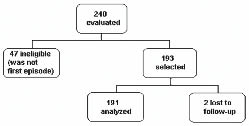
OBJECTIVE: To assess the relationship between lifestyle prior to the event and functional recovery at hospital discharge after acute stroke.
DESIGN: Cohort study.
Patients: A sample of 191 patients with first stroke episode (87.4% ischaemic).
METHODS: Severity of the event at admission was measured by the Modified National Institutes of Health Stroke Scale. The Frenchay Activity Index was used to evaluate the patients’ previous lifestyles. Functional recovery was assessed using the Modified Rankin scale on discharge from hospital. A Rankin score ≤ 2 was the main outcome.
RESULTS: At discharge, 37.2% of the patients were functionally independent. A receiver operating characteristic curve analysis established a value of ≥ 18 on the Frenchay Activity Index scale as the best cut-off point to predict favourable outcome (specificity 62%; 95% confidence interval (CI) 54–69% and a sensibility of 60%; 95% CI 49–69%) with an area under the curve of 0.65 (95% CI 0.57–0.71). There was a positive association between Frenchay Activity Index ≥ 18 and a Rankin score ≤ 2, after controlling for potential confounders (odds ratio 2.62; 95% CI 1.21–5.66; p = 0.001).
CONCLUSION: This result emphasizes the protective effect of mental, physical and social activity for the prevention of functional damage after an acute cerebrovascular event.
Key words: stroke, lifestyle, disability evaluation.
J Rehabil Med 2008; 40: 195–199
Correspondence address: Miguel Gus, Hospital de Clínicas de Porto Alegre (HCPA), Serviço de Cardiologia, Ramiro Barcelos, 2350, 90035-003, Porto Alegre, RS, Brazil. E-mail: mgus@terra.com.br
Submitted May 6, 2007; accepted October 16, 2007
INTRODUCTION
Stroke is a major health problem in many countries (1, 2). It is responsible for approximately 50% of all neurological admissions in the USA (3, 4). In Brazil, an incidence of 150,000 cases a year reflects the burden of this disease (5). Stroke is also a major cause of work incapacity; 30–60% of patients have some degree of physical disability after the event (2, 3).
Intervention related to modifiable risk factors for cardiovascular disease, such as hypertension, smoking, dyslipidaemia, diabetes and atrial fibrillation (1) have been the mainstay for primary and secondary prevention of stroke. Variables such as gender, age, previous health condition, intellectual basis, environmental aspects, time of hospitalization in the acute phase, and involved brain area have been associated with the degree of functional recovery (4, 6, 7). Other predictors, including the initial clinical picture, the degrees of paresis, urinary incontinence, disorientation in time and place, level of consciousness during the first 48 hours, scores for activities of daily living and sitting balance, have been identified (8, 9).
Few data have been published regarding the protective effect of pre-morbid physical, cognitive and social activity on neurological damage after an acute cerebral event. Maintenance of cerebral blood flow to the area involved may play an important role in functional recovery, as appropriate cerebral perfusion would preserve neural structure and strengthen the expansion of cerebral synapses. According to some authors, an active lifestyle, including physical and cognitive activity could enhance cerebral perfusion (10–13). This hypothesis has been tested more frequently in longitudinal studies of degenerative dementia, such as in Alzheimer’s disease (14, 15).
The aim of the present study was to determine the association between pre-morbid lifestyle and functional recovery at hospital discharge. The pre-morbid lifestyle included cognitive and social potentialities, measured by the Frenchay Activities Index (FAI), prior to an acute stroke. The length of hospital stay, presence of vascular risk factors and co-morbidities were also recorded.
METHODS
This prospective cohort study was carried out at the emergency department, intensive care and internment units of 2 public general hospitals in Porto Alegre, Brazil, between February 2005 and May 2006. A total of 191 patients with a clinical diagnosis of stroke were enrolled by convenience sampling. The study protocol was approved by the medical ethics committees of the participating hospitals.
Patients were selected if they had experienced onset of symptoms between 24 and 76 hours. All underwent cerebral tomography prior to admission to confirm the diagnosis, and those with a first episode of stroke were included in the present analysis. The data were collected in the emergency department and intensive therapy centres of the Hospital de Clinicas and Hospital Pronto-Socorro, in Porto Alegre. A questionnaire on socio-demographic factors, including education (years in school), the presence of cardiovascular risk factors (history of systemic arterial hypertension, diabetes mellitus and smoking), and co-morbidities (depression and dementia) was completed by the patient and/or a family member. Previous heart disease was defined as a positive history of angina, acute myocardium infarction and arrhythmia. During admission, blood pressure, presence of atrial fibrillation and infections of the respiratory and urinary systems were recorded. These data were obtained through the admission charts. The investigators were trained by a stroke neurologist certified on the National Institutes of Health Stroke Scale (NIHSS) score, and checked neurological deficits at baseline through the modified NIHSS-5 scale (NIHSS-M) (16). This is a simplified version of the NIHSS-15 scale, where only the 5 main prognostic items are taken into account: sight deviation, visual field impairment, paresis and/or plegia and aphasia (17). The scale ranges from 0 to 16. Higher scores indicate greater severity.
Pre-morbid levels of independence, physical activity and individual’s social interaction were measured using the FAI (18), translated and back-translated into Portuguese, with punctuation based on the frequency of accomplishment of 15 activities during a specific period (in the present study represented by the last 6 months). In most cases information was based on next-of-kin responses (19). The FAI scale was developed for quantitative measurement of incapacities and deficiencies in patients. It embraces domestic, cognitive and social areas. The scale ranges from 0 to 45, with a higher score indicating greater activity (20).
Functional independence was assessed on the Modified Rankin scale (21) at hospital discharge, or immediately afterwards by telephone. This 6-item scale constitutes a referential for limitations in the activities and changes of lifestyle. In the present study, we attributed grade 6 to patient’s decease according to ref (22). Patients with a Rankin score ≤ 2 were considered as having a favourable degree of functional dependence. The length of hospitalization in relation to the referred episode was also recorded.
Statistical analysis
Continuous variables were presented as mean and standard deviation or median and percentile 25th–75th (interquartile range). Categorical variables were presented as absolute frequencies and percentages. Comparison between groups was made using a χ2 test for categorical variables. The Student’s-t test was used for normally distributed variables. Otherwise the Mann-Whitney U test was used. A receiver operator characteristic (ROC) curve was constructed to estimate the best cut-off point of the FAI scale considering favourable outcome (Rankin ≤ 2). A logistic regression model was carried out to assess the independent predictors of favourable recovery. The variables with statistical significance in univariate analysis or in agreement with the theoretical presupposition (age, systolic and diastolic blood pressures) were candidates for inclusion in the model. Analyses were performed using SPSS for Windows version 13 (SPSS, Chicago, IL, USA).
Results of a pilot study showed a 60% and 40% prevalence of Rankin ≤ 2 after a stroke in those patients considered active vs not active (23), respectively, measured on the FAI scale. A sample of 168 patients were established considering 2-tailed significance level of 5%, a power of 80% and a possible 40% protective effect of a higher pre-morbid activity level. An additional sample of 20% was added to maintain the power in case of potential refusals, losses and multivariable analysis.
RESULTS
A total of 191 patients were included in the analysis (Fig. 1). Of these, 165 (86.4%) had a diagnosis of ischaemic stroke and 26 (13.6%) of haemorrhagic stroke. Table I shows the baseline demographic and clinical characteristics of the cohort. The level of education was low (62.8% of the patients had less than 4 years of formal education), with 20.4% of the patients reading a newspaper once a week. Only 25% of patients scored 24 points or more on the FAI scale (percentile 75% = 24). The median hospital stay was 10 days (25th–75th percentiles: 8–12) for the total sample. Patients with Rankin scores ≤ 2 spent significantly less time in hospital (median 6 days; 25th–75th percentiles 5–7) in comparison with those with Rankin scores > 2 (median 16 days; 25th–75th percentiles 13–19).
| Table I. Baseline characteristics of the cohort studied |
| Variable | n = 191 |
| Age (years), mean (SD) | 65.5 (13.4) |
| Gender, n (%) Men Women | 84 (44.0) 56 (84.0) |
| SBP (mmHg), mean (SD) | 167.3 (37.6) |
| DBP (mmHg), mean (SD) | 98.5 (22.4) |
| NIHSS-M median (q1–q3) | 4.0 (2.0–7.0) |
| FAI median (q1–q3) | 18.0 (9.0–24.0) |
| Education (year) n (%) | |
| ≤ 4 | 120 (62.8) |
| 5–9 | 52 (27.2) |
| ≥ 10 | 19 (9.9) |
| Read newspaper least 1/ week, n (%) | 39 (20.4) |
| Smokers, n (%) | 77 (40.3) |
| Hypertension, n (%) | 140 (73.7) |
| Heart disease, n (%) | 73 (38.2) |
| Atrial fibrillation, n (%) | 23 (12.0) |
| Diabetes mellitus, n (%) | 61 (31.9) |
| Depression, n (%) | 47 (24.6) |
| Death, n (%) | 34 (17.8) |
| SD: standard deviation; SBP: systolic blood pressure; DBP: diastolic blood pressure; NIHSS-M: modified National Institutes of Health Stroke Scale; FAI: Frenchay Activities Index. |
Table II shows that 71 (37.2%) patients had made a good neurological recovery at discharge. There was a significant association between NIHSS-M and FAI values and functional recovery. Individuals with favourable outcomes presented lower NIHSS-M and higher FAI scores, respectively, and a tendency to lower systolic and diastolic blood pressures.
| Table II. Functional recovery of patients’ at hospital discharge measured on the Modified Rankin scale |
| Variable | n = 71 | n = 120 | p |
| Age (year), mean (SD) | 64.9 (12.1) | 65.8 (14.1) | 0.677 |
| Gender, n (%) Male Women | 31 (43.7) 40 (56.3) | 53 (44.2) 67 (55.8) | 1.000 |
| Systolic blood pressure (mmHg), mean (SD) | 161.3 (36.2) | 170.8 (38.1) | 0.094 |
| Diastolic blood pressure (mmHg), mean (SD) | 96.0 (19.5) | 99.9 (23.9) | 0.248 |
| NIHSS-M, median (q1–q3) | 2.0 (1.0–3.0) | 5.0 (3.0–9.0) | < 0.001 |
| FAI, median (q1–q3) | 21.0 (15.0–25.0) | 14.0 (6.3–23.0) | 0.003 |
| Frenchay ≥ 18, n (%) | 50 (70.4) | 55 (45.8) | 0,001 |
| Education (year) n (%) | | | |
| ≤ 4 | 44 (62.0) | 76 (63.3) | 0.975 |
| 5–9 | 20 (28.2) | 32 (26.7) | |
| ≥ 10 | 7 (9.9) | 12 (10.0) | |
| Read newspaper 1/week, (n (%)) | 16 (22.5) | 23 (19.2) | 0.710 |
| Smoker, n (%) | 30 (42.3) | 47 (39.2) | 0.789 |
| Heart disease, n (%) | 27 (38.0) | 46 (38.3) | 1.000 |
| Atrial fibrillation, n (%) | 10 (14.1) | 13 (10.8) | 0.662 |
| Diabetes mellitus, n (%) | 23 (32.4) | 38 (31.7) | 1.000 |
| Depression, n (%) | 15 (21.1) | 32 (26.7) | 0.493 |
| SBP: systolic blood pressure; DBP: diastolic blood pressure; NIHSS-M: modified National Institute of Health Stroke Scale; FAI: Frenchay Activities Index. |
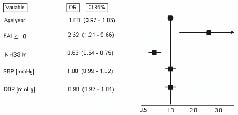
An FAI score ≥ 18 had a sensibility of 62% (95% confidence interval (CI); 54–69%) and a specificity of 60% (95% CI; 49–69%) to predict a Rankin ≤ 2 at hospital discharge, with an area under the curve of 0.65 (95% CI; 0.57–0.71). In a logistic regression model, adjusting for age, severity of stroke (assessed by the NIHSS-M scale), systolic and diastolic blood pressures, FAI ≥ 18 was independently associated with a better functional recovery (Fig. 2). A total of 175 patients (91.6%) were assessed in the hospital. A sensitive analysis excluding those who were contacted by telephone showed the same trend: odds ratio (OR) = 2.23; 95% CI 1.02–4.85; p = 0.04.
Fig. 2. Frenchay Activities Index (FAI) scale and functional recovery in a logistic regression model. SBP: Systolic blood pressures; DBP: Diastolic blood pressure; NIHSS-M: modified National Institutes of Health Stroke Scale; OR: Odds Ratio; FAI: Frenchay Index; CI: confidence interval.
The inclusion of other variables in the model (presence of risk factors, depression and associated co-morbidities at admission) did not modify the results.
DISCUSSION
In this study, 67.8% of patients with a first episode of acute stroke died or were left with substantial functional disability. This result reinforces the high degree of morbidity and mortality associated with this clinical condition. Data from multiple institutions throughout the USA have shown that, in the context of thrombolytic therapy used in the acute phase, 57% of patients present a Rankin score higher than 2 in 30 days, a figure not substantially different from ours (24). Our sample did not include patients submitted to reperfusion therapy, because during the recruitment and follow-up period, a reperfusion protocol had been introduced recently in only one of the centres. Surveys performed in different North American communities have indicated that the use of this therapeutic strategy reaches, at best, 10% of all patients (25). Therefore, our sample represents the outcomes of most cases of acute stroke.
Traditionally, the approach to managing or controlling risk factors for cardiovascular disease has been the main focus in the primary or secondary prevention of stroke (1). Besides the use of thrombolytic therapy, aspirin, and the implementation of specific care units in the treatment of acute stroke, few approaches have demonstrated a capacity to influence prognosis during the period of hospitalization (26). Even optimal blood pressure control is a controversial issue. Therefore there is considerable need for research into new treatment strategies for the acute phase.
In our study 71 patients (37.2%) showed favourable neurological recovery. They reached higher levels on the FAI scale compared with those with functional dependence at discharge. This positive association was independent of other factors, such as the initial clinical picture, blood pressure at the time of admission, or age. The level of education was not different between the 2 groups, indicating that, more than the degree of instruction, global cognitive activity prior to the event could induce a protective effect during the episode. This seems to corroborate the theory proposed by Holbrook & Skilbeck (18), that patients with low pre-morbid scores in the FAI experience poor rehabilitation. Cockburn & Smith (20), analysing 119 patients with stroke, found that the fluid intelligence and memory performance, estimated by Ravens matrix, were independently associated with domestic, social and leisure activities. They concluded that the FAI scale is a valuable instrument to measure daily activities and cognitive abilities.
Several mechanisms may explain the relationship between the level of physical, social and cognitive activity and the higher degree of functional independence. Changes in the metabolism and brain blood flow during motor and mental activity have been investigated in many pathological conditions, particularly in stroke (11, 12). Studies involving the aspects of the brain’s physiopathology and evolution of functional deficits hypothesize that alterations in the brain blood flow (induced by playing board and card games, reading, practicing musical instruments and other cognitive activities) can be used as brain reorganization inducers. Bragoni et al. (11) found an increase in blood flow in brain arteries in 29 patients after stroke during the performance of cognitive tasks. There was a positive association between blood flow in the ipsilateral hemisphere of the lesion and functional recovery.
The underlying mechanisms involved in neurological recovery after stroke are not clear. It is possible that a physical activity regime prior to stroke may lead to an increase blood flow and decrase in neurological damage during the event, through optimization of the brain cells and reorganization of neuronal activities (26). In an experimental study in mice, Endres et al. (13) observed that the size of the brain lesion was significantly smaller in the group that had performed physical activity in the 3 weeks prior to the stroke (34 ± 7 mm3) than in the control group (37 ± 5 mm3).
Longitudinal studies focusing on the development of degenerative conditions, such as Alzheimer’s disease, have clearly identified a protective effect of global cognitive activity. Wilson et al. (14) observed, in a study of 801 elderly people followed, on average, for 4.5 years, that a 1-point increase in global cognitive score activity was associated with a 33% decrease in risk of Alzheimer’s disease (relative risk 0.67; 95% CI 0.49–0.92), independent of age, gender and education level. Laurin et al. (15), in another cohort involving 9008 elderly people (≥ 65 years) in Canada, showed that those who performed physical activity presented a lower risk of cognitive weakness (OR 0.58, 95% CI 0.41–0.85), and a lower risk of developing Alzheimer’s disease.
Some limitations of this study must be considered. Data analysed from a convenience sample could be prone to selection bias. However, the patient’s characteristics and the outcomes do not indicate significant differences in relation to other samples studied in the context of clinical trials or epidemiological data of acute stroke (23, 27). The presence of depression or cognitive disturbances previous to the event was not properly measured and was analysed from the information included in admission files. These clinical situations, obviously, could be associated with sustained lower global cognition scores. However, those diagnoses would equally indicate lower values in the FAI scale. The use of the FAI scale, an instrument that is not usually applied in Brazilian communities, could decrease the accuracy of identification of the real levels of our patients’ physical, cognitive and social activities. The FAI scale seems to be suitable for use with proxy respondents (28). However, it must be remembered that the FAI scale based on next-of-kin responses has good results for the total score, but there is less agreement between each item level (28, 29). The outcome was assessed using the disability Modified Rankin scale. This is a simple and accepted way to measure functional recovery after stroke, but its ability to capture the full spectrum of limitation may be questioned (30).
Finally, the same investigators who performed the initial evaluation collected the outcomes. Although the Modified Rankin scale is an objective tool to assess functional recovery, this fact could have affected the blinding. However, at the moment of the evaluation of the Modified Rankin score, the researchers did not have access to clinical or follow-up data, only to the identification files. An analysis including only those patients evaluated in the hospital environment showed the same trend.
In summary, the present study indicates that, as in chronic-degenerative situations, pre-morbid global cognitive activity may exert a protective effect on the acute cerebrovascular event leading to better functional recovery. Therefore, a lifestyle that promotes this favourable profile could be included in the recommendations regarding the prophylactic treatment of acute stroke. New studies, with different instruments validated in different populations, with a longer follow-up and with a larger number of patients, are needed to confirm our results.
ACKNOWLEDGEMENTS
We thank the emergency department and intensive unit of the Hospital de Clinicas of Porto Alegre. We also thank the Neurological and Intensive Therapy Units of the Hospital Pronto-Socorro of Porto Alegre.
REFERENCES
1. Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 2006; 37: 1583–1633.
2. Martins T, Ribeiro JP, Garret C. Disability and quality of life of stroke survivors: evaluation nine month after discharge. Rev Neurol 2006; 42: 655–659.
3. Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke 1998; 19: 1497–1500.
4. Hendricks HT, Limbeek J, Gertus AC. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil 2002; 83: 1629–1637.
5. Oliveira GM, Klein CH, Souza e Silva NA. Mortality from cardiovascular diseases in three Brazilian states from 1980 through 2002. Rev Panam Salud Publica 2006; 19: 85–93.
6. Ohlsson A-L, Johansson BB. Environment influences functional outcome of cerebral infarction in rats. Stroke 1995; 26: 644-649.
7. Chamorro A, Vila N, Ascaso C, Saiz A, Montalvo J, Alonso P. Early prediction of stroke severity. Role of erythrocyte sedimentation rate. Stroke 1995; 26: 573–576.
8. Wade DT, Langton-Hewer R. Functional abilities after stroke: measurements natural history and prognosis. J Neurol Neurosurg Psychiatry 1987; 50: 177–182.
9. Hsieh CL, Sheu CF, Hsueh P, Wang CH. Trunk control as an early predictor of compreehensive activities of daily living function in stroke patients. Stroke 2002; 33: 2626–2630.
10. Verghese J, Lipton RB, Katz MJ. Leisure activities and risk of dementia in the elderly. N Engl J Med 2003; 348: 2508–2516.
11. Bragoni M. Caltagirone C, Troisi E, Matteis M, Varnieri F, Silvestrini M. Correlation of cerebral hemodynamic changes during mental activity and recovery after stroke. Neurology 2000; 55: 35–40.
12. Kemperman G, Kuhn GH, Gage FH. More hippocampal neurons in adult mice living in enriched environment. Nature 1997; 386: 493–495.
13. Endres M, Gertz K, Lindauer U. Mechanisms of stroke protection by physical activity. Ann Neurol 2003; 54: 582–590.
14. Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA. Participation in cognitive stimulation activities and risk of incident Alzheimer disease. JAMA 2002; 287: 742–748.
15. Laurin D, Verreault R, Lindsay Y, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol 2001; 58: 498–504.
16. Golstein LB, Bertels C, Davis JN. Interrater reability of the NIH stroke scale. Arch Neurol 1989; 46: 660–662.
17. Tirschwell DL, Longstreth WT, Becker KJ, Gammans RE, Sabounjian LA, Hammilton S, Morgenstern LB. Shortening the NIH stroke scale for use in the prehospital setting. Stroke. 2002; 33; 2801–2806.
18. Holbrook M, Skilbeck CE. An activities index for use with stroke patients. Age Ageing 1983; 12: 166–170.
19. Segal ME, Schall RR. Determining functional health status and its relation to disability in stroke survivors. Stroke 1994; 25: 2391–2397.
20. Cokburn J, Smith PT. Influence of cognitive function on social, domestic, and leisure activities of community-dwelling older people. Int Disabil Studies 1990; 12: 169–172.
21. Rankin J. Cerebral vascular accidents in patients over the age of 60: II. Prognosis. Scot Med J 1957; 2: 200–205.
22. Uyttenboogaart M, Stewart RE, Vroomen PCAJ, De Keyser J, Luijckx G-J. Optimizing cutoff scores for the Barthel index and the modified Rankin Scale for defining outcome in acute stroke trials. Stroke 2005; 36: 1984–1987.
23. Turnbull JC, Kersten P, Habib M, McLellan L, Mullee MA, George S. Validation of the Frenchay Activities Index in a general population aged 16 years and older Arch Phys Med Rehabil 2000; 81: 1034–1038.
24. Albers GW, Bate VE, Clark WM, Bell R, Verro P, Hamilton S. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the standard treatment with atepease to reverse stroke (STARS) study. JAMA 2000; 283: 1145–1150.
25. Reeves MJ, Arora S, Broderick JP, Frankel M, Heinrich JP, Hickenbottom S, et al. Acute stroke care in the US. Results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke 2005; 36: 1236–1240.
26. Adams HP Jr, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, et al. Guidelines for the early management of patients with ischemic stroke. A scientific statement of the Stroke Council of the American Stroke Association. Stroke 2003; 34: 1056–1083.
27. Di Carlo A, Lamasso M, Baldereschi M, Pracucci G, Consoli D, Wolfe DAC, Giroud M. Risk factors and outcome of subtypes of ischemic stroke. Data from a multicenter multinational hospitals-based registry. The European Community Stroke Project. J Neurol Sci 2006; 244: 143–150.
28. Wyller TB, Sveen U, Bautz-Holter E. The Frenchay Activities Index in stroke in patients: agreement between scores by patients and by relatives. Disabil Rehabil 1996; 18: 454–459.
29. Tooth LR, Mcenna KT, Smith M, O’Rourke P. Further evidence for the agreement between patients with stroke and their proxies on the Frenchay Activities Index. Clin Rehabil 2003; 17: 656–665.
30. Uyttenboogaart M, Luijckx GJ, Vroomen PC, Stewart RE, De Keyser J. Measuring disability in stroke: relationship between the modified Rankin scale and the Barthel index. J Neurol 2007; 254: 1113–1117.