OBJECTIVE: To investigate changes in brain activation related to tone and speech sound processing during aphasia rehabilitation.
DESIGN: Longitudinal study investigating patients with stroke, subarachnoid hemorrhage and traumatic brain injury 3 and 7 months post-injury.
SUBJECTS: Eight patients with aphasia, reflecting a wide range of auditory comprehension impairment.
METHODS: Token test and Norwegian Basic Aphasia Assessment were used to measure auditory comprehension function. Brain event-related potentials were recorded in passive paradigms with harmonically rich tones and syllables in order to obtain the mismatch negativity component that reflects automatic stimulus discrimination. In an active syllable discrimination paradigm, stimulus feature integration (N1), attended stimulus discrimination and classification (N2), and target detection (P3) were studied.
RESULTS: Auditory comprehension scores improved approximately 10% during the observation period. Ipsilesional frontal P3- and N2-amplitude increased significantly. A significant shift in topographical distribution from the contralesional to the ipsilesional hemisphere was observed for the N2 component. The study of individual waveforms indicates inter-individual differences in reorganization after brain injury.
CONCLUSION: Hemispherical distribution of brain activation correlating with speech sound processing in aphasia can change during the first months after brain injury. Event- related potentials are a potentially useful method for detecting individual activation patterns relevant to recovery in aphasia rehabilitation.
Key words: aphasia, rehabilitation, event-related potentials, mismatch negativity, MMN, N1, N2, P3, plasticity.
J Rehabil Med 2007; 39: 658–661
Correspondence address: Frank Becker, Sunnaas Rehabilitation Hospital, NO- 1450 Nesoddtangen, Norway. E-mail: frank.becker@sunnaas.no
Submitted September 22, 2006; accepted May 24, 2007.
INTRODUCTION
Recent research has illustrated the ability of the brain to change both structurally and functionally in response to exogenous stimuli; so-called plasticity (1). Increased knowledge about reorganization processes after brain injury is important for rehabilitation because it is likely to impact on therapeutic strategies. For example, brain reorganization patterns might be identified that are associated with successful recovery, while other patterns might be less favourable. One method of studying plasticity is to examine event-related brain potentials (ERPs); this is an electroencephalogram (EEG)-based method that measures brain activity correlated to stimuli presented to the investigated subject. Different waveforms, elicited by different stimuli and in different conditions or tasks, can be identified as so-called components that can be related to sensory and cognitive processes. Clinical application of ERPs has been advocated for components reflecting the stimulus feature integration (N1) (2), passive stimulus discrimination (mismatch negativity (MMN) (3)), and the target detection (P3) components (4). In rehabilitation medicine, ERPs might, for example, be used as prognostic tools or for longitudinal monitoring (5); they are especially useful as a supplement to neuropsychological methods in the assessment of cognitive function (6). A particular advantage of ERPs is that they allow the investigation of cognitive processes even in patients who are unable to give verbal or motor responses (3).
In the present study, we explored electrophysiological aspects of speech sound processing during aphasia rehabilitation. Based on earlier reports of significant reductions in ERP-amplitudes in aphasia (e.g. (7) for MMN and N1, (8) for N2) and of significant correlations of amplitude reductions with aphasia severity (8), we expected to find increasing amplitudes during the observation period. Since these findings were often made in ipsilesional frontal areas, we were especially interested in changes in these brain regions, which might also lead to altered hemispheric distribution of the investigated components.
METHODS
Eight patients with aphasia admitted to our hospital due to stroke, subarachnoid haemorrhage or traumatic brain injury were recruited consecutively for this study. They participated in a standard rehabilitation programme, which, besides treatment for other functions, included approximately 8–12 h of speech and language therapy per week, 50–80% delivered as one-to-one therapy. All were native speakers of Norwegian; none reported a history of neurological or psychiatric disease, hearing loss, or speech and language disorders. The study was approved by the regional research ethics committee of Eastern Norway; all participants gave their informed consent. The patient’s age range was 18–66 years; 3 were men and 5 women. All subjects were examined in 2 sessions, at admission (mean 90 days post-injury) and at discharge or shortly afterwards (mean 208 days, cf. Table I). Language function was investigated using the Norwegian Basic Aphasia Assessment (Norsk grunntest for afasi, NGA; (9)) and the Token test (10). Event-related potentials were recorded with 3 different paradigms, as described previously (8, 11): a passive syllable discrimination paradigm, a passive tone discrimination paradigm, and an active syllable discrimination and identification paradigm. The harmonically rich tones differed in duration, the speech sound stimuli were the Norwegian syllables /ba:/ and /ta:/. In the unattended paradigms, the subjects were instructed not to attend to the stimuli, but to leaf through richly illustrated magazines. In the attended syllable paradigm, the task was to push a button as fast as possible in response to the target syllable /ta:/. Stimuli were delivered binaurally via headphones.
Using a nose reference, EEG was recorded continuously from 17 electrode sites (Fz, Cz, Pz, F3/4, C3/4, P3/4, F7/8, T3/4, T5/6; M1/2). Standard post-hoc analysis was performed including band-pass filtering (1–15 Hz), correction for ocular artefacts and removal of sweeps containing large amplitudes (±100 μV). The individual sweeps were averaged separately for each paradigm and stimulus; pre-stimulus baseline correction was performed. Grand average waveforms were computed separately for each paradigm, stimulus and session. Mean latencies were calculated as group means of the individual peak latencies and served as centres of time-windows, which were used to compute mean amplitudes. (The size of the time windows was 30 millisec for N1, 40 millisec for MMN and 50 millisec for P3. See Table II for centre latencies. The time windows were centered at the mean of the respective 2 mean group latencies from each session. For P3 only, 2 different time windows were applied.) Attended syllable discrimination was studied by analysing the difference waveform (target–standard) in 50 millisec intervals in the 75–325 millisec time-range. For statistical analysis, paired t-tests, analyses of variance (ANOVA) with Greenhouse-Geisser corrections and Spearman’s rank correlation test were used. ANOVA-models with the within group factors “session” (1st vs 2nd test), “line” (frontal vs central vs parietal) and “electrode” (5 electrodes per line, e.g. F7, F3, Fz, F4, and F8 for the frontal line) were used; for analysis of the MMN components, models with 2 lines (frontal and central) and 3 electrodes (F3, Fz, F4 and C3, Cz, C4) were applied.
Two patients (patients 4 and 7) were left-handed, had computerized tomography-verified right hemisphere lesions and left hemiparesis; for these patients, electrodes were swapped between hemispheres. Results are reported as ipsilesional (odd electrode numbers) and contralesional (even numbers) in reference to the language-dominant, brain-damaged hemisphere.
RESULTS
Table I gives an account of the aphasia assessment results. The aphasic subjects represent a wide range of aphasia, both regarding aphasia type and severity. Despite significant improvement in all 3 aphasia scores, the clinical improvement was rather small. All patients were able to discriminate the speech sounds and detect the target syllable /ta:/ except one subject (patient 5) who could perform the task in the 2nd session only. There were no differences between sessions with regard to correct hits (29.7 vs 29.9 of 30 targets) and false alarms (1.1 vs 0.6). The reaction time increased non-significantly from 487 to 575 ms.
| Table I. Characteristics of the patients |
| No. | Sex/age | Site of lesion | Aetiology | Aphasia type | Session 1 | Session 2 |
| Days post- onset | NGAb | NGA acc | Tokend | Days post-onset | NGAb | NGA acc | Tokend |
| 1 | F/66 | F, T | BI | TSA | 100 | 209 | 68 | 19 | 266 | n.d. | 66 | 21.5 |
| 2 | M/54 | P | BI | WA | 130 | 116 | 46 | 6 | 297 | n.d. | 52 | 7.5 |
| 3 | F/18 | F, T, P, O | TBI | AA | 106 | 209 | 69 | 30.5 | 149 | n.d. | 70 | 34.0 |
| 4 | M/53 | Pa | BI | MTA | 93 | 161 | 55 | 8 | 162 | 194 | 62 | 14.5 |
| 5 | F/48 | F | SAH, BI | GA | 68 | 93 | 39 | 10.5 | 142 | 163 | 54 | 11.0 |
| 6 | M/61 | F, T, P | BI | BA | 63 | 155 | 65 | 21.5 | 200 | 194 | 67 | 26.5 |
| 7 | F/63 | F, Ta | BI | GA | 80 | 165 | 52 | 21 | 166 | 181 | 60 | 26.0 |
| 8 | F/49 | F, T, P | SAH | GA | 76 | 35 | 13 | 4 | 281 | 85 | 37 | 11.5 |
| Mean: | | | | | 90 | 122 | 50.9 | 15.1 | 208 | 163** | 58.5* | 17.2* |
| Average scores for the clinical aphasia results increased significantly (*p < 0.05, **p < 0.01). aPatients with right hemisphere lesions. bMaximum score 217. cMaximum score 71. dToken test score corrected for years of education (maximum uncorrected score = 36). F: frontal; T: temporal; P: parietal; O: occipital; BI: brain infarction; SAH: subarachnoid haemorrhage; TBI: traumatic brain injury; AA: anomic aphasia; BA: Broca’s aphasia; GA: global aphasia; MTA: mixed transcortical aphasia; TSA: transcortical sensory aphasia; WA: Wernicke’s aphasia; NGA: Norwegian Basic Aphasia Assessment, total score; ac: subsection auditory comprehension; n.d.: data missing. |
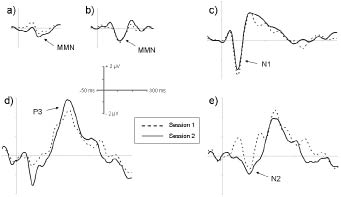
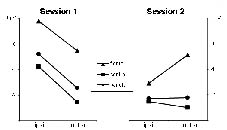
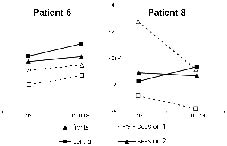
All waveforms of interest (MMN, N1, P3, and N2) were observed in both sessions (Fig. 1). At midline electrodes, neither latencies nor amplitudes differed significantly between the 2 sessions (Table II). For the 75–125 millisec time-window of the attended difference waveform, a significant session * electrode interaction was observed (F [1, 7] = 4.43; p < 0.05), which was caused by changes in hemispherical distribution between sessions. While the processing difference was more negative over the contralesional hemisphere in the first session, it peaked ipsilesionally in the second session. This effect was most prominent at central and parietal sites; the increase in negativity at the P3 site was almost significant (1.14 vs –1.18 μV; p = 0.051). These hemisphere differences between sessions were confirmed when a hemisphere model with the electrodes F3/C3/P3 vs F4/C4/P4 was used (F [1, 7] = 7.54; p < 0.05). Similar changes in topographical distribution were also found for the 175–225 millisec interval (Fig. 2; F [1, 7] = 3.40; p < 0.05; hemisphere model: F [1, 7] = 12.17; p < 0.05); in this interval, F7 amplitude increased significantly from the first to the second session (–0.18 vs –2.59 μV; p < 0.05). The hemisphere findings were also statistically significant when the 2 left-handed patients with right hemisphere lesions were excluded from the analysis (75–125 millisec: F [1, 5] = 11.16, p < 0.05; 175–225 millisec: F [1, 5] = 23.35, p < 0.01). A revision of the single case waveforms revealed individual differences in hemisphere patterns, which are illustrated by patient 6, who was a moderate to mild aphasic and improved only slightly, and patient 8, who had a very severe aphasia and showed the largest improvement in auditory comprehension (cf. Table I). For the N2 component in the 175–225 millisec time-window, patient 6 showed a slight ipsilesional overweight in the first session, while this component had a clear contralesional maximum in patient 8. During the follow-up period, the hemispherical pattern of patient 6 remained rather unchanged (Fig. 3), while in patient 8 a reversal of the contralesional overweight was observed, resulting in an almost balanced hemispherical distribution in session 2, with even ipsilesional overweight at central sites.
Fig. 1. Group grand average waveforms for session 1 (dashed line) and 2 (solid line): (a) Unattended harmonically rich tones, difference waveform. (b) Unattended syllables, difference waveform. (c) Attended standard syllable /ba:/. (d) Attended target syllable /ta:/. (e) Attended difference waveform (target–standard). Same electrodes as in Table II.
| Table II. Event-related brain potential results |
| Component | Session 1 | Session 2 |
| MMN tones | Latency (μV) | 154 | 160 |
| Amplitude (millisec) | –0.81 | –0.50 |
| MMN syllables | Latency (μV) | 161 | 162 |
| Amplitude (millisec) | –1.22 | –1.36 |
| N1 | Latency (μV) | 114 | 112 |
| Amplitude (millisec) | –3.48 | –2.72 |
| P3 | Latency (μV) | 432 | 369 |
| Amplitude (millisec) | 2.10 | 5.45 |
| N2 amplitude | 75–125 millisec | 1.67 | –0.15 |
| 125–175 millisec | 0.09 | –1.14 |
| 175–225 millisec | –0.73 | –1.73 |
| Electrodes used: Fz (MMN tones), Cz (MMN syllables, N1, N2), Pz (P3). MMN: mismatch negativity. |
Fig. 2. Hemispherical distribution of the processing difference (target–standard) in the 175–225 millisec time-window of the attended syllable paradigm; mean N2 amplitudes from electrodes adjacent to the midline are shown (F3/4, C3/4, P3/4).
Fig. 3. Mean amplitudes at frontal (F3/4) and central (C3/4) sites of the 175–225 millisec time-window of the processing difference of the attended paradigm (target–standard syllable) in 2 selected patients illustrating different patterns of hemispherical distribution.
N1 amplitude from the first session at the F7 site predicted outcome as assessed by the auditory comprehension subsection of the NGA (r = 0.881; p < 0.01): the smaller N1 in the first session, the larger was the NGA improvement. At the F7 electrode site, mean P3 amplitude was significantly larger in the second session (2.20 vs 0.36 μV; p < 0.05). A positive correlation between increase in NGA comprehension sub-score and decrease in tone-MMN amplitude was observed (C4: r = 0.905, p < 0.01; P4: r = 0.905; p < 0.01).
DISCUSSION
During the observation period, the participants showed only limited progress in language comprehension function, but this was consistent with previous data using the same measures of recovery in a comparable time window (9). Only small changes in ERP amplitudes and latencies were observed. However, a shift in hemispherical distribution of amplitudes from 2 time-windows of the N2 component was seen. The contralesional overweight from the first session, 3 months post-injury, developed into an ipsilesional overweight 3 months later, suggesting bilateral brain reorganization processes during aphasia rehabilitation. In an earlier study, we observed a left-hemispherical peak in a healthy control group for the 175–225 millisec time-window (8); the observed hemispherical shift in this present study can thus be interpreted as a normalization of the electrophysiological pattern. Bi-hemispherical activation changes resulting in a significant increase in an early negativity have also been reported in a study with 9 patients with chronic aphasia who underwent a short-term intensive therapeutic intervention (12). Our present findings, though based on a small number of patients recruited from an aetiologically heterogeneous group, are consistent with compensatory increased contralesional activation at 3 months after brain damage that decreases over time, and thus support the results of a recent functional magnetic resonance imaging (fMRI)-study of successfully recovering aphasic patients: hyper-activation in contralesional brain areas was present after 2 weeks, but decreased during 1-year follow-up (13). The presented single cases illustrate that the shift from contralesional to ipsilesional overweight was not present in all subjects. While ipsilesional overweight was seen at 3 months in a patient with only mild to moderate auditory comprehension impairment (patient 6), a patient with a very severe aphasia (patient 8) showed a clear contralesional overweight in the first session and approached a more physiological distribution pattern as she improved during aphasia rehabilitation. Inter-individual variation in brain reorganization during recovery from aphasia might be due to a number of factors, such as lesion site and size. In addition, pre-morbid differences in functional-anatomical conditions are discussed; for example, there might be differences in how parts of the bi-hemispherical language network are used (14). This study illustrates that ERPs can reveal individual differences in hemispherical activation, making the method potentially useful in aphasia rehabilitation.
Ipsilesional frontal N1 amplitude at the first session predicted recovery: the smaller N1 was in the first session, the larger was the improvement in auditory comprehension. The N1 component is known to be attenuated in aphasic subjects, both in response to tones (e.g. (7)) and speech sounds (8). In an earlier study, we have shown that ipsilesional frontal N1 amplitudes correlate positively with auditory comprehension function (8). In the present study, amplitudes of both P3 and N2 (175–225 millisec time-range) increased significantly between sessions at the ipsilesional frontal F7 electrode site. These findings suggest that ipsilesional frontal areas should be a focus of future ERP-studies of aphasia rehabilitation, which preferably should investigate more homogenous samples with regard to post-injury time.
Acknowledgements
This project was financed with the aid of EXTRA funds from the Norwegian Foundation for Health and Rehabilitation and by Sunnaas Rehabilitation Hospital and the University of Oslo.
References
1. Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci 2005; 28: 377–401.
2. Hyde M. The N1 response and its applications. Audiol Neurootol 1997; 2: 281–307.
3. Näätänen R. Mismatch negativity: clinical research and possible applications. Int J Psychophysiol 2003; 48: 179–188.
4. Polich J. Clinical application of the P300 event-related brain potential. Phys Med Rehabil Clin N Am 2004; 15: 133–161.
5. Giaquinto S. Evoked potentials in rehabilitation. A review. Funct Neurol 2004; 19: 219–225.
6. Mazzini L. Clinical applications of event-related potentials in brain injury. Phys Med Rehabil Clin N Am 2004; 15: 163–175.
7. Ilvonen TM, Kujala T, Tervaniemi M, Salonen O, Näätänen R, Pekkonen E. The processing of sound duration after left hemisphere stroke: event-related potential and behavioral evidence. Psychophysiology 2001; 38: 622–628.
8. Becker F, Reinvang I. Successful syllable detection in aphasia despite processing impairments as revealed by event-related potentials. Behav Brain Funct 2007; 3: 6.
9. Reinvang I, editor. Aphasia and brain organization. New York: Plenum Press; 1985.
10. De Renzi E, Faglioni P. Normative data and screening power of a shortened version of the Token test. Cortex 1978; 14: 41–49.
11. Becker F, Reinvang I. Mismatch negativity elicited by tones and speech sounds: Changed topographical distribution in aphasia. Brain Lang 2007; 100: 69–78.
12. Pulvermüller F, Hauk O, Zohsel K, Neininger B, Mohr B. Therapy-related reorganization of language in both hemispheres of patients with chronic aphasia. Neuroimage 2005; 28: 481–489.
13. Saur D, Lange R, Baumgärtner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain 2006; 129: 1371–1384.
14. Knecht S, Floel A, Drager B, Breitenstein C, Sommer J, Henningsen H, et al. Degree of language lateralization determines susceptibility to unilateral brain lesions. Nat Neurosci 2002; 5: 695–699.