OBJECTIVE: To identify the impairments and limitations that indicate loss of independence in older patients after discharge from post-acute rehabilitation.
DESIGN: Prospective cohort study.
SUBJECTS/PATIENTS: A total of 128 patients in the development cohort and 137 patients in the validation cohort.
methods: Data on functioning and previous living situation were collected at admission; data on prospective living situation were collected at discharge. Multivariable logistic and non-parametric (CART) analyses were carried out with the development cohort. The resulting models were validated in a validation cohort.
RESULTS: Development cohort: mean age was 80.3 years (95% confidence interval (CI) 79.1–81.6), 52% of patients experienced loss of independence. The International Classification of Functioning, Disability and Health (ICF) category d465 “moving around using equipment” (odds ratio (OR) = 2.7, 95% CI 1.2–5.8) and a dichotomous indicator variable for fractures or joint replacement (OR = 2.3, 95% CI 1.1–4.9) remained in the logistic model. CART yielded the ICF categories d465 “moving around using equipment”, and b765 “involuntary movement functions” conditional on d465. Validation cohort: mean age was 72.6 years (95% CI 70.3–74.9). 40% experienced loss of independence. d465 (OR = 7.6, 95% CI 1.6–35.5) and b765 (OR = 5.9, 95% CI 2.6–13.4) were also significant predictors in the logistic model.
CONCLUSION: Older patients who are not able to move around with the help of equipment at the beginning of post-acute
rehabilitation are 3 times as likely to lose independence when discharged. It may be important for patients’ independent living to encourage the use of wheelchairs and walking aids at very early stages of rehabilitation.
Key words: aged, rehabilitation, disability, outcome assessment (healthcare), ICF.
J Rehabil Med 2007; 39: 591–597
Correspondence address: Gerold Stucki, Department of Physical Medicine and Rehabilitation, Ludwig Maximilian University, Marchioninistr. 15, DE-81377 München, Germany. E-mail: gerold.stucki@med.uni-muenchen.de
Submitted October 16, 2006; accepted March 26, 2007
Introduction
The objectives of post-acute rehabilitation in older patients are to sustain physical functioning during the acute episode of illness and to initiate the restoration of physical functioning as early as possible (1). The broader goals are to prevent disability, to maintain or restore patients’ autonomy and to prevent the need for long-term care (2). Therefore, independence, i.e. the possibility to continue living at home, is one of the major outcomes concerning older people.
With growing numbers of frail older persons and decreasing financial resources of the health system, there is an increasing need for efficient geriatric rehabilitation services. Loss of independence and the prolonged need for nursing care implies both decreased quality of life and increased costs (3, 4). Thus, by analysing indicators for loss of independence, treatment targets can be set at the beginning of the rehabilitation process and efficient discharge planning can be arranged. This includes conceivable needs, such as modifications of the home environment or prolonged follow-up and assistance (5).
Many different indicators for independence after discharge have been identified. They include physiological, socio-
demographic, and psychosocial indicators, such as age, primary diagnosis, number of co-morbidities, emotion and cognition (3, 6–8). Equally, parameters of functioning, such as urinary incontinence, visual impairment, and impaired mobility, are associated with the need for nursing care after discharge (9).
Therefore, functioning at the beginning of rehabilitation therapy, usually assessed by measures of activities of daily living and self-care, such as the Barthel Index, or the Functional Independence Measure (FIMTM), can predict if the patient will be able to live independently (3, 10–12). For example, patients after stroke with low FIMTM scores at admission had a 12-fold increased risk of loss of independence after their discharge from rehabilitation, i.e. the need for additional assistance or placement in a nursing home (13). Nevertheless, the specific components of patients’ functioning associated with loss of independence are not known. More detailed models for discharge destination are therefore warranted.
The International Classification of Functioning, Disability and Health (ICF) (14) categories potentially facilitate the description and classification of all aspects of function and health in individuals, independent of a specific instrument (15). Based on the ICF a so-called geriatric ICF Core Set was developed by a formal decision-making and consensus process (16, 17). This ICF Core Set (see Appendix) is a selection of categories out of the whole classification that can serve as the minimal standard for the assessment and reporting of functioning and health.
The objective of this study was to develop a model for loss of independence after discharge based on the ICF Core Set for post-acute geriatric rehabilitation facilities. More specifically, the objective was to identify the specific and readily identifiable impairments and limitations that indicate loss of independence and that may be amenable to intensified rehabilitation interventions.
Methods
Study design
Development cohort. As development cohort a convenience sample of older patients requiring rehabilitation care in a post-acute rehabilitation facility was included. Patients were recruited from the geriatric rehabilitation hospital of the Arbeiterwohlfahrt Bezirksverband Unterfranken e.V. Würzburg between July and December 2002. This freestanding hospital is a model project of the Federal Republic of Germany and the State of Bavaria for the rehabilitation of elderly people and is equipped with 84 in-patient beds on 3 wards.
Validation cohort. The validation cohort comprised a convenience sample of patients admitted to 5 post-acute rehabilitation facilities. Patients were recruited from the geriatric rehabilitation hospital Würzburg and 4 rehabilitation wards of acute hospitals in the cities of München, Ingolstadt and Nürnberg between July 2004 and December 2005. Those 4 wards specialize in post-acute rehabilitation, but do not focus specifically on older patients. They are equipped with 10–40 beds each.
For both cohorts informed consent was obtained from patients or, if the responsible physician indicated that a patient was unable to make an informed decision, from the patient’s carer. Criteria for admission to each facility were old age, need and capacity for rehabilitation (assessed by the responsible physician at the acute hospital), patients’ consent and reimbursement provided. Approval was obtained from the institutional ethics committees.
Measures
Data were collected by interview within the first 14 days after admission (mean 4.6, standard deviation (SD) 3.4) with a standardized case record form.
The ICF has 2 parts, each divided into 2 components. Part 1 covers: 1. Body Functions (b), Body Structures (s) and 2. Activities and Participation (d).
Part 2 covers contextual factors including the components: 1. Environmental Factors (e) and 2. Personal Factors. The geriatric ICF Core Set is a list of categories of the ICF that were chosen in a multi-stage consensus process on which aspects of functioning are relevant for aged patients, integrating evidence from empirical studies and input from experts (17). The categories were coded 1 if any limitation/restriction/impairment was present and 0 if absent. Although there is no valid operational specification of the ICF categories at the moment, there is empirical evidence that ICF categories yield valid results if this dichotomized qualifier is used (18). Length of stay (LOS), medical diagnosis, the patients’ prior living conditions and discharge living conditions were collected from the hospital records. Candidate variables for inclusion into a multiple regression model included the 123 categories of the geriatric ICF Core Set, age, gender, LOS, and diagnosis leading to rehabilitation.
Loss of independence was defined as follows: if the patient was discharged home but needed more assistance than before, if the patient had to move either to a carer’s household, or to an adult or nursing home, or if the patient was transferred to another rehabilitation facility or to acute medical care. A patient who had already been at a nursing home prior to admission to the rehabilitation facility was not defined as having lost independence.
Statistical analysis
Model development. To screen for potential predictors we used bivariate χ2 tests and t-tests exploring the association between independent variables and the dependent variable (loss of independence/stable living situation). A variable would be a candidate for entering the regression model if it had a p-value < 0.20 in the bivariate test. To avoid collinearity, variables would be selected for entering the multiple logistic model only if the Spearman correlation coefficient was < 0.5. Between 2 correlated variables, the variable with the stronger association with the dependent variable would enter the model.
A logistic model was then used to select the final set of predictors based on backward elimination (p < 0.05 to remove).
Model fit was tested by the Hosmer-Lemeshow statistic, which should be non-significant (p > 0.05) to maintain the null hypothesis of adequate fit (19). Predictive power of the logistic model was determined using Receiver Operating Characteristics (ROC) curves (20). The ROC curve indicates how well any model is able to distinguish between events and non-events, yielding the c-value as a measure for the area under the curve. By definition, the c-value varies between 0.5 and 1; the higher the c-value the better the model.
Model validation. Logistic regression poses several challenges. There are few diagnostic procedures to assess model fit within one sample. Variable selection becomes difficult in case of collinearity, which is to be expected in human functioning, and detection of interaction, which is also to be expected in the case of many variables, may become onerous, yielding non-intuitive interpretations of the interaction parameters. Non-parametric regression methods, such as Classification and Regression Trees (CART), work without assumptions on the underlying data and simplify the identification of interactions and specific risk groups (21).
We therefore followed a stepwise strategy for model validation. We first applied the CART procedure to the development sample. CART divides a population into several subpopulations depending on certain characteristics. Subpopulations are as homogenous as possible with regard to the outcome variable; here the outcome was discharge to the same living situation as before. There are many different ways to construct CART. We employed the technique proposed by Lausen et al. (22). In brief, the data set is partitioned according to the predictor variable with the smallest p-value. After the partitioning the subsets are reconsidered for partitioning based on the remaining predictor variables. This algorithm is repeated until a pre-set stop criterion is reached. The recursive partitioning strategy results in a tree, where the root is the whole data set and the leaves are the final subsets, which are as homogenous as possible with regard to the dependent outcome variable. We defined the following stop criterion: the partitioning would have to stop if the significance level of the best split exceeded 0.10. To avoid spurious results we additionally defined that subsets with less than 25 persons should not be considered for a further split. Model performance was evaluated by a ROC curve. The SAS macro %treedisc (23) was used to create the tree.
We hypothesized that CART would show hidden interactions not detected by logistic regression. Based on the variables retained in the CART model, a second logistic model was built and its model fit was evaluated.
Furthermore, the resulting models were evaluated in a new dataset. A different population was used to gain information on external validity of the models.
Statistical analyses were carried out with SAS for Windows version 9.1 (SAS Institute, Inc., Cary, NC, USA).
Results
Model development
A total of 128 patients were included in the development phase of this study, 52% of whom experienced loss of independence. Patients’ ages ranged from 62 to 98 years, with a median of 81 years. The mean age in the sample was 80.3 years (95% CI 79.1–81.6), mean length of stay was 31.2 days (95% CI 28.4–34.0). Women represented 69% of the patients. The most common reasons for admission were simple or complex fractures and joint replacement (43%), cerebrovascular conditions, mainly stroke (25%), cardiovascular and oncological conditions (33%). All patients had at least one additional diagnosis with a mean of 6.5 (95% CI 5.9–7.1 (SD 3.5)). Before the event leading to rehabilitation most patients (98%) lived in at home, while 2% lived in nursing homes.
Table I shows the sociodemographic characteristics of the study population stratified by living situation after discharge (worse/stable).
| Table I. Sociodemographic characteristics of development and validation sample |
| | Total | Living situation stable | Loss of independence |
| Development sample, n | 128 | 61 | 67 |
| Mean age (SD) | 80.3 (7.2) | 79.7 (6.7) | 80.8 (7.6) |
| Female gender, % | 69 | 69 | 69 |
| Musculoskeletal condition, % | 43 | 36 | 48 |
| Validation sample, n | 137 | 82 | 55 |
| Mean age (SD) | 72.6 (13.4) | 73.6 (13.8) | 71.1 (12.7) |
| Female gender, % | 54 | 63 | 40 |
| Musculoskeletal condition, % | 30 | 34 | 24 |
| SD: standard deviation. |
Eighteen variables met the inclusion criteria for the multivariable logistic model (see Table II). Categories b110, b176 and b450 were not included in the logistic model because of zero cell count. As a rule, a logistic model should hold at least 10 events per parameter included (24). We therefore started with 2 subsets of the 18 variables, one consisting of the categories from the component Body Functions, one consisting of the categories from the component Activities and Participation. Of all included variables, only the ICF category d465 “moving around using equipment” controlled for diagnosis (dichotomous indicator variable for fractures of joint replacement – musculoskeletal condition (msk)) remained in the final model (see Table III for parameter estimates) after backward selection. Patients with limitations in moving around using equipment had a three-fold increased risk of experiencing a loss of independence after discharge. These estimates did not change substantially when using age or gender as forced-in variables. Two-way interactions were not significant. The model fit was adequate (Hosmer-Lemeshow statistic p = 0.5891, c-value = 0.649).
| Table II. Details on variables selected for logistic regression. A variable would be candidate for entering the regression model if it had a p-value of < 0.20 in the bivariate test. Percentages are percentages of patients with impairment or limitation |
| ICF Code | Description | Total (n) | Total (%) | Living situation stable (%) | Loss of
independence (%) |
| – | Musculoskeletal diagnosis | 128 | 42 | 40 | 59 |
| b110 | Consciousness functions* | 127 | 3 | 0 | 6 |
| b147 | Psychomotor functions | 120 | 23 | 14 | 31 |
| b167 | Mental functions of language | 124 | 9 | 5 | 12 |
| b176 | Mental functions of sequencing complex movements* | 125 | 3 | 0 | 6 |
| b230 | Hearing functions | 124 | 35 | 25 | 42 |
| b260 | Proprioceptive functions | 118 | 19 | 14 | 24 |
| b320 | Articulation functions | 127 | 14 | 10 | 18 |
| b410 | Heart functions | 126 | 51 | 57 | 45 |
| b420 | Blood pressure functions | 126 | 77 | 83 | 71 |
| b450 | Additional respiratory functions* | 123 | 2 | 0 | 5 |
| b530 | Weight maintenance functions | 119 | 50 | 59 | 41 |
| b735 | Muscle tone functions | 97 | 44 | 36 | 52 |
| d130 | Copying | 99 | 15 | 9 | 21 |
| d315 | Communicating – receiving nonverbal messages | 121 | 12 | 5 | 17 |
| d335 | Communicating – producing non-verbal messages | 125 | 18 | 12 | 24 |
| d465 | Moving around with equipment | 117 | 36 | 26 | 45 |
| d570 | Looking after one’s health | 118 | 28 | 21 | 36 |
| d860 | Basic economic transactions | 84 | 25 | 17 | 31 |
| *Did not enter the logistic model because of zero cell count. |
| Table III. Model parameter estimates |
| Model | Variables included | Odds ratio | 95% CI |
| Development sample | | | |
| Logistic model | Moving around with equipment (d465) | 2.7 | 1.2; 5.8 |
| | Musculoskeletal condition (msk) | 2.3 | 1.1; 4.9 |
| Logistic model with CART variables | Moving around with equipment (d465) | 3.4 | 1.5; 7.9 |
| | Non-voluntary movement functions*) (b765) | 3.0 | 1,1; 8.1 |
| Validation sample | | | |
| Logistic model | Moving around with equipment (d465) | 10.4 | 2.3; 46.5 |
| | Musculoskeletal condition (msk) | 0.6 | 0.3; 1.3 |
| Logistic model with CART variables | Moving around with equipment (d465) | 7.6 | 1.6; 35.5 |
| | Non-voluntary movement functions (b765) | 5.9 | 2.6; 13.4 |
| (coding: 1 = impaired/limited, 0 = not impaired/not limited) *Conditional on d465 = 0. CI: confidence interval; CART: Classification and Regression Trees. |
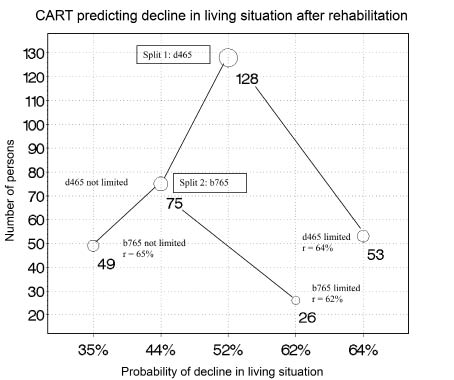
CART yielded a classification tree displayed in Fig. 1. First split was by ICF category d465 “moving around using equipment”. The subset with patients with limitations in this category yielded no further split. Sixty-four percent of patients experienced a loss of independence in this group. The subset with patients without limitation in this category could be further split into a group whose involuntary movement functions were impaired (category b765, proportion of worse living situation: 62%) and a group without impairment in involuntary movement functions (proportion of worse living situation: 35%). No further split could be made.
Fig. 1. Classification and Regression Tree (CART) splitting the development sample into subpopulations that are as homogenous as possible with regard to the outcome variable. Splits could be made for the variables moving around with equipment (d465) and involuntary movement functions (b765). This indicates that persons with limitations in d465 have the highest risk to experience loss of independence/decline in living situation, whereas persons who are neither limited in d465 nor in b765 have the smallest risk. Bubble size and numbers next to the bubbles indicate the number of persons in a specific subgroup. For each subpopulation the classification accuracy r (number of correctly classified cases out of the total number of cases) is given.
A second logistic regression model to confirm the results of the CART model contained the independent variable d465 and a new variable that was constructed to mirror the interaction with b765 conditional on d465. This new variable was set to 0 in patients with limitation in d465 and set to equal b765 for patients without limitation in d465. All terms were significant (see Table II for parameter estimates). This model displayed similar fit and calibration (Hosmer-Lemeshow statistic p = 0.9998, c-value = 0.643).
Model validation
The model was validated on a cohort of 137 patients. 54% were female, 40% experienced loss of independence. Mean age was 72.6 days (age range 23–92 years, 95% CI 70.3–74.9), mean LOS was 27.2 (95% CI 25.2–29.2). Thirty percent of the patients had a msk.
The logistic model with the independent variables d465 and a dichotomous indicator variable for fractures of joint replacement (msk) yielded a significant parameter estimate for d465 but not for msk. The estimates did not change substantially when using age or gender as forced-in variables. Parameter estimates are displayed in Table II (Hosmer-Lemeshow statistic p = 0.7235, c-value = 0.656). The logistic model with the variables d465 and the interaction variable yielded a significant parameter estimate for d465 but not for the interaction (Hosmer-Lemeshow statistic p = 0.9995, c-value = 0.623). Including d465 and b765 as main effects resulted in significant parameter estimates for both variables (Hosmer-Lemeshow statistic p = 0.8742, c-value = 0.764). Parameter estimates are shown in Table II.
Discussion
This study has demonstrated that older patients’ ability to move around with the help of equipment at the beginning of the post-acute rehabilitation process was associated with regained autonomy after discharge. The ICF more precisely defines this category d465 as “moving the whole body from place to place … by using specific devices designated to facilitate moving”. The ICF allows categories of the component Activities and Participation to be coded as activity (a), the execution of a task or action, or as participation (p), the involvement in a life situation, or both. In this case, d465 would be defined rather as a465, since it would be assessed at the onset of rehabilitation as the execution of a single task. This was the only factor having an independent and stable effect on discharge destination across different patient populations. Additionally, the validation models revealed an increased risk for nursing home placement in patients with age- or disease-related involuntary movement disorders, such as tremor (ICF category b765).
In patients after stroke, the ability to propel a wheelchair shortly after admission was shown to be associated with the ability to walk by the time of discharge (25, 26). Several previous studies have shown that impaired mobility influences activities of daily living and predicts morbidity and mortality (27). In particular, the association between the ability to walk and independent living has been reported previously (28, 29). Also, stroke patients with weakness in the lower extremities, thus experiencing limitations in mobility, are at higher risk of being discharged to a nursing home than patients without this weakness (30). Involuntary movement disorders, such as tremor, may indicate frailty and impede mobility and efficient use of assistive devices.
Our study illustrates the importance of assistive devices for human functioning. An individual may not be able to move without aids. Nevertheless, products to enhance mobility are a major key to his or her independent living. The findings regarding the importance of assistive devices is in line with the literature showing that assistive devices may reduce disability to a greater extent than personal assistance, especially for tasks involving the lower extremities and body transfer (31).
While mobility predisposes independent living, it remains open whether appropriate interventions regarding the use of assistive devices would also translate into better functioning. The potential for interventions aiming at mobility with aids cannot be answered based on our study. Indeed, evidence is scarce on how any kind of assistive device improves functioning and reduces the risk of institutionalization in geriatric rehabilitation (32). Equally, mobility with the help of equipment may be only a proxy for other, more fundamental, body functions, which could be enhanced by specific interventions.
The predictive power of cognition and emotional status has been discussed controversially in the literature (7, 33). We did not find evidence that cognitive impairment or depression influence the loss of independence. The patients in our study, however, were not necessarily representative of the geriatric rehabilitation population with respect to cognitive status, since more than two-thirds of them had at least average cognitive capacity.
The underlying findings can therefore be generalized only for older patients without severe cognitive impairment or symptoms of depression.
In this paper we have illustrated how CART analysis can support and indeed improve logistic regression analysis because it unravels interactions that would never have been detected by regression techniques. The interaction of category b765 with d465 conditional on d465 would not appear in any stepwise variable selection procedure. Based on the results of the CART analysis it may then be possible to introduce appropriate interaction terms and therefore achieve a more comprehensive regression model, for example in the context of clinical prediction. Although this interaction could not be reproduced in the validation data set, its main effects remained significant, indicating that there are several components of mobility that determine patient autonomy.
Several potential limitations merit consideration. First, the sample was not drawn at random; this study was conducted on a small number of patients, and reflects the experience of a single rehabilitation hospital. Thus, the results may not be representative for other facilities. Nevertheless, the distribution of diagnosis, age and age-related impairments reflect the typical situation found in a geriatric rehabilitation setting (8, 34). Thus it is likely that the results of our study are based on a representative study population.
Another point of concern is that information bias could have been introduced by the method of collection of patient data. During the interview, answers could have been biased by social desirability. Additionally, interviews were rather long. Answers given at the end of the interview could have been less precise than at the beginning. Yet, acceptable validity of self-reports in very old people has been reported previously (35) and we have no reason to doubt this.
Another source of potential information bias could have been introduced by the definition of the dependent variable “loss of independence”, dichotomized for analysis purposes, which could have resulted in misclassification and could have underestimated the effect of the independent variables. In fact persons who can live in the community, even with the help of others, will have a different profile of disability compared with nursing home residents. We argued, however, that any change in living situation has to be prevented or, if not preventable, has to be provided for as early as possible.
Although parametric regression models are appropriate to evaluate outcome predictors, their model assumptions are very restrictive. Non-parametric approaches, such as CART, are another way to analyse data; however, they do not yield easily interpretable effect measures. Since human functioning is multi-faceted, complex interactions between predictor variables are to be expected that are not necessarily retrievable by a logistic model. Our exercise confirms that it is necessary to validate predictive models in different populations (36).
Walking is arguably one of the major preconditions of independence after an acute event (25, 26). It may be important for patients’ independent living to encourage the use of wheelchairs and walking aids at very early stages of rehabilitation. Although this is seen rather as an activity, i.e. execution of a task, at the early stages, a definition of walking with aids as a specific participation issue may improve outcome orientation in rehabilitation interventions.
In principle these findings are not new. Even so, this is the first time the ICF could be operationalized for this group of patients and provided a useful framework to describe functioning in older persons. Further studies should evaluate interactions and factors associated with decreased mobility at the onset of rehabilitation and their influence on discharge destination, hopefully within the ICF framework.
AcknowledgementS
We thank Ralf Strobl, statistician, for his advice on model building and interpretation. Funding: this project was supported by the German Ministry of Health and Social Security (BMGS), grant no 124-43164-1/501.
REFERENCES
1. Stucki G, Stier-Jarmer M, Grill E, Melvin J. Rationale and principles of early rehabilitation care after an acute injury or illness. Disabil Rehabil 2005; 27: 353–359.
2. Williams ME, editor. The American Geriatrics Society‘s complete guide to aging & health. New York: Harmony Books; 1995.
3. Hager K, Nennmann U. Rehabilitation of the elderly – influence of age, sex, main diagnosis and activities of daily living (ADL) on the elderly patients‘ return to their previous living conditions. Arch Gerontol Geriatr 1997; 25: 131–139.
4. Kramer AM, Steiner JF, Schlenker RE, Eilertsen TB, Hrincevich CA, Tropea DA, et al. Outcomes and costs after hip fracture and stroke. A comparison of rehabilitation settings. JAMA 1997; 277: 396–404.
5. Oczkowski WJ, Barreca S. The functional independence measure: its use to identify rehabilitation needs in stroke survivors. Arch Phys Med Rehabil 1993; 74: 1291–1294.
6. Hanks RA, Lichtenberg PA. Physical, psychological, and social outcomes in geriatric rehabilitation patients. Arch Phys Med Rehabil 1996; 77: 783–792.
7. Landi F, Bernabei R, Russo A, Zuccala G, Onder G, Carosella L, et al. Predictors of rehabilitation outcomes in frail patients treated in a geriatric hospital. J Am Geriatr Soc 2002; 50: 679–684.
8. Patrick L, Knoefel F, Gaskowski P, Rexroth D. Medical comorbidity and rehabilitation efficiency in geriatric inpatients. J Am Geriatr Soc 2001; 49: 1471–1477.
9. Aditya BS, Sharma JC, Allen SC, Vassallo M. Predictors of a nursing home placement from a non-acute geriatric hospital. Clin Rehabil 2003; 17: 108–113.
10. Hajek VE, Gagnon S, Ruderman JE. Cognitive and functional assessments of stroke patients: an analysis of their relation. Arch Phys Med Rehabil 1997; 78: 1331–1337.
11. Kagaya H, Takahashi H, Sugawara K, Dobashi M, Kiyokawa N, Ebina H. Predicting outcomes after hip fracture repair. Am J Phys Med Rehabil 2005; 84: 46–51.
12. Sager MA, Rudberg MA, Jalaluddin M, Franke T, Inouye SK, Landefeld CS, et al. Hospital admission risk profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization. J Am Geriatr Soc 1996; 44: 251–257.
13. McKenna K, Tooth L, Strong J, Ottenbacher K, Connell J, Cleary M. Predicting discharge outcomes for stroke patients in Australia. Am J Phys Med Rehabil 2002; 81: 47–56.
14. World Health Organisation (WHO). International Classification of Functioning, Disability and Health: ICF. Geneva: WHO; 2001.
15. Stucki G, Ewert T, Cieza A. Value and application of the ICF in rehabilitation medicine. Disabil Rehabil 2002; 24: 932–938.
16. Grill E, Ewert T, Chatterji S, Kostanjsek N, Stucki G. ICF Core Sets development for the acute hospital and early post-acute rehabilitation facilities. Disabil Rehabil 2005; 27: 361–366.
17. Grill E, Hermes R, Swoboda W, Uzarewicz C, Kostanjsek N, Stucki G. ICF Core Set for geriatric patients in early post-acute rehabilitation facilities. Disabil Rehabil 2005; 27: 411–417.
18. Grill E, Stucki G, Boldt C, Joisten S, Swoboda W. Identification of relevant ICF categories by geriatric patients in an early post-acute rehabilitation facility. Disabil Rehabil 2005; 27: 467–473.
19. Hosmer DW, Lemeshow S. A goodness-of-fit test for the multiple logistic regression model. Communications in Statistics 1980; A10: 1043–1069.
20. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983; 148: 839–843.
21. Breiman L, Friedman J, Olshen R, Stone C, editors. Classification and regression trees. Boca Raton, Florida: CRC Press; 1984.
22. Lausen B, Sauerbrei W, Schumacher M. Classification and regression trees (CART) used for the exploration of prognostic factors measured on different scales. In: Dirschedl P, Ostermann R, editors. Computational statistics. Heidelberg: Physica; 1994, p. 483–496.
23. SAS Institute. %treedisc macro. 1995 [cited 2006 March 25]. Available from: http://www.stat.lsu.edu/faculty/moser/exst7037/treedisc.html
24. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–1379.
25. Blower PW, Carter LC, Sulch DA. Relationship between wheelchair propulsion and independent walking in hemiplegic stroke. Stroke 1995; 26: 606–608.
26. Singh R, Hunter J, Philip A, Todd I. Predicting those who will walk after rehabilitation in a specialist stroke unit. Clin Rehabil 2006; 20: 149–152.
27. Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc 2004; 52: 1263–1270.
28. Brosseau L, Potvin L, Philippe P, Boulanger YL. Post-stroke inpatient rehabilitation. II. Predicting discharge disposition. Am J Phys Med Rehabil 1996; 75: 431–436.
29. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995; 332: 556–561.
30. Lai SM, Alter M, Friday G, Lai SL, Sobel E. Disposition after acute stroke: who is not sent home from hospital? Neuroepidemiology 1998; 17: 21–29.
31. Verbrugge LM, Rennert C, Madans JH. The great efficacy of personal and equipment assistance in reducing disability. Am J Public Health 1997; 87: 384–392.
32. Hoenig H, Siebens HC. Geriatric rehabilitation: new frontiers in geriatrics research: an agenda for surgical and related medical specialties. 2004 [cited 2006 June 20]. Available from: http://www.frycomm.com/ags/rasp/chapter.asp?ch = 12
33. Dodge HH, Kadowaki T, Hayakawa T, Yamakawa M, Sekikawa A, Ueshima H. Cognitive impairment as a strong predictor of incident disability in specific ADL-IADL tasks among community-dwelling elders: the Azuchi Study. Gerontologist 2005; 45: 222–230.
34. Hoenig H, Siebens H. Research agenda for geriatric rehabilitation. Am J Phys Med Rehabil 2004; 83: 858–866.
35. Sager MA, Dunham NC, Schwantes A, Mecum L, Halverson K, Harlowe D. Measurement of activities of daily living in hospitalized elderly: a comparison of self-report and performance-based methods. J Am Geriatr Soc 1992; 40: 457–462.
36. Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med 2000; 19: 453–473.
| APPENDIX: Categories of the geriatric ICF Core Set |
| Body Functions |
| b110 Consciousness functions |
| b114 Orientation functions |
| b117 Intellectual functions |
| b130 Energy and drive functions |
| b134 Sleep functions |
| b140 Attention functions |
| b144 Memory functions |
| b147 Psychomotor functions |
| b152 Emotional functions |
| b156 Perceptual functions |
| b167 Mental functions of language |
| b176 Mental function of sequencing complex movements |
| b180 Experience of self and time functions |
| b210 Seeing functions |
| b215 Function of structures adjoining the eye |
| b230 Hearing functions |
| b240 Sensations associated with hearing and vestibular function |
| b260 Proprioceptive function |
| b265 Touch function |
| b270 Sensory functions related to temperature and other stimuli |
| b280 Sensation of pain |
| b320 Articulation functions |
| b410 Heart functions |
| b415 Blood vessel functions |
| b420 Blood pressure functions |
| b430 Haematological system functions |
| b435 Immunological system functions |
| b440 Respiration functions |
| b450 Additional respiratory functions |
| b455 Exercise tolerance functions |
| b460 Sensations associated with cardiovascular and respiratory functions |
| b510 Ingestion functions |
| b525 Defecation functions |
| b530 Weight maintenance functions |
| b535 Sensations associated with the digestive system |
| b540 General metabolic functions |
| b545 Water, mineral and electrolyte balance functions |
| b620 Urination functions |
| b630 Sensations associated with urinary functions |
| b710 Mobility of joint functions |
| b715 Stability of joint functions |
| b730 Muscle power functions |
| b735 Muscle tone functions |
| b755 Involuntary movement reaction functions |
| b760 Control of voluntary movement functions |
| b765 Involuntary movement functions |
| b770 Gait pattern functions |
| b780 Sensations related to muscles and movement functions |
| b810 Protective functions of the skin |
| b820 Repair functions of the skin |
| b840 Sensation related to the skin |
| Body Structures |
| s110 Structure of brain |
| s120 Spinal cord and related structures |
| s320 Structure of mouth |
| s410 Structure of cardiovascular system |
| s430 Structure of respiratory system |
| s610 Structure of urinary system |
| s620 Structure of pelvic floor |
| s710 Structure of head and neck region |
| s720 Structure of shoulder region |
| s740 Structure of pelvic region |
| s750 Structure of lower extremity |
| s760 Structure of trunk |
| s770 Additional musculoskeletal structures related to movement |
| s810 Structure of areas of skin |
| Activities and Participation |
| d130 Copying |
| d155 Acquiring skills |
| d177 Making decisions |
| d230 Carrying out daily routine |
| d240 Handling stress and other psychological demands |
| d310 Communicating with – receiving – spoken messages |
| d315 Communicating with – receiving – nonverbal messages |
| d330 Speaking |
| d335 Producing nonverbal messages |
| d360 Using communication devices and techniques |
| d410 Changing basic body position |
| d415 Maintaining a body position |
| d420 Transferring oneself |
| d440 Fine hand use (picking up, grasping) |
| d445 Hand and arm use |
| d450 Walking |
| d460 Moving around in different locations |
| d465 Moving around using equipment |
| d510 Washing oneself |
| d520 Caring for body parts |
| d530 Toileting |
| d540 Dressing |
| d550 Eating |
| d560 Drinking |
| d570 Looking after one’s health |
| d760 Family relationships |
| d770 Intimate relationships |
| d860 Basic economic transactions |
| d930 Religion and spirituality |
| d940 Human rights |
| Environmental Factors |
| e110 Products or substances for personal consumption |
| e115 Products and technology for personal use in daily living |
| e120 Products and technology for personal indoor and outdoor mobility and transportation |
| e125 Products and technology for communication |
| e140 Products and technology for culture, recreation and sport |
| e145 Products and technology for the practice of religion or spirituality |
| e150 Design, construction and building products and technology of buildings for public use |
| e240 Light |
| e245 Time-related changes |
| e250 Sound |
| e310 Immediate family |
| e315 Extended family |
| e320 Friends |
| e325 Acquaintances, peers, colleagues, neighbours and community members |
| e330 People in position of authority |
| e355 Health professionals |
| e360 Health related professionals |
| e410 Individual attitudes of immediate family members |
| e415 Individual attitudes of extended family members |
| e420 Individual attitudes of friends |
| e425 Individual attitudes of acquaintances, peers, colleagues, neighbours and community members |
| e430 Individual attitudes of people in positions of authority |
| e450 Individual attitudes of health professionals |
| e455 Individual attitudes of other professionals |
| e460 Societal attitudes |
| e465 Social norms, practices and ideologies |
| e570 Social security, services, systems and policies |
| e580 Health services, systems and policies |