OBJECTIVE: To examine self-perceived health status during the first year following mild closed head injury.
METHODS: At 1 week, and at 3, 6 and 12 months post-injury, 37 patients with mild closed head injury completed written versions of the Rivermead Post-Concussion Symptoms Questionnaire (RPSQ), the Rivermead Head-Injury Follow-up Questionnaire (RHIFQ) and the SF-36 Health Survey. Thirty-seven controls provided baselines for the SF-36 and the RPSQ.
RESULTS: The 3 questionnaires conveyed differing impressions of recovery. On the RPSQ, the patients exhibited ongoing symptomatic complaints and higher scores compared with controls. The RHIFQ conveyed a better recovery in terms of everyday function. The SF-36 showed the best recovery, with the mild closed head injury group achieving normal scores at 3, 6 and 12 months. Regression analyses indicated an influence of IQ, but not of age, education, or clinical measures of injury severity, on long-term health status.
CONCLUSION: Recovery after mild closed head injury can involve a dichotomy of persistent post-concussional symptoms but relatively normal functionality and quality of life. In addition to indicating an influence of IQ on perception of recovery in mild closed head injury, our findings demonstrate that the nature of self-report questionnaires considerably influences the picture of recovery. This emphasizes the importance of methods unaffected by IQ and self-evaluative accuracy in the assessment of mild closed head injury.
Key words: head injury, recovery, IQ, rehabilitation, health status.
J Rehabil Med 2007; 39: 612–621
Correspondence address: Marcus Heitger, Van der Veer Institute for Parkinson’s & Brain Research, PO Box 2682, Christchurch, New Zealand.
E-mail: marcus.heitger@chmeds.ac.nz
Submitted May 26, 2006; accepted April 19, 2007.
Introduction
Mild closed head injury (mCHI) is one of the most common causes for visits to emergency departments and hospital admissions, with admission rates between 100 and 300 cases per year per 100,000 population (1, 2). Most patients with mCHI have a good outcome and recover to normal levels of physical, cognitive and emotional function, the majority of patients after mCHI being independent of care by others and able to return to work or resume social activities within a few weeks, if not days, post-injury (1–3). While this may suggest that having a mild head injury is consistently associated with an uncomplicated and complete recovery, patients with mCHI may struggle with overcoming post-concussional symptoms and performing previous social and leisure activities, or executing their work to a pre-morbid level of efficiency (1–4). Although post-concussional symptoms usually resolve within days or weeks, mCHI may have a persistent long-term impact comprising physical, cognitive and emotional sequelae for several months or years post-injury (5–13). With regard to this notion of prolonged health problems after mCHI it is important to make the distinction between subjective symptoms and objectively quantified functional deficits identified by neurological examination or cognitive testing. Whilst studies using functional imaging (14) and electroencephalography (15) indicate that mild head trauma may be associated with abnormalities in brain function beyond the first weeks post-injury, self-reported symptoms or functional complaints should not be misinterpreted as definite indicators of decreased brain function or cerebral injury.
Despite evidence that patients with mCHI can experience disabling symptoms beyond the first few weeks post-injury, there is controversy about the extent and duration of such ongoing symptoms and complaints. Several studies have indicated that, in most patients, any symptoms or cognitive impairment have resolved by 3 months post-injury (16–20), whilst others have shown that a considerable proportion of patients (> 20%) have ongoing problems beyond 3 months post-injury (7–13, 21–24). There is agreement across studies that the proportion of patients with ongoing complaints decreases over the first year post-injury, but the stated proportions of patients with ongoing problems differ widely between studies, varying between 10% and 80% (7–13, 21, 22). Some studies report only the “presence” of symptoms without rating their severity (21, 24) whilst others do not include a control group to provide a normal baseline independent from patient self-report (7, 9, 11, 21, 24, 25). In addition, there is considerable variation between studies with regard to differing time points/intervals for collecting data and different criteria for reporting symptoms.
This prospective study collected 4 data points across the first year post-injury in a group of 37 participants with mCHI and 37 matched controls. We used the Rivermead Post-Concussion Symptoms Questionnaire (RPSQ) (26), Rivermead Head Injury Follow-up Questionnaire (RHIFQ) (25) and Short Form 36 Health Survey, version 2 (SF-36v2) (27) in consecutive assessments (one week, 3, 6 and 12 months) to collect data on post-concussive symptoms as well as quality of, and functionality in, everyday life. The RPSQ is a widely used tool to assess and quantify the presence of post-concussional complaints in head trauma patients and has been deployed by several previous studies (e.g. 10, 13, 22–24, 26, 28, 29). Similar to the RPSQ, the RHIFQ was developed by the Oxford Head Injury Service as an outcome measure focussing on social disability and has been used by multiple previous studies (e.g. 22, 24, 25, 29). The SF-36 has also been deployed successfully by multiple studies in the context of head trauma (e.g. 8, 23, 30, 31).
In the light of the controversy on the extent and duration of ongoing problems after mild head trauma, it was hoped that the data of this study would be able to help clarify questions on the time-line of symptomatic recovery after mild head trauma and how health complaints are reflected in everyday functionality and quality of life (QoL) during the first year post-injury. We hypothesized that, if prolonged health problems are associated with mild head trauma, such problems were likely to manifest equally in symptomatic complaints as well as decreased functional status and poorer QoL compared with healthy subjects, and that problems in these 3 domains would improve in parallel over the course of the first year post-injury. As the symptoms and complaints commonly associated with mild head trauma are not specific to this type of injury but may be observed in other clinical presentations, such as chronic pain, depression, anxiety disorders, substance abuse, whiplash (1, 3), and may further be affected by factors relating to litigation and monetary compensation (1), we aimed to exclude the influence of such factors through our patient selection criteria.
We further examined the influence of age, years of education, intellectual ability and clinical measures of injury severity on self-perceived health status in the first year post-injury. Whilst there is evidence supporting the contribution of age and lower educational level (1, 3, 6, 17) in determining poorer outcome after mCHI, there is little evidence for the impact of intellectual ability on shaping the perception of recovery after mCHI, contrasting the evidence available on this topic in more severe head trauma (32, 33). Similarly, studies on the relationship between clinical measures of injury severity such as Glasgow Coma Scale (GCS) and duration of post-traumatic amnesia (PTA) and outcome following mCHI are inconclusive (34). The current study, with a sample purely consisting of patients with mCHI and selection criteria aiming to mitigate the number of confounding factors affecting recovery after mild head trauma, provided an opportunity to re-examine the relationship of clinical measures of injury severity and intellectual ability with outcome in mCHI.
The present data were collected as part of a wider study examining abnormalities of eye and arm motor performance and neuropsychological function during the first 12 months after mild head trauma, evaluating the ability of these modalities to track functional recovery as well as predict outcome at an early stage after mCHI (35, 36).
Methods
Participants
Thirty-seven subjects (13 females and 24 males) with mCHI (GCS score 13: 4 cases, 14: 12 cases, 15: 21 cases) were recruited from persons presenting with acute head injury to Christchurch Hospital (the principal hospital for a regional population of over 400,000). The GCS used was the score on first assessment (i.e. the first recorded GCS post-injury). In most cases, this was at time of admission to the emergency department. In some cases, the GCS was first assessed by the ambulance team prior to arrival at the hospital. In order to be included in the study, patients had to have a score of between 13 and 15 on first assessment without falling below 13 at any consecutive assessment at the hospital. At the time of recruitment, no standardized method was in practice to assess duration of PTA in patients presenting with mild head injury to the emergency department at Christchurch Hospital and PTA duration was not routinely noted in patient files (apart from brief comments on the lack of recall of the injury-event if applicable). Hence, an iterative process was used to: (i) confirm that PTA was less than 24 h (the only required screening criterion) and (ii) provide an approximate duration of PTA (the GCS score was the principal factor for mCHI classification). At the initial pre-recruitment interview at the hospital, patients were asked about their first memory following the injury. If the remembered event fell within a 24-hour period, it was assessed whether the patient remembered being at the scene after the accident/regaining consciousness, being helped by others (e.g. extraction from a vehicle, somebody clearing their bicycle off the street or calling an ambulance), the arrival of the ambulance (if applicable, standard response time of ambulances within city borders taken as time approximation), being in the ambulance, arriving at the hospital (time was recorded on admission sheet as was time of accident in the case of motor vehicle accidents and most sport accidents), treatment events for which the time was noted on the patient chart, being served a meal (usually dinner or breakfast for patients who stayed overnight). All patients had experienced PTA ranging between 2 min and 22 h (median 15 min) and 32 had a confirmed loss of consciousness (LOC) (median 2.0 min, range 0.5–15 min). Duration of LOC was, in most cases, established from the available patient records. Most of the patients, in particular those involved in sports and motor vehicle accidents, had a witnessed LOC, with duration recorded in the ambulance/patient notes. Mean age was 29.1 years (standard deviation (SD) 12.7, range 15–56 years) and mean years of education 13.6 (SD 2.56, range 8–19 years). The injury causes included motor vehicle accidents (9 cases), bicycle accidents (8), rugby (6), horse riding (4), falls (6), netball (2), soccer (1) and roller blading (1). All patients were either employed or attended institutions for secondary or tertiary education and none was involved in litigation. Injury-related costs for the head injured participants were covered by the New Zealand Accident Compensation Corporation (ACC), a government-funded public insurer. Every New Zealand resident is automatically insured by ACC. ACC operates based on a “no-blame” policy and will pay for medical treatment costs, post-injury assessments, and provide monetary compensation to patients who are unable to return to work. To our knowledge, none of our participants were involved in any dispute or seeking monetary compensation beyond the standard provisions covered within the mandate of ACC. Before participating in the study, all prospective participants were made aware that their future healthcare or treatment, including access to free public healthcare and coverage by ACC, would not be affected by their decision whether or not to take part in the study.
Other potential participants were excluded if there was evidence of any influence of alcohol or psychoactive drugs at time of injury (because of possible distortion effects of alcohol/drugs on the GCS), regular intake of psychoactive drugs or history of drug abuse (influence of pyschoactive medications on eye movement control), central neurological disorder or psychiatric condition (several such conditions are known to affect eye movement patterns in particular), structural brain damage or haematoma on computed tomography (CT) head scan (where obtained), skull fractures, or prior history of mild, moderate or severe head injury with persisting symptoms or complaints (2 of the participants had a history of mCHI but were free of ongoing complaints, and none had any history of moderate or severe head trauma). These criteria were consistent with a previous study which examined the adverse impact of mild head trauma on eye and arm motor control specifically at one week post-injury (37).
The control group consisted of 37 subjects with no history of mild, moderate or severe head injury with persisting symptoms or complaints, no central neurological disorder or psychiatric condition, and no regular intake of psychoactive drugs or history of drug abuse. The controls were individually matched to each mCHI case with respect to age (within 3 years for patients > 18 years, within one year for patients < 18 years), gender and years of formal education (within 2 years for patients > 18 years, within one year for subjects < 18 years). The mean age for the control group was 29.2 years (SD 12.6, range 15–57 years) and mean years of formal education was 13.7 (SD 2.71, range 9–19 years). Controls were recruited via a volunteer database made available by the Department of Psychology at the University of Canterbury, Christchurch, New Zealand. These volunteers are interested in taking part in research studies and have agreed to be contacted for this purpose. In cases where a subject with head injury could not be matched with a control from the database, controls were recruited amongst siblings, relatives or friends of the head injured participant (patients were happy to suggest and contact potential controls having been explained the necessity for having a control and the matching criteria).
Throughout the study, none of the participants was hospitalized or developed secondary health problems related to other causes which could have affected any of the measures. All of the 37 participants with head injury had returned to work or school/study at the time of the 3 month follow-up. One of the patients had changed from a “hands-on” job to an administrative position in the same company due to increasing attacks of dizziness related to the level of physical activity in his previous position. Another patient took early retirement as his employer was “down- sizing”, but subsequently opened his own business. Subjects were offered compensation for travel costs to and from the hospital but received no other payment. The project was approved by the Canterbury Ethics Committee and written consent was obtained from all participants.
Health status questionnaires
On the RPSQ (26), the patients rated the presence and problem-status of 16 possible post-concussional symptoms on a scale from 0 to 4, comparing the presence and problem-status of each symptom with its pre-morbid status (0 = not experienced at all after the injury, 1 = experienced but no more of a problem compared with before the injury, 2 = a mild problem, 3 =a moderate problem, and 4 = a severe problem). Because the RPSQ asks the subject to rate the problem-status for the 16 symptoms “compared with before the injury”, the controls were given a slightly altered version of the RPSQ, wherein subjects were asked to rate the current incidence/problem-status for each of the 16 RPSQ items, but which contained no reference to the subject having sustained a head injury. The scale was equivalent to the standard RPSQ (i.e. 0 = not experienced at all, 1 = symptom was present but not perceived as a problem, 2 = symptom was present and perceived as a mild problem, 3 = symptom was present and perceived as a moderate problem, 4 = symptom was present and perceived as a severe problem). Subjects had to read the questions, select and mark their answers in presence of the examiner. Since the RPSQ refers to the presence of the listed symptoms (in relation to their pre-morbid status) over the last 24 hours, all subjects were asked to state whether their RPSQ answers could be considered representative of their current symptom status. This was indeed confirmed by all subjects.
On the RHIFQ (25), the patients rated their perceived change on 10 items of everyday activity on a scale from 0 to 4 (0 = no change compared with before the injury, 1 = no change, but somewhat more difficult, 2 = a mild change, 3 = a moderate change, and 4 = a very marked change compared with before the injury).
The SF-36v2 (27) was also administered as written questionnaire. Subjects read the questions and selected their answers in the presence of the examiner. The standard SF-36 form was used for assessments at 3, 6 and 12 months. As the standard form refers to the preceding 4-week time period in some of the question categories, the acute SF-36 form was used for the first assessment (in this form, the “4-week” time period is replaced by the term “1 week”). If less than a week had passed between injury and first assessment, patients were asked to refer only to the time period since injury when answering the questionnaire items. The questionnaires were scored according to the SF-36v2 manual. The key measure for each scale was the “Transformed Scale Score” with a best score of 100. In addition, there were 2 normed summary scores (centered around 50), termed Physical Function and Mental Function.
Assessment of IQ
The 2-subtest form of the Wechsler Abbreviated Scale of Intelligence (WASI©; The Psychological Corporation, San Antonio, USA), comprising the subtests Vocabulary Test and Matrix Reasoning, was administered to all patients and controls at one week and at 6 months post-injury. Standardized instructions were followed for the tests.
Procedures
Patients were assessed within one week of injury (mean 5.5 days (SD 3.0)) and then at 3 months (90 days (SD 5.5)), 6 months (182 days (SD 15)) and 12 months (365 days (SD 14)), completing the SF-36, RPSQ and RHIFQ each time. The controls completed the SF-36 4 times (i.e. same number of assessments at the same time intervals as the patients), and the RPSQ at least once (i.e. controls were asked to rate the current “problem-status” for each of the 16 RPSQ items using the same scale of 0 to 4). Ten controls completed the RPSQ multiple times; the examination of these controls (i.e. with multiple RPSQ reports) confirmed that the presence of post-concussional complaints amongst these 10 subjects was consistent and did not vary between assessments. Hence, the RPSQ data of the initial assessment of each control was accepted as a normal baseline for the incidence and degree of RPSQ symptoms in the control group. This baseline was used for the serial comparison with the mCHI group through the first 12 months. The RHIFQ was not completed by the controls as this questionnaire examines perceived change in ability to perform certain tasks as a result of having sustained a head injury and is linked to a specific trauma event as time reference.
Statistical analysis
Non-parametric Wilcoxon matched-pairs tests were used to compare the health status measures between the mCHI group and controls at one week, and at 3, 6 and 12 months post-injury. The analysis at 12 months included only 31 matched pairs, due to 6 patients not returning for their one-year follow up. Differences between groups were considered significant at a 2-tailed p value ≤ 0.05.
Stepwise-forward linear regression was applied to explore the role of age, IQ and years of formal education (pre-morbid factors) as well as injury severity (as measured by GCS, PTA and duration of LOC) in explaining variance in patients' scores on the questionnaires at one week as well as at 3, 6 and 12 months post-injury. Independent variables were the pre-morbid factors and clinical measures of injury severity. The dependent measures were the patients' scores on the SF-36 Physical and Mental Summaries and the totals on the RPSQ and RHIFQ throughout the year. Separate models were calculated for each of these 4 dependent measures at one week, 3, 6 and 12 months. The "F-to-enter” was 2.0, the “F-to-remove” 0.5. The interpretation of the strength of association of each model with the respective dependent variable was based on the adjusted R2, which takes into account the number of independent variables and measures the amount of variance in outcome explained by the in-model variables. The relative contribution of each of the respective in-model variables was quantified by standardized regression coefficients (beta). Normal-probability plots (plotting the residuals vs their expected normal value) calculated for the regression models showed that the residual values in all models were normally distributed. This confirmed that the distribution of variables in these models was sufficiently normal and that the relationships between the dependent and independent variables was appropriately linear, thereby providing a reliable quantification of the relationship between the respective independent measures and each dependent variable. As the impact of mCHI may alter the performance on IQ tests in the short-term, we used the WASI scores at 6 months post-injury as a measure of subjects’ IQ for the regression analyses. There was no difference in IQ between the patient group and the controls, either at one week or at 6 months (means at 6 months: IQ mCHI group: 114.1 (SD 12.3) vs controls: 115.9 (SD 10.6), p = 0.29).
Results
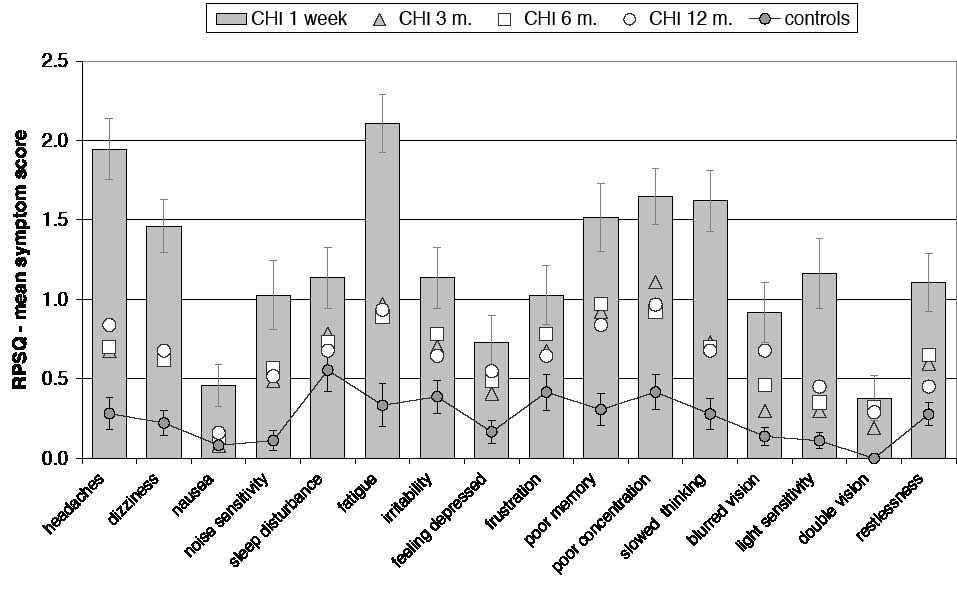
At one week post-injury, the mCHI group reported problems on all items of the RPSQ compared with their self-rated pre-injury symptom status (Fig. 1, Tables I and II). More than 91% of patients assigned a score of 2 or higher to one or more post-concussional symptoms on the RPSQ (a symptom with a score of 2 is more pronounced than pre-injury and is considered at least a mild problem by the reporting patient) and no patient was entirely symptom-free post-injury (i.e. scoring 0 on all symptoms) (in the control group of n = 37, 35% of subjects were entirely symptom-free). The most frequently reported symptoms at one week were fatigue, headaches, dizziness, poor concentration and slowed thinking (Table I). The patients had significantly higher scores on all RPSQ symptoms at one week compared with the control baseline (Fig. 1 and Table II).
| Table I. Frequency of symptoms/problems on Rivermead Post-Concussion Symptoms Questionnaire (RPSQ) and Rivermead Head-Injury Follow-up Questionnaire (RHIFQ) throughout the first year |
| | Patients reporting symptoms/problems (%) | Controls (%) |
| | 1 week | 3 months | 6 months | 12 months | | |
| Symptom / problem | Overall | Score of 2 or higher* | Overall | Score of 2 or higher* | Overall | Score of 2 or higher* | Overall | Score of 2 or higher* | Overall | Score of 2 or higher* |
| RPSQ | | | | | | | | | | |
| Fatigue/tiring more easily | 89 | 76 | 59 | 27 | 59 | 22 | 55 | 26 | 22 | 8 |
| Headaches | 86 | 68 | 46 | 14 | 46 | 22 | 52 | 26 | 24 | 3 |
| Dizziness | 84 | 43 | 35 | 16 | 41 | 19 | 39 | 23 | 22 | 3 |
| Poor concentration | 84 | 59 | 62 | 32 | 59 | 27 | 58 | 26 | 35 | 5 |
| Taking longer to think | 81 | 54 | 49 | 16 | 49 | 16 | 45 | 16 | 24 | 3 |
| Forgetfulness/poor memory | 76 | 41 | 51 | 27 | 54 | 27 | 61 | 16 | 24 | 8 |
| Restlessness | 65 | 30 | 41 | 11 | 43 | 14 | 35 | 3 | 30 | 0 |
| Frustration/Impatience | 62 | 24 | 43 | 19 | 57 | 16 | 35 | 16 | 35 | 5 |
| Irritability/easily angered | 59 | 35 | 46 | 19 | 51 | 22 | 42 | 13 | 32 | 8 |
| Sleep disturbance | 57 | 41 | 43 | 22 | 46 | 14 | 39 | 19 | 43 | 8 |
| Light sensitivity | 54 | 35 | 19 | 8 | 16 | 11 | 32 | 6 | 14 | 0 |
| Noise sensitivity | 49 | 30 | 32 | 14 | 32 | 16 | 29 | 13 | 11 | 3 |
| Feeling depressed | 49 | 14 | 27 | 11 | 38 | 11 | 32 | 19 | 16 | 3 |
| Blurred vision | 46 | 32 | 19 | 8 | 24 | 16 | 39 | 23 | 16 | 0 |
| Nausea/vomiting | 30 | 14 | 5 | 3 | 8 | 5 | 10 | 3 | 11 | 0 |
| Double vision | 19 | 14 | 14 | 5 | 16 | 11 | 19 | 6 | 0 | 0 |
| RHIFQ | | | | | | | | | | |
| Finding work more tiring | 86 | 73 | 41 | 8 | 41 | 14 | 35 | 23 | - | - |
| Maintaining previous work load | 84 | 65 | 38 | 8 | 32 | 14 | 19 | 10 | - | - |
| Enjoying previous leisure activities | 78 | 70 | 16 | 8 | 14 | 8 | 10 | 6 | - | - |
| Routine domestic activities | 65 | 46 | 8 | 3 | 8 | 5 | 10 | 3 | - | - |
| Participation in previous social activity | 65 | 41 | 11 | 3 | 11 | 5 | 3 | 3 | - | - |
| Coping with family demands | 62 | 27 | 27 | 5 | 19 | 8 | 19 | 10 | - | - |
| Conversation with 2 or more | 49 | 8 | 16 | 5 | 14 | 5 | 13 | 10 | - | - |
| Conversation with 1 person | 41 | 8 | 11 | 5 | 11 | 5 | 6 | 6 | - | - |
| Relationships with previous friends | 27 | 11 | 11 | 3 | 8 | 5 | 3 | 3 | - | - |
| Relationship with partner | 24 | 8 | 8 | 3 | 3 | 3 | 6 | 6 | - | - |
| *”Score of 2 or higher” = subjects who reported a score of 2, 3 or 4 for this symptom (scale = 0–4), i.e. the symptom was at least a mild problem. |
Fig. 1. Mean symptom scores on the Rivermead Post-Concussion Symptoms Questionnaire (RPSQ) throughout the first year post-injury. The control baseline includes the entire control group (n = 37). Error bars show standard errors. At one week, the mild closed head injury (mCHI) group had higher scores on all symptoms (see also Table II). At 3 months, the mCHI group had significantly higher mean scores (compared with the control baseline) on the symptoms headaches, dizziness, noise sensitivity, fatigue, poor memory, poor concentration, slowed thinking and double vision. At 6 months, the mCHI group continued to report, compared with the controls, higher scores on the symptoms headaches, dizziness, noise sensitivity, fatigue, irritability, depression, poor memory, poor concentration, slowed thinking, double vision and restlessness. At 12 months, significant group differences remained on the symptoms headaches, fatigue, poor memory, poor concentration, blurred vision and double vision.
| Table II. Mean symptom scores on the Rivermead Post-Concussion Symptoms Questionnaire |
| Symptom | Mild closed head injury |
| 1 week (n = 37) | 3 months (n = 37) | 6 months (n = 37) | 12 months (n = 31) | Controls (n = 37) | Controls 12 months sample (n = 31) |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Headaches | 1.9*** | 1.2 | 0.7* | 0.9 | 0.7** | 0.9 | 0.8* | 1.0 | 0.3 | 0.6 | 0.3 | 0.7 |
| Dizziness | 1.5*** | 1.0 | 0.6* | 1.0 | 0.6* | 0.9 | 0.7 | 1.0 | 0.2 | 0.5 | 0.3 | 0.5 |
| Nausea | 0.5* | 0.8 | 0.1 | 0.4 | 0.1 | 0.5 | 0.2 | 0.6 | 0.1 | 0.3 | 0.1 | 0.2 |
| Noise sensitivity | 1.0** | 1.3 | 0.5* | 0.8 | 0.6** | 1.0 | 0.5 | 1.0 | 0.1 | 0.4 | 0.1 | 0.4 |
| Sleep disturbance | 1.1* | 1.2 | 0.8 | 1.1 | 0.7 | 1.1 | 0.7 | 1.0 | 0.6 | 0.8 | 0.6 | 0.8 |
| Fatigue | 2.1*** | 1.1 | 1.0** | 1.0 | 0.9** | 0.9 | 0.9* | 1.1 | 0.3 | 0.8 | 0.4 | 0.9 |
| Irritability | 1.1** | 1.2 | 0.7 | 0.9 | 0.8* | 0.9 | 0.6 | 1.0 | 0.4 | 0.6 | 0.4 | 0.7 |
| Feeling depressed | 0.7* | 1.0 | 0.4 | 0.8 | 0.5* | 0.7 | 0.5 | 0.9 | 0.2 | 0.4 | 0.2 | 0.5 |
| Frustration | 1.0** | 1.1 | 0.7 | 0.9 | 0.8 | 0.9 | 0.6 | 1.1 | 0.4 | 0.7 | 0.4 | 0.7 |
| Poor memory | 1.5*** | 1.3 | 0.9** | 1.1 | 1.0** | 1.1 | 0.8* | 0.9 | 0.3 | 0.6 | 0.3 | 0.6 |
| Poor concentration | 1.6*** | 1.1 | 1.1** | 1.1 | 0.9* | 1.0 | 1.0* | 1.1 | 0.4 | 0.7 | 0.4 | 0.7 |
| Slowed thinking | 1.6*** | 1.2 | 0.7* | 1.0 | 0.7* | 0.9 | 0.7 | 0.9 | 0.3 | 0.6 | 0.3 | 0.6 |
| Blurred vision | 0.9** | 1.1 | 0.3 | 0.7 | 0.5 | 0.9 | 0.7* | 1.0 | 0.1 | 0.3 | 0.2 | 0.4 |
| Light sensitivity | 1.2*** | 1.3 | 0.3 | 0.7 | 0.4 | 0.9 | 0.5 | 0.8 | 0.1 | 0.3 | 0.1 | 0.3 |
| Double vision | 0.4* | 0.9 | 0.2* | 0.5 | 0.3* | 0.8 | 0.3* | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| Restlessness | 1.1*** | 1.1 | 0.6 | 0.9 | 0.6* | 0.9 | 0.5 | 0.8 | 0.3 | 0.4 | 0.3 | 0.4 |
| *p < 0.05, **p < 0.01, ***p < 0.001. SD: standard deviation. |
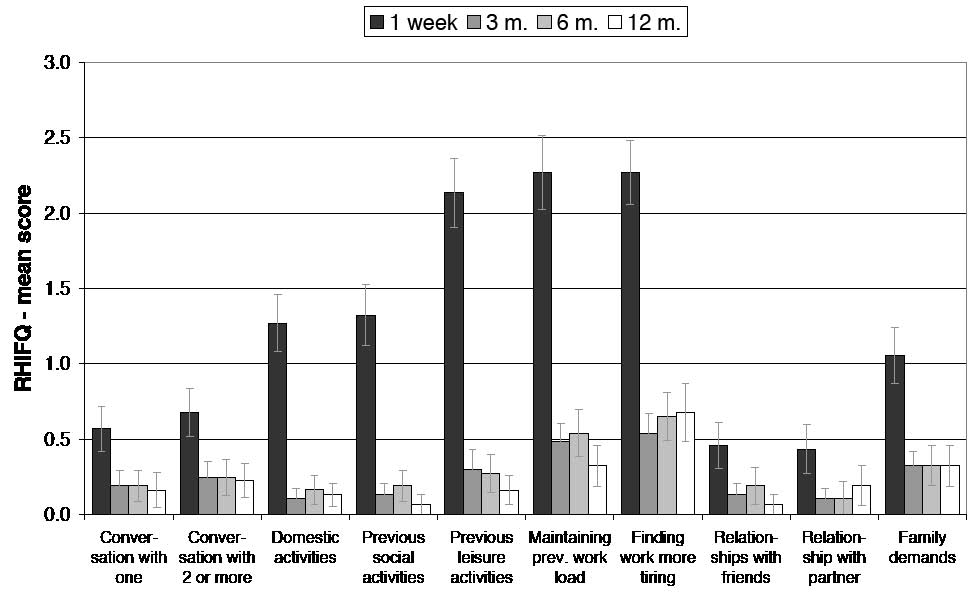
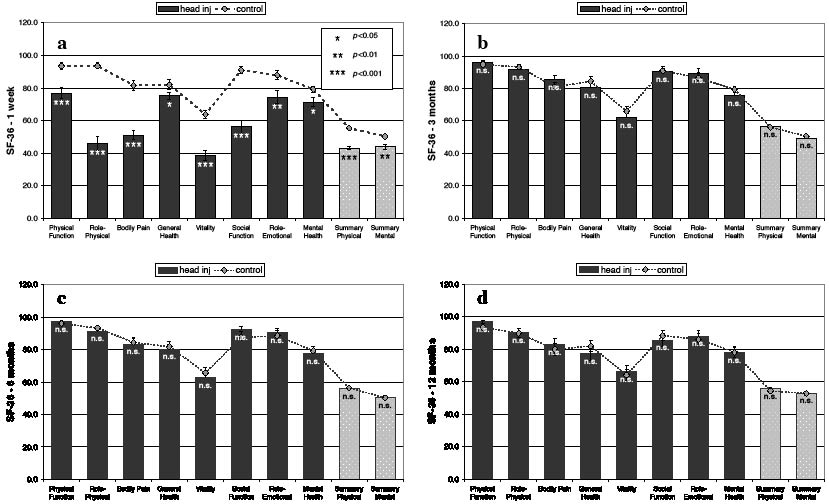
Similar to the findings on the RPSQ, the mCHI group reported an injury-related increase in problems with many activities on the RHIFQ at one week post-injury (Table I and Fig. 2), with 84% of patients reporting problems on RHIFQ items with a score of 2 or higher and only 3% of patients being entirely complaint-free on the RHIFQ at one week. Items most frequently associated with problems were: finding work more tiring, maintaining previous work load, enjoying previous leisure activities, problems with routine domestic activities and with participation in previous social activities (Table I). Similarly, the mCHI group had significantly lower scores on the SF-36 at one week post-injury (Fig. 3a), this including Physical Function (mCHI mean (SD): 76.89 (21.7) vs control 93.51 (12.0), p < 0.001), Role Physical (46.11 (26.4) vs 93.58 (11.4), p < 0.001), Bodily Pain (51.05 (17.7) vs 81.68 (19.2), p < 0.001), General Health (75.19 (14.1) vs 82.0 (18.0), p = 0.03), Vitality (38.68 (19.0) vs 63.68 (16.3), p < 0.001), Social Function (56.08 (23.5) vs 90.88 (13.7), p < 0.001), Role Emotional (74.32 (23.5) vs 87.84 (17.4), p = 0.007), Mental Health (71.22 (16.8) vs 79.19 (12.4), p = 0.048), Physical Summary (43.13 (7.5) vs 55.31 (5.2), p < 0.001) and Mental Summary (43.75 (8.5) vs 50.31 (7.8), p = 0.003).
At 3 months, the mCHI group had improved on the RPSQ, although 59% of patients continued to report symptoms with a score of 2 or higher and the incidence of many RPSQ symptoms remained high (Table I). Only 16% of patients were entirely symptom-free on the RPSQ at 3 months. The mCHI group continued to report significantly higher mean scores (compared with the control baseline level) on the symptoms headache, dizziness, noise sensitivity, fatigue, poor memory, poor concentration, slowed thinking and double vision (Fig. 1 and Table II). Conversely, the mCHI group had improved to normal levels (i.e. same as the controls) on the symptoms nausea, sleep disturbance, irritability, depression, frustration, light sensitivity, blurred vision and restlessness (Fig. 1 and Table II).
The mean scores on all RHIFQ items had improved markedly at 3 months compared with one week post-injury (Fig. 2). However, 19% of patients continued to report scores of 2 or higher on at least one of RHIFQ items (i.e. there was at least a mild change compared with pre-injury status), with only 32% of patients having no problems whatsoever on tasks listed on the RHIFQ. As observed at one week, work-related complaints, such as finding work more tiring and maintaining the previous work load, were the items most commonly associated with complaints at 3 months, followed by coping with family demands and enjoying previous leisure activities. In contrast to the problems documented by the patients’ scores on the RPSQ and RHIFQ at 3 months, the mCHI group had improved to normal on all SF-36 scales, having the same scores as the controls (Fig. 3).
Fig. 2. Mean scores of the patient group on the Rivermead Head Injury-Follow-up Questionnaire (RHIFQ) throughout the first year post-injury. At one week, the patients reported a considerable level of problems on most items. The consecutive sessions at 3, 6 and 12 months showed considerably lower (i.e. improved) mean scores, which, however, did not improve to 0 at any stage. Error bars show standard errors.
Fig. 3. SF-36 scales at: (a) one week, (b) 3 months, (c) 6 months, and (d) 12 months post-injury. Contrasting the markedly poorer scores of the mild closed head injury (mCHI) group on all scales at one week, ratings on the SF-36 had improved to normal levels at 3 months. The mCHI group continued to show a good recovery on all SF-36 scales at 6 months and remained at almost identical levels at 12 months post-injury. Error bars show standard errors.
ns: not significant.
At 6 months, over half (51%) of patients continued to report post-concussional symptoms on the RPSQ with a score of 2 or higher, while only 13% were entirely symptom-free. Although the mean scores of the mCHI group of most RPSQ symptoms had improved to below 1.0 (Fig. 1 and Table II), the mCHI group continued to report, compared with the control baseline level, significantly higher scores on the symptoms headache, dizziness, noise sensitivity, fatigue, irritability, depression, poor memory, poor concentration, slowed thinking, double vision and restlessness (Fig. 1 and Table II). At 6 months, 27% of patients reported problems on the RHIFQ with a score of 2 or higher, while 49% were entirely complaint-free. As observed at 3 months, the mCHI group did not differ from the controls on the SF-36 at 6 months (Fig. 3).
At 12 months, 52% of the 31 patients evaluated reported scores of 2 or higher on at least one post-concussional symptom on the RPSQ, while only 39% were entirely symptom-free. The patient mean scores on several RPSQ symptoms remained slightly elevated compared with the control baseline (Fig. 1 and Table II), these symptoms being headaches, fatigue, poor memory, poor concentration, blurred vision and double vision. However, 23% of the 31 patients continued to report scores of 2 or higher on RHIFQ items at 12 months, with 61% being entirely complaint-free on this questionnaire. Scores on the SF-36 continued to remain normal at 12 months, the mean scores of both groups being practically identical to the levels observed at 6 months (Fig. 3).
Age, education, IQ and clinical measures of injury severity
The results of the regression analysis with the factors age, years of education, IQ and clinical measures of injury severity (i.e. GCS, LOC, PTA) as independent variables showed that these factors could only explain a very limited amount of variance in patients’ scores on the SF-36 summary scores and the totals on the Rivermead questionnaires at any stage throughout the first year post-injury (Table III). The amounts of explained variance (equivalent to the adjusted R2 of each regression model) were 10–16% for the SF-36 Physical Summary, 7–32% for the SF-36 Mental Summary, 8–14% for the RPSQ total and 8–11% for the RHIFQ. In addition, several of the calculated models were unable to reach a significant relationship with the respective health status measure, this applying to the RHIFQ (one week and 3 months (no variables in model) and 12 months) and the RPSQ (one week (no variables in model) and 6 months), but also the SF-36 Mental Summary (3 and 6 months) and Physical Summary (12 months) (Table III). The profile of the in-model variables showed that most of the examined factors were not useful in terms of explaining variance in scores on the applied questionnaires throughout the first year post-injury.
| Table III. Associations of age, IQ, years of education and measures of injury severity with the Rivermead Post-Concussion Symptoms Questionnaire (RPSQ), Rivermead Head-Injury Follow-up Questionnaire (RHIFQ) and Short-Form 36 Health Survey (SF-36) |
| Dependent measures | Time delay post-injury |
| 1 week | | p | 3 months | | p | 6 months | | p | 12 months | | p |
| SF-36 Physical | Adjusted R² | 0.16 | 0.032 | Adjusted R² | 0.19 | 0.008 | Adjusted R² | 0.11 | 0.038 | Adjusted R² | 0.10 | 0.065 |
| Summary | In-model variables* | | | | | | | | | | | |
| | | Beta** | | | Beta | | | Beta | | | Beta | |
| | PTA | –0.47 | 0.012 | IQ | 0.46 | 0.008 | IQ | 0.37 | 0.038 | IQ | 0.37 | 0.065 |
| | GCS score | –0.28 | 0.123 | | | | | | | | | |
| | | | | | | | | | | | | |
| SF-36 Mental | Adjusted R² | 0.32 | 0.005 | Adjusted R² | 0.08 | 0.064 | Adjusted R² | 0.07 | 0.075 | Adjusted R² | 0.12 | 0.044 |
| Summary | In-model variables* | | | | | | | | | | | |
| | | Beta | | | Beta | | | Beta | | | Beta | |
| | GCS score | –0.42 | 0.010 | GCS score | –0.33 | 0.064 | IQ | 0.32 | 0.075 | IQ | 0.40 | 0.044 |
| | Age | 0.30 | 0.054 | | | | | | | | | |
| | IQ | 0.42 | 0.023 | | | | | | | | | |
| | Years of educ. | –0.36 | 0.052 | | | | | | | | | |
| | | | | | | | | | | | | |
| RPSQ | - | - | - | Adjusted R² | 0.12 | 0.030 | Adjusted R² | 0.08 | 0.109 | Adjusted R² | 0.14 | 0.033 |
| | In-model variables* | | | | | | | | | | | |
| | No variables in model | | | | Beta | | | Beta | | | Beta | |
| | | | | IQ | –0.38 | 0.030 | IQ | –0.31 | 0.085 | IQ | –0.42 | 0.033 |
| | | | | | | | LOC | –0.26 | 0.140 | | | |
| | | | | | | | | | | | | |
| RHIFQ | - | - | - | - | - | - | Adjusted R² | 0.11 | 0.034 | Adjusted R² | 0.08 | 0.094 |
| | In-model variables* | | | | | | | | | | | |
| | No variables in model | | | No variables in model | | | | Beta | | | Beta | |
| | | | | | | | IQ | –0.38 | 0.034 | IQ | –0.34 | 0.094 |
| *In-model variables = all independent variables that make unique, non-redundant contributions to explaining variance in the dependent measure. **Beta = standardized regression coefficients for in-model variables. PTA: post-traumatic amnesia; GCS: Glasgow Coma Scale; LOC: loss of consciousness. |
It was apparent from the composition of the in-model variables that, amongst the examined factors, IQ was the most useful independent variable, this measure being present in 11 of the 16 models. In particular in the context of explaining patients’ scores at 6 and 12 months, IQ appeared to be more useful than other variables, with IQ being the sole variable in 7 of the 8 “longer-term” models (i.e. examining report status at 6 and 12 months). Conversely, factors such as the GCS score, PTA, age and years of education were only present in models relating to “shorter-term” health status at one week and, in the case of the GCS, 3 months.
Discussion
This study is the first to have administered the RPSQ, RHIFQ and SF-36 in parallel after mCHI, pairing this with serial assessments in patients and matched controls over the course of one year post-injury. Through the year of follow-up, the questionnaires conveyed differing impressions of recovery. On the RPSQ, the mCHI group exhibited ongoing symptomatic complaints and significantly higher group scores on many symptoms compared with the controls up to 12 months post-injury. The RHIFQ appeared to convey better recovery on its 10 tasks of everyday function, whilst the SF-36 showed even better and quicker recovery so that, having reported markedly lower scores compared with the controls at one week, the mCHI group achieved normal scores at 3, 6 and 12 months. Hence, whilst our findings partially support previous notions of symptomatology persisting beyond the first few weeks post-injury, they do not confirm our hypothesis that ongoing health problems after mild head trauma should manifest equally in all 3 domains of recovery (i.e. symptomatic complaints, everyday functionality and QoL) assessed by the questionnaires applied in this study.
Comparison of the symptom levels and functional status of the current patient group with earlier studies shows that the incidence of symptoms on the RPSQ throughout the first 12 months was generally in agreement with other reports on the incidence of post-concussional symptoms and ongoing health problems during the first year after mCHI (6–12, 21, 22, 28). However, the variation in presence and degree of ongoing complaints after mild head trauma apparent from previous studies was mirrored in the present findings.
Judging by the “presence” of complaints in terms of percentages of patients experiencing problems, our results support previous notions that mild head trauma can be associated with ongoing health problems and persistent symptoms in 10–30% of patients with mCHI beyond 3 months post-injury (7–13, 21, 22). This conclusion is supported by the finding that statistically significant group differences remained between the group with mCHI and the non-injured controls on several symptoms of the RPSQ throughout the year post-injury, even up to 12 months post-injury. Although the ranking of the RPSQ symptoms most endorsed by the mCHI group slightly changed across the 4 assessments, poor concentration, fatigue, taking longer to think, poor memory and headaches were consistently amongst the “top 5” symptoms. This endorsement pattern contains several symptoms, such as fatigue, headaches and taking longer to think, frequently endorsed by patient groups in previous studies on mild head trauma (e.g. 16, 17), although other symptoms frequently reported after mild head trauma, such as irritability, did not rank quite as highly. Similarly, the mean scores of the RPSQ symptoms reported were comparable to previous findings from other longitudinal studies on mild head trauma (16, 22).
On the other hand, it has to be acknowledged that, in spite of persistent group differences, there was considerable improvement in the mean scores of the mCHI group on the RPSQ, and a marked improvement on the RHIFQ items between one week and 3 months post-injury. After 3 months post-injury, the means of many items on both the RPSQ and RHIFQ had fallen to or below 1.0. This indicates that while these complaints were present in many patients as evident from the data presented in Table I, and in the case of the RPSQ to a statistically significantly higher degree than in controls, the majority of patients did not necessarily rate these symptoms as a problem in their everyday functionality beyond 3 months post-injury. This aspect of our study is consistent with previous studies concluding that any clinically relevant symptoms or complaints should have resolved by 3 months after mild head injury (16–20). This interpretation is supported by the patients’ reports of good functionality and QoL evident from the SF-36, which showed markedly poorer health status of the group with mCHI at one week post-injury, but a lack of group differences at 3, 6 and 12 months. Yet, it was apparent that our mCHI group did much better on the SF-36 throughout the year compared with previous studies that have used the SF-36 after mild head trauma (8, 30, 31) and found long-term deficits on the SF-36 after mCHI in comparison with healthy controls (8).
The apparent contrast between the presence of symptoms on the RPSQ and the different impression of recovery portrayed by the same patients’ perception of everyday tasking and QoL on the RHIFQ and SF-36 show that apparently irreconciliatory aspects of recovery after mild head trauma found in the literature can be present in the same patient group. This may suggest that the variations in the literature on the occurrence and degree of persistent problems with post-concussional symptoms and problems on everyday tasks may not necessarily be mutually exclusive but reflect different, yet equally applicable, aspects of recovery after mild head trauma, consistent with Bernstein’s suggestion that “good recovery” after mCHI may actually present a behavioural adaptation rather than a return to pre-injury levels of functioning (4).
The multiple regression analyses aimed to determine the extent to which several factors might affect recovery, and perception of recovery, after mCHI. Based on the selection criteria for the participants in this study, we were able to exclude pre-morbid factors such as substance abuse, neurological disorders, litigation and problems with mental health such as the pre-injury presence of psychological or mood disorders as differentiating factors in the current group. Examination of the variance in scores on the applied questionnaires that can be explained by factors such as years of formal education, age and IQ suggested only a limited impact of these specific factors on self-reported health status, as none of these variables showed a strong relationship with self-reported health status across the first year. This was equally true for clinical measures of injury severity such as GCS, PTA and duration of LOC. GCS and PTA were only present in models relating to “shorter-term” QoL (i.e. the SF-36 summary scores) at one week and, in the case of the GCS, the SF-36 Mental Summary at 3 months. LOC was only present in one model relating to scores on the RPSQ at 6 months. These findings indicate a very limited association of clinical measures of injury severity with self-reported health status throughout the first year post-injury, and are consistent with evidence from several previous studies having shown no or very poor associations between outcome after mCHI and initial clinical measures of injury severity such as GCS (38), PTA (10, 17, 28) and LOC (1). Amongst the examined variables, IQ was a dominant factor in terms of associations with questionnaire scores beyond one week post-injury. This suggests that intellectual ability contributes more than the other factors to shaping the profile of self-reported health status after mCHI, especially when reporting the degree of symptoms or functional disability at 6 and more months post-injury.
The relationship between IQ and self-reported health status apparent in the present study indicates that the association of intellectual ability with return to work and rehabilitation outcome found after more severe head trauma (32, 33) also exists in mCHI. Our findings are consistent with reports by Luis et al. (39) who, in a retrospective study, examined the presence of post-concussional symptoms in a cohort of male veterans and found such symptoms to be more likely present in individuals with “lower intelligence”.
The current study has several limitations. Our patients did not undergo repeated clinical evaluation or expert rating (e.g. repeated examination by a neurologist or clinical psychologist) and, therefore, the responses on our health status measures were not quantified in terms of an external validation of problem status. Patients’ perception and subsequent report of symptoms on such health status measures is subject to a number of factors that influence perception of recovery but may not be related to the injury itself. “Post-concussive” symptoms and complaints are not specific to concussion, but can occur in chronic pain, depression, anxiety disorders, substance abuse and whiplash (1, 3), and can also be influenced by factors relating to litigation and monetary compensation (1). Our recruitment criteria aimed to mitigate the influence of such confounding factors. However, whilst such precautions were taken in recruiting our participants, caution may be advised in attributing symptoms beyond 3 months to the head trauma impact alone (16). Also, it has to be acknowledged that our control subjects reported very little health problems. Whilst this may be expected from healthy control subjects, there are a number of studies that examined the presence and intensity of post-concussional symptoms and complaints in healthy control samples and found that the rates of these symptoms can be quite high in the general population (1, 3). This, in turn, raises the question of whether our results are representative of the typical health status of patients having suffered a mild head injury. In addition, “expectation-factors” may contribute to the perception of symptoms after mild head trauma, these factors relating to what recovery after mild head trauma “should look like” as well as “the good old times” theory (40) whereby it is not uncommon for patients with mCHI to overestimate their pre-morbid health status when completing questionnaires as used in the present study. However, these limiting factors come with an intrinsic problem regarding their quantification in, and applicability to, any given sample of patients and controls. Whilst we consider it important to mention these limitations, it is also difficult to assess the extent to which any of the above factors contributed to the results of this study.
In conclusion, the current discrepancies between the presence of symptomatic complaints on the RPSQ and the relatively normal level of functionality and QoL conveyed by the RHIFQ and SF-36 suggest that recovery from mCHI can be subject to a dichotomy of experiencing (persistent) post-concussional symptoms whilst being able to achieve a good level of everyday functionality. This finding reiterates previous notions that “good recovery” after mCHI may involve a behavioural adaptation rather than a complete return to pre-injury health status, but it also indicates that nature and composition of questionnaires applied after mCHI may considerably influence the picture of recovery apparent from the patients’ perception of their health and functional status. This emphasizes the susceptibility of self-reported health status to factors relating to subjective self-awareness and self-evaluative accuracy, and that it might be warranted, particularly in patients reporting very high levels of persisting post-concussional symptoms and/or functional disabilities, to apply measures of brain function that are independent of patient self-report and unaffected by variables such as IQ, education and personal expectations about recovery after mild head trauma, such as imaging (14), electroencephalography (15) and screening for the presence of functional abnormalities by way of advanced (oculo)motor screening (35–37).
Acknowledgements
The study was hosted by the Canterbury District Health Board and the Christchurch School of Medicine & Health Sciences, University of Otago, New Zealand. Funding for patients’ travel costs was provided by the Neurological Foundation of New Zealand (NFNZ grant number 0027/SPG). We wish to thank these institutions for their support of this research.
References
1. Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004; 43: 84–105.
2. Anderson TJ, Heitger MH, Macleod AD. Concussion and mild head injury. Prac Neurol 2006; 6: 342–357.
3. Iverson GL. Outcome from mild traumatic brain injury. Curr Opin Psychiatry 2005; 18: 301–317.
4. Bernstein DM. Recovery from mild head injury. Brain Inj 1999; 13: 151–172.
5. Mittenberg W, Canyock EM, Condit D, Patton C. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol 2001; 23: 829–836.
6. Evans RW. The postconcussion syndrome and the sequelae of mild head injury. Neurol Clin 1992; 10: 815–847.
7. van der Naalt J, van Zomeren AH, Sluiter WJ, Minderhoud JM. One year outcome in mild to moderate head injury: the predictive value of acute injury characteristics related to complaints and return to work. J Neurol Neurosurg Psychiatry 1999; 66: 207–213.
8. Emanuelson I, Andersson Holmkvist E, Bjorklund R, Stålhammar D. Quality of life and post-concussion symptoms in adults after mild traumatic brain injury: a population-based study in western Sweden. Acta Neurol Scand 2003; 108: 332–338.
9. Stålnacke BM, Björnstig U, Karlsson K, Sojka P. One-year follow-up of mild traumatic brain injury: post-concussion symptoms, disabilities and life satisfaction in relation to serum levels of S-100B and neurone-specific enolase in acute phase. J Rehabil Med 2005; 37: 300–305.
10. Bazarian JJ, Wong T, Harris M, Leahey N, Mookerjee S, Dombovy M. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj 1999; 13: 173–189.
11. De Kruijk JR, Leffers P, Menheere PPCA, Meerhoff S, Rutten J, Twijnstra A. Prediction of post-traumatic complaints after mild traumatic brain injury: early symptoms and biochemical markers. J Neurol Neurosurg Psychiatry 2002; 73: 727–732.
12. Thornhill S, Teasdale GM, Murray GD, McEwen J, Roy CW, Penny KI. Disability in young people and adults one year after head injury: prospective cohort study. BMJ 2000; 320: 1631–1635.
13. Sterr A, Herron KA, Hayward C, Montaldi D. Are mild head injuries as mild as we think? Neurobehavioral concomitants of chronic post-concussion syndrome. BMC Neurol 2006; 6: article 7. Available from: http://www.biomedcentral.com/1471-2377/6/7
14. McAllister TW, Sparling MB, Flashman LA, Saykin AJ. Neuroimaging findings in mild traumatic brain injury. J Clin Exp Neuropsychol 2001; 23: 775–791.
15. Duff J. The usefulness of quantitative EEG (QEEG) and neurotherapy in the assessment and treatment of post-concussion syndrome. Clin EEG Neurosci 2004; 35: 198–209.
16. Kashluba S, Paniak C, Blake T, Reynolds S, Toller-Lobe G, Nagy J. A longitudinal, controlled study of patient complaints following treated mild traumatic brain injury. Arch Clin Neuropsychol 2004; 19: 805–816.
17. Ponsford J, Willmott C, Rothwell A, Cameron P, Kelly AM, Nelms R, et al. Factors influencing outcome following mild traumatic brain injury in adults. J Int Neuropsychol Soc 2000; 6: 568–579.
18. Dikmen S, McLean A, Temkin N. Neuropsychological and psychosocial consequences of minor head injury. J Neurol Neurosurg Psychiatry 1986; 49: 1227–1232.
19. Macciocchi SN, Barth JT, Alves W, Rimel RW, Jane JA. Neuropsychological functioning and recovery after mild head injury in collegiate athletes. Neurosurgery 1996; 39: 510–514.
20. Pellman EJ, Lovell MR, Viano DC, Casson IR. Concussion in professional football: recovery of NFL and high school athletes assessed by computerized neuropsychological testing – part 12. Neurosurgery 2006; 58: 263–274.
21. Rutherford WH, Merrett JD, McDonald JR. Symptoms at one year following concussion from minor head injuries. Injury 1979; 10: 225–230.
22. Lundin A, Boussard C, Edman G, Borg J. Symptoms and disability until 3 months after mild TBI. Brain Inj 2006; 20: 799–806.
23. Stulemeijer M, van der Werf S, Bleijenberg G, Biert J, Brauer J, E Vos P. Recovery from mild traumatic brain injury: a focus on fatigue. J Neurol 2006; 253: 1041–1047.
24. Nolin P, Villemure R, Heroux L. Determining long-term symptoms following mild traumatic brain injury: method of interview affects self-report. Brain Inj 2006; 20: 1147–1154.
25. Crawford S, Wenden FJ, Wade DT. The Rivermead head injury follow up questionnaire: a study of a new rating scale and other measures to evaluate outcome after head injury. J Neurol Neurosurg Psychiatry 1996; 60: 510–514.
26. King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 1995; 242: 587–592.
27. Ware JE, Jr., Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol 1998; 51: 903–912.
28. Ingebrigtsen T, Waterloo K, Marup-Jensen S, Attner E, Romner B. Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J Neurol 1998; 245: 609–612.
29. Wade DT, King NS, Wenden FJ, Crawford S, Caldwell FE. Routine follow up after head injury: a second randomised controlled trial. J Neurol Neurosurg Psychiatry 1998; 65: 177–183.
30. De Kruijk JR, Leffers P, Meerhoff S, Rutten J, Twijnstra A. Effectiveness of bed rest after mild traumatic brain injury: a randomised trial of no versus six days of bed rest. J Neurol Neurosurg Psychiatry 2002; 73: 167–172.
31. Paniak C, Phillips K, Toller-Lobe G, Durand A, Nagy J. Sensitivity of three recent questionnaires to mild traumatic brain injury-related effects. J Head Trauma Rehabil 1999; 14: 211–219.
32. Powell JM, Machamer JE, Temkin NR, Dikmen SS. Self-report of extent of recovery and barriers to recovery after traumatic brain injury: a longitudinal study. Arch Phys Med Rehabil 2001; 82: 1025–1030.
33. Ip RY, Dornan J, Schentag C. Traumatic brain injury: factors predicting return to work or school. Brain Inj 1995; 9: 517–532.
34. van der Naalt J. Prediction of outcome in mild to moderate head injury: a review. J Clin Exp Neuropsychol 2001; 23: 837–851.
35. Heitger MH, Jones RD, Dalrymple-Alford JC, Frampton CM, Ardagh MW, Anderson TJ. Motor deficits and recovery during the first year following mild closed head injury. Brain Inj 2006; 20: 807–824.
36. Heitger MH, Jones RD, Dalrymple-Alford JC, Frampton CM, Ardagh MW, Anderson TJ. Mild head injury – a close relationship between motor function at one week post-injury and overall recovery at three and six months. J Neurol Sci 2007; 253: 34–47.
37. Heitger MH, Anderson TJ, Jones RD, Dalrymple-Alford JC, Frampton CM, Ardagh MW. Eye movement and visuomotor arm movement deficits following mild closed head injury. Brain 2004; 127: 575–590.
38. Balestreri M, Czosnyka M, Chatfield DA, Steiner LA, Schmidt EA, Smielewski P, et al. Predictive value of Glasgow Coma Scale after brain trauma: change in trend over the past ten years. J Neurol Neurosurg Psychiatry 2004; 75: 161–162.
39. Luis CA, Vanderploeg RD, Curtiss G. Predictors of postconcussion symptom complex in community dwelling male veterans. J Int Neuropsychol Soc 2003; 9: 1001–1015.
40. Gunstad J, Suhr JA. “Expectation as etiology” versus “the good old days”: postconcussion syndrome symptom reporting in athletes, headache sufferers, and depressed individuals. J Int Neuropsychol Soc 2001; 7: 323–333.