rehabilitation; t2: end of inpatient rehabilitation; t3: 1 year after t2). (a) forced vital capacity (FVC), (b) forced expiratory volume in 1 second (FEV1), (c) forced inspiratory volume in 1 second (FIV1), (d) peak expiratory flow (PEF), (e) peak inspiratory flow (PIF)) for the 4 lesion level groups separately; HT: subjects with high-level tetraplegia (C3–C5); LT: subjects with low-level tetraplegia (C6–C8); HP: subjects with high-level paraplegia (T1–T6); LP: subjects with low-level paraplegia (T7–T12). Values were calculated for males with average age and height of the tested group. Age, gender and height-corrected able-bodied subjects’ reference values (100% predicted) with 95% confidence intervals (CI) are given for FVC, FEV1 and PEF.
Fig. 2. Calculated estimates from the final multi-level regression models for time-courses of respiratory muscle pressure generating capacity (Pemax, Pimax) for men with paraplegia (PP) and tetraplegia (TP) (t1 = start of active rehabilitation,t2 = end of inpatient rehabilitation, t3 = 1 year after t2).
Fishburn et al. (5) found increases in respiratory tract infections with increasing lesion levels. Furthermore, respiratory tract infections seem to be associated with the ability to cough as well as with PEF and Pemax (5, 34). Coughing is important for airway clearance and prevention of pulmonary complications (35). Fugl-Meyer (36) further reported that a PEF of at least 5–6 l/sec is necessary to produce an effective cough. Even if this is much below ABS 100% predicted, our subjects with high-level tetraplegia did not reach this level throughout the whole analysed time-course (Fig. 1d). Therefore, subjects with a high motor complete tetraplegia seem to be at high risk for respiratory tract infections. For optimization of cough capacity, inspiratory muscle function should also be considered. A deep inspiration increases pulmonary recoil pressure and is therefore an important precondition of an effective cough (37).
Influence of personal factors
The influence of personal factors, such as gender, age and height, on respiratory function is generally known from ABS reference equations (19, 24). In the present study, gender had a significant effect on all lung function parameters, leading to 0.5–0.7 l higher FVC, FEV1 and FIV1 and 0.8–0.9 l/sec higher PEF and PIF estimates for men than for women (Table II). Gender differences in the able-bodied population are somewhat higher for FVC (0.9 l) and PEF (2.1 l/sec). However, relative to the lower values in subjects with SCI, gender differences seem to be quite similar (19). Regarding respiratory muscle pressure generating capacity, gender has a large effect in ABS (24), with 30% higher Pimax and 38% higher Pemax estimates for men than for women. In our Dutch SCI sample, gender had a 5% higher effect on respiratory muscle pressure generating capacity than in ABS, resulting in differences of 35% for Pimax and 43% for Pemax (Table III).
To our knowledge there have been few studies addressing issues regarding ageing and SCI (38). Furthermore, studies assessing the interaction of ageing and respiratory function in SCI are non-existent. The influence of ageing on lung function, i.e. the decline in respiratory function with age, seems to be even lower in subjects with SCI (–0.012 to –0.021 l per year; Table II) than in the able-bodied population (–0.026 to –0.043 l per year) (19). Nevertheless, this study only assessed changes during the first 2 years after injury. To obtain reliable data on the influence of ageing on lung function for subjects with SCI, longitudinal studies of at least 10 years are needed.
Height had, similar to ABS reference equations, only significant influences on lung function, but not on respiratory muscle pressure generating capacity (Table II). Taking the absolute lower values of subjects with SCI in mind, influences of height on lung function seems to be quite similar to the ABS population (19).
In accordance with ABS reference equations, body mass had no significant effect on any of the measured parameters, probably since most of our subjects were within the range of normal body mass index (Table I). It is known that in obese ABS lung function is diminished (39). Former or current smoking also had no significant effect on the tested lung function and respiratory muscle pressure generating capacity parameters. One possible reason for that finding could be that many subjects stopped smoking after SCI (Table I). Results of 2 other studies provided equivocal findings concerning effects of smoking on lung function in SCI (32, 40).
Clinical relevance
Results of the present study clearly show that during inpatient rehabilitation lung function and respiratory muscle pressure generating capacity generally increase without specific respiratory muscle training, but are on a very low level in subjects with high-level tetraplegia. Changes in muscle tone, spasticity, chest wall and abdominal compliance, body position and physical exercise training are factors that may additionally affect trajectories of respiratory function, especially during the initial time after injury. Individuals with high-level tetraplegia are those at highest risk for respiratory complications, since they did not reach the lower limit to produce an effective cough. Therefore, regular screening and early training interventions in at least subjects with high-level, motor complete tetraplegia is important. Since the commonly used screening parameters FVC and FEV1 are the least affected, especially PEF, Pemax and Pimax should also become part of regular lung function testing.
In conclusion, all estimates for lung function and maximal respiratory muscle pressure generating capacity showed significant increases during inpatient rehabilitation, but to a different extent. During the first year after discharge from inpatient rehabilitation, only FVC, FEV1 and Pimax improved further, while all other parameters remained constant or even decreased (Pemax). Subjects with high-level tetraplegia generally showed the highest impairments, while PEF and Pemax were the most affected parameters in all groups of subjects with SCI. Thus, this study should motivate healthcare professionals to improve follow-up measurements as well as motivation and possibilities for training of the SCI population.
Acknowledgements
This study was supported by the Dutch Health Research and Development Council, ZON-MW Rehabilitation program, grant number 1435.0003.
The contribution of the 8 participating rehabilitation centres and especially the research assistants for collecting all the data is highly appreciated: Sacha van Langeveld (De Hoogstraat, Utrecht), Annelieke Niezen/ Peter Luthart (Rehabilitation Centre Amsterdam), Marijke Schuitemaker (Het Roessingh, Enschede), Karin Postma (Rijndam Revalidatiecentrum, Rotterdam), Jos Bloemen (Hoensbroeck Revalidatiecentrum, Hoensbroeck), Hennie Rijken (Sint Maartenskliniek, Nijmegen), Ferry Woldring (Beatrixoord, Haren) and Linda Valent (Heliomare, Wijk aan Zee).
We thank Professor Dr Hans Folgering for critically reading this manuscript.
References
1. Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil 1994; 75: 270–275.
2. DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil 1993; 74: 248–254.
3. Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005; 43: 408–416.
4. Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 2003; 82: 803–814.
5. Fishburn MJ, Marino RJ, Ditunno JF, Jr. Atelectasis and pneumonia in acute spinal cord injury. Arch Phys Med Rehabil 1990; 71: 197–200.
6. McMichan JC, Michel L, Westbrook PR. Pulmonary dysfunction following traumatic quadriplegia. JAMA 1980; 243: 528–531.
7. De Troyer A, Estenne M. Functional anatomy of the respiratory muscles. Clin Chest Med 1988; 9: 175–193.
8. Saltzstein R, Melvin J. Ventilatory compromise in spinal cord injury – a review. J Am Paraplegia Soc 1986; 9: 6–9.
9. Fugl-Meyer AR. Effects of respiratory muscle paralysis in tetraplegic and paraplegic patients. Scand J Rehabil Med 1971; 3: 141–150.
10. Sinderby C, Weinberg J, Sullivan L, Borg J, Lindstrom L, Grassino A. Diaphragm function in patients with cervical cord injury or prior poliomyelitis infection. Spinal Cord 1996; 34: 204–213.
11. Axen K, Pineda H, Shunfenthal I, Haas F. Diaphragmatic function following cervical cord injury: neurally mediated improvement. Arch Phys Med Rehabil 1985; 66: 219–222.
12. Bluechardt MH, Wiens M, Thomas SG, Plyley MJ. Repeated measurements of pulmonary function following spinal cord injury. Paraplegia 1992; 30: 768–774.
13. Haas F, Axen K, Pineda H, Gandino D, Haas A. Temporal pulmonary function changes in cervical cord injury. Arch Phys Med Rehabil 1985; 66: 139–144.
14. Loveridge B, Sanii R, Dubo HI. Breathing pattern adjustments during the first year following cervical spinal cord injury. Paraplegia 1992; 30: 479–488.
15. Lucke KT. Pulmonary management following acute SCI. J Neurosci Nurs 1998; 30: 91–104.
16. Bach JR, Wang TG. Pulmonary function and sleep disordered breathing in patients with traumatic tetraplegia: a longitudinal study. Arch Phys Med Rehabil 1994; 75: 279–284.
17. de Groot S, Dallmeijer AJ, Post MW, van Asbeck FW, Nene AV, Angenot EL, et al. Demographics of the Dutch multicenter prospective cohort study ‘Restoration of mobility in spinal cord injury rehabilitation’. Spinal Cord 2006; 44: 668–675.
18. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995; 152: 1107–1136.
19. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16: 5–40.
20. Karvonen J, Saarelainen S, Nieminen MM. Measurement of respiratory muscle forces based on maximal inspiratory and expiratory pressures. Respiration 1994; 61: 28–31.
21. Roth EJ, Lu A, Primack S, Oken J, Nusshaum S, Berkowitz M, et al. Ventilatory function in cervical and high thoracic spinal cord injury. Relationship to level of injury and tone. Am J Phys Med Rehabil 1997; 76: 262–267.
22. Twisk JW, editor. Applied longitudinal data analysis for epidemiology, a practical guide. Amsterdam: Cambridge University Press; 2003.
23. Rasbash J, Browne W, Goldstein H, Yang M, Plewis I, Healey M, et al, editors. A user’s guide to MLwin. London: Centre for Multilevel Modelling, Institute of Education, University of London; 2000.
24. Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis 1969; 99: 696–702.
25. Jain NB, Brown R, Tun CG, Gagnon D, Garshick E. Determinants of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1/FVC in chronic spinal cord injury. Arch Phys Med Rehabil 2006; 87: 1327–1333.
26. Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care 2006; 51: 853–868; discussion 869–870.
27. Estenne M, De Troyer A. Mechanism of the postural dependence of vital capacity in tetraplegic subjects. Am Rev Respir Dis 1987; 135: 367–371.
28. Vidal J, Javierre C, Segura R, Lizarraga A, Barbany JR, Perez A. Physiological adaptations to exercise in people with spinal cord injury. J Physiol Biochem 2003; 59: 11–18.
29. Sinderby C, Ingvarsson P, Sullivan L, Wickstrom I, Lindstrom L. The role of the diaphragm in trunk extension in tetraplegia. Paraplegia 1992; 30: 389–395.
30. Urmey W, Loring S, Mead J, Slutsky AS, Sarkarati M, Rossier A, et al. Upper and lower rib cage deformation during breathing in quadriplegics. J Appl Physiol 1986; 60: 618–622.
31. Kang SW, Shin JC, Park CI, Moon JH, Rha DW, Cho DH, et al. Relationship between inspiratory muscle strength and cough capacity in cervical spinal cord injured patients. Spinal Cord 2006; 44: 242–248.
32. Linn WS, Adkins RH, Gong H, Jr, Waters RL. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch Phys Med Rehabil 2000; 81: 757–763.
33. Mateus SR, Beraldo PS, Horan TA. Maximal static mouth respiratory pressure in spinal cord injured patients: correlation with motor level. Spinal Cord 2007; 45: 569–575.
34. Braun SR, Giovannoni R, O’Connor M. Improving the cough in patients with spinal cord injury. Am J Phys Med 1984; 63: 1–10.
35. Wang AY, Jaeger RJ, Yarkony GM, Turba RM. Cough in spinal cord injured patients: the relationship between motor level and peak expiratory flow. Spinal Cord 1997; 35: 299–302.
36. Fugl-Meyer AR. The respiratory system. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. New York: American Elsevier; 1976, p. 335–349.
37. Kang SW, Kang YS, Sohn HS, Park JH, Moon JH. Respiratory muscle strength and cough capacity in patients with duchenne muscular dystrophy. Yonsei Med J 2006; 47: 184–190.
38. Weitzenkamp DA, Jones RH, Whiteneck GG, Young DA. Ageing with spinal cord injury: cross-sectional and longitudinal effects. Spinal Cord 2001; 39: 301–309.
39. Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest 2006; 130: 827–833.
40. Almenoff PL, Spungen AM, Lesser M, Bauman WA. Pulmonary function survey in spinal cord injury: influences of smoking and level and completeness of injury. Lung 1995; 173: 297–306.