OBJECTIVE: To assess upper extremity functioning of children with unilateral transverse upper limb reduction deficiency, using standardized instruments, and to investigate their validity and reliability.
DESIGN: Cross-sectional study.
SUBJECTS: Twenty subjects aged 4–12 years; 9 prosthetic users and 11 non-users.
METHODS: The Assisting Hand Assessment, Unilateral Below Elbow Test, Prosthetic Upper extremity Functional Index and ABILHAND-Kids were assessed in all children. Users were tested with and without their prosthesis. We compared results of users and non-users, and of users with and without their prosthesis. Validity was determined by testing hypotheses and correlations with other measures. Test-retest reliability was assessed from repeated measurements in 10 children.
RESULTS: Children with an upper limb reduction deficiency performed well on daily activities. They could use their prosthesis in 68% of the activities, but were currently using it in only 30%. Children find their prosthesis useful for specific activities, rather than for daily activities in general. The Assisting Hand Assessment and Prosthetic Upper extremity Functional Index showed best validity; test-retest reliability was good to excellent.
CONCLUSION: The use of standardized instruments adds relevant information on functioning of children with an upper limb reduction deficiency. We found additional support for validity and reliability of, in particular, the Assisting Hand Assessment and Prosthetic Upper extremity Functional Index.
Key words: upper extremity, prostheses and implants, activities of daily living, outcome assessment (healthcare), reproducibility of results.
J Rehabil Med 2007; 39: 379–386
Correspondence address: Laurien M. Buffart, Erasmus MC, Department of Rehabilitation Medicine, PO box 2040, NL-3000 CA Rotterdam, The Netherlands. E-mail: l.buffart@erasmusmc.nl
Submitted February 22, 2006; accepted January 12, 2007
INTRODUCTION
In children with transverse upper limb reduction deficiencies (ULRD), 44–66% wear a prosthetic device to enhance their ability to perform functional activities (1–3). However, empirical evidence as to whether prostheses yield improved functional outcomes in these children is scarce (4).
Assessment of arm and prosthetic functioning largely relies on clinical observation of task performance (5). To adequately measure arm and prosthetic functioning in children with ULRD, standardized measures to assess activities are required. Based on clinical experience, it is known that what a child “can do” in a clinical setting (capacity) and “does do” in daily life (performance) may differ (6). Thus, for an adequate evaluation, both capacity and performance of activities should be measured. Functional tests can measure capacity, whereas self-reported or parent-reported questionnaires or assessment of spontaneous arm or prosthetic use can measure performance. Several instruments have been developed recently to measure arm/hand functioning in children with different diagnoses. In a previous study we selected 4 instruments for children with ULRD, which met the following criteria: (i) they assess bimanual activities of daily living; (ii) they measure quality of movement and/or difficulty in performing activities; and (iii) they are attractive to children aged 4–12 years (7). The instruments selected were: the Unilateral Below Elbow Test (UBET) (8); the Assisting Hand Assessment (AHA) (9); and 2 questionnaires, the Prosthetic Upper extremity Functional Index (PUFI) (10, 11) and ABILHAND-Kids (12).
The first objective of the present study was to assess upper extremity functioning of children with ULRD with and without a prosthesis, as measured with these instruments. Two of the instruments, the UBET and PUFI, were developed specifically for children with ULRD, whereas the AHA and ABILHAND-Kids have been found valid and reliable in other patient groups, i.e. cerebral palsy (CP) and obstetric brachial plexus palsy (OBPP). We expect that these instruments might also be useful to assess upper extremity functioning of children with ULRD. A second objective of the study was to obtain additional insight into the validity and reliability of the selected instruments for children with ULRD.
METHODS
Patients
Children with ULRD between 4 and 12 years old were consecutively recruited from the Department of Rehabilitation Medicine of Erasmus MC – University Medical Centre Rotterdam from March to October 2004. A total of 25 children and their parents were invited to participate in the study, of whom 20 (10 boys, 10 girls) were willing to participate; a response rate of 80%. Responders and non-responders did not differ regarding age, gender, level of reduction deficiency and prosthetic device.
The mean age (standard deviation) of the participating children was 8.7 (2.9) years. One child had a below-shoulder reduction deficiency, 16 children had a reduction deficiency below the elbow and 3 had a partial hand. Eight children used a myoelectric device, 1 used a passive device and 11 did not use a prosthetic device.
Parents of all the children gave their informed consent in writing prior to the children being included in the study. The study was approved by the ethics committee of Erasmus MC.
Procedures
Each subject had 3 visits to the hospital; during each visit one test and one questionnaire were assessed. To limit the number of visits, repeated measurements for one test and one questionnaire were performed at the third visit in 10 children and for the other test and questionnaire in the other 10 children. The time interval between the test and retest was 14 days (range 11–18 days). All functional tests were administered by the same occupational therapist (VVH). Parents of all children completed the questionnaires.
Measurements
Functional activities of the upper limb were assessed with 2 tests, focusing on capacity (UBET) and performance (AHA) of activities. The UBET, consisting of 2 rating scales, addressed the method of arm (or prosthetic) use during tasks and the ease of task completion (8). The AHA addressed spontaneous arm or prosthetic functioning by evaluating the effectiveness of a child’s assisting arm or prosthesis in bi-manual play (9). For children wearing a prosthesis, functional tests were first executed with the prosthesis, followed by a test without the prosthesis. Two questionnaires were also assessed: the ABILHAND-Kids, focusing on the ease of performing daily activities, irrespective of use or non-use of a prosthesis, and the PUFI, including 4 rating scales addressing the method of use, ease of performance with and without a prosthesis and the usefulness of the prosthesis. For children who do not wear a prosthesis, only the method of use and ease of performance without a prosthesis was assessed. Detailed information on tests and questionnaires is given in the Appendix. To compare sum scores of different instruments, sum scores from 0 to 100% were normalized.
The usefulness of the prosthesis was assessed using the PUFI (11). Of each activity, the usefulness of the prosthesis was rated on a 3-level scale (not useful, somewhat useful, and very useful).
Prosthetic wearing time was assessed using 4 categories: (i) 0–2 hours/day; (ii) 3–5 hours/day; (iii) 6–10 hours/day; and (iv) 11–15 hours/day.
At the final visit, the parents were asked the following question about prosthetic functioning of their child: “Are you happy with the way your child’s prosthesis helps him/her perform daily activities?” We selected this question from the Child Amputee Prosthetics Projects – Prosthesis Satisfaction Inventory; Satisfaction with functioning was rated on a 5-level scale (0 = “not at all satisfied’; 4 = “very much satisfied”) (13).
After the first visit, the therapist judged the arm and prosthetic functioning of the child on a 10-point numeric rating scale without knowing the final sum scores on the instruments (14).
Data analysis
Functional activities. Twenty children (9 users and 11 non-users) and their parents completed the tests and questionnaires. One prosthetic user did not complete the AHA without the prosthesis because she ran out of time, and another prosthetic user did not complete the AHA with the prosthesis because the myoelectric prosthesis battery ran out on the day of testing. For the AHA, results of 8 users (with and without prosthesis) and 11 non-users are presented.
For the PUFI scales addressing ease of performance with prosthesis and usefulness of prostheses we calculated additional sum scores for those activities for which a child actually used the prosthesis (actively or passively). Since children used their prosthesis mainly in specific activities, this allowed fair judgements to be made about prosthetic use and functioning.
We compared the performance of activities for children who wear a prosthesis (users) with those of children who do not wear a prosthesis (non-users) using the Mann-Whitney U test. A Wilcoxon matched-pairs signed-rank test was used to compare the performance of children who have a prosthesis, tested with and without their device. Exact p-values ≤ 0.05 were considered significant.
Validity. In the absence of a gold standard, the construct validity and convergent validity of the 4 instruments were explored (15). Addressing construct validity, we expected strong (rs ≥ 0.70) or good (rs ≥ 0.60) correlations between sum scores on the instruments and other characteristics of prosthetic functioning, i.e. prosthetic wearing time, usefulness of prosthesis and parent satisfaction with prosthetic functioning, (see Table III). For gender and age, no correlations were expected with sum scores on the instruments, since items were considered age specific (see Appendix). In case of the ABILHAND-Kids this applies to children aged ≥ 6 years.
Analyses addressing convergent validity focused on inter-relationships between instruments and on their relationship with the therapist’s global assessment of arm and prosthetic functioning.
Relationships were assessed using the Spearman rho coefficients (rs). Since we expected the relationships to be in one direction, we tested one-tailed. The Mann-Whitney U test (two-tailed) was used to assess differences between boys and girls.
Test-retest reliability. Test-retest reliability was estimated from variance between patients and error variance between visits, using analysis of variance. For sum scores on instruments, the intraclass correlation coefficient (ICC), standard error of measurement (SEM) and associated smallest detectable difference at a 95% confidence level were calculated (SDD95) (16–19).
Reliability was considered excellent when values of ICC exceeded 0.75, values of ICC from 0.60 to 0.74 were evaluated as good, from 0.40 to 0.59 as moderate, and less than 0.40 as poor (20). In order to compare SDDs of different instruments, SDDs were expressed as percentage of the total possible measurement range of the instrument (21, 22). The measurement error of an instrument is considered small enough when the instrument is able to distinguish 7 steps (with a range from 5 to 9) on the total measurement range (21, 23–25). Therefore, we considered instruments with SDD/range ratios ≤ 0.20 adequate for clinical practice.
All analyses were performed using statistical software (SPSS 11).
RESULTS
Functional activities
Use of prosthesis. Of the 9 prosthetic users, 3 wore their prosthesis 0–2 hours/day, 3 wore it 3–5 hours/day, 2 wore it 6–10 hours/day and 1 wore it 11–15 hours/day. The median score of parents’ satisfaction with their child’s functioning was 2.5 (range: 1 (“a little satisfied”) to 4 (“very much satisfied”)). The median score of the therapist’s global assessment of functioning was 6 (range 2–8) with prosthesis and 8 (range 6–9) without prosthesis.
Overall, the median score on usefulness of the prosthesis was 37.5 (range 1.9–58.5). For those activities in which the prosthesis is actually used, such as riding a bicycle and using scissors, the prosthesis was found very useful (median score 75; range 66.7–100).
Method of performance. The way children can and do use their prosthesis or residual limb in performing activities is scored in the UBET and PUFI, respectively (Table I). Several results are worth mentioning. First, most activities (94%) are performed independently, as reported by the PUFI; on average 2% was performed with some help and 4% could not be performed. Secondly, children can use their prosthesis actively or passively in 68% of activities, as shown by the UBET. However, in daily life these children use the prosthesis in 30% of activities as reported by the PUFI (Table I). Children used their prosthesis mainly for riding a bicycle (78% of users), stringing beads (60% of users), using scissors (50% of users), and for sports and play (48% of users).
| Table I. Method of use of prosthetic device or residual limb assessed with Unilateral Below Elbow Test (UBET) and Prosthetic Upper extremity Functional Index (PUFI). |
| Method of use (% of activities) | Can do (UBET) | Method of performance (% of activities) | Does do (PUFI) |
| Users with (n = 9) Mean (SD) | Users without (n = 9) Mean (SD) | Non-users (n = 11) Mean (SD) | Users (n = 9) Mean (SD) | Non-users (n = 11) Mean (SD) |
| Actively | 30 (28) | 44 (24) | 55 (30) | Prosthesis actively | 15 (18) | 0 (0) |
| Passively | 38 (25) | 19 (15) | 17 (13) | Prosthesis passively | 15 (21) | 0 (0) |
| Elbow/trunk | 11 (14) | 16 (17) | 27 (27) | Residual limb | 41 (34) | 85 (12) |
| One-handed | 21 (13) | 21 (23)* | 1 (3) | One-handed | 23 (18)* | 5 (6) |
| | | | | Some help | 2 (4) | 3 (3) |
| | | | | Cannot do | 4 (4) | 7 (9) |
| *Significant difference between users and non-users. |
Thirdly, both the UBET and PUFI show that users (with and without prosthesis) performed more activities one-handed compared with non-users (p < 0.009). Finally, both instruments show that non-users performed most activities using the residual limb: the UBET reports active use of the residual limb in 55% of activities and passive use in 17%, and the PUFI shows residual end limb manipulation in 85% of activities.
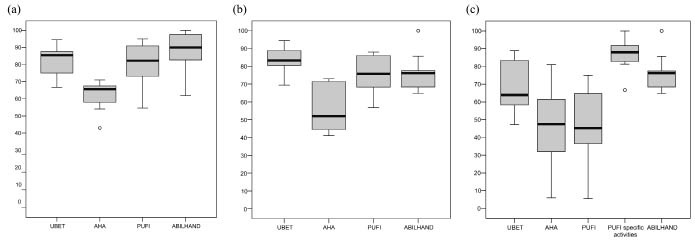
Effectiveness and ease of performance. Children with ULRD had high sum scores on tests and questionnaires regarding effectiveness or ease of performance, especially without a prosthesis (Table II). Normalized sum scores are presented in Fig. 1.
| Table II. Effectiveness and ease of performance of functional activities. |
| Instrument | Median | Min | Max | Difference non-users/users without prothesis | Difference non-users/users with prothesis | Difference users with prothesis/without prothesis |
| UBET |
| Completion of task score | | | | | | |
| Non-users | 31 | 24 | 34 | p = 0.545 | p = 0.03* | p = 0.02* |
| Users – without prosthesis | 30 | 25 | 34 | | | |
| Users – with prosthesis | 23 | 17 | 32 | | | |
| AHA |
| Effectiveness | | | | | | |
| Non-users | 67 | 43 | 71 | p = 0.656 | p = 0.02* | p = 0.09 |
| Users – without prosthesis | 52 | 41 | 73 | | | |
| Users – with prosthesis | 47.5 | 6 | 81 | | | |
| PUFI |
| Ease of performance | | | | | | |
| Non-users | 79.7 | 54.6 | 95 | p = 0.331 | p = 0.001* | p = 0.02* |
| Users – without prosthesis | 75.8 | 56.8 | 88 | | | |
| Users – with prosthesis | 45.2 | 5.6 | 75 | | | |
| Ease, specific activities | | | | | | |
| Users – with prosthesis | 88.0 | 66.7 | 100 | | †p = 0.41 | †p = 0.04* |
| ABILHAND-Kids |
| Ease of performance | | | | | | |
| Non-users | 38 | 26 | 42 | | | |
| Users | 32 | 27 | 42 | p = 0.046* | | |
| Median values of 11 non-users and 9 users of a prosthesis. †Difference calculated for users with prosthesis for specific activities. *Significant differences between groups. Min: minimum; Max: maximum; UBET: Unilateral Below Elbow Test; AHA: Assisting Hand Assessment; PUFI: Prosthetic Upper extremity Functional Index. |
Fig. 1. Effectiveness and ease of performance of functional activities: comparison of 4 instruments. Normalized sum scores of Assisting Hand Assessment (AHA), Unilateral Below Elbow Test (UBET), Prosthetic Upper extremity Functional Index (PUFI) and ABILHAND-Kids for (a) non-users (n = 11); (b) users without prosthesis (n = 9) and (c) users with prosthesis (n = 9). Median (bold lines) with lower and upper quartiles (lower and upper edge of boxes) and range (whiskers) are indicated; the circles represent outliers.
A comparison of non-users and users of a prosthesis showed that non-users had better capacity of performing tasks (p = 0.03), used their arm more effectively (p = 0.02) and performed tasks with more ease (p = 0.001; Table II). No differences were found in capacity and performance between non-users and users without the prosthesis. The ABILHAND-kids showed higher scores for non-users than for users (p = 0.046).
A comparison of users with and without a prosthesis showed that their capacity (UBET) was lower when users performed the activities with the prosthesis than without the prosthesis (p = 0.02), but performance did not differ according to the AHA (p = 0.09; Table II). On the PUFI, lower sum scores were found for users assessing the performance of activities with their prosthesis than without (p = 0.02). However, when taking into account only the activities for which the children actually use the prostheses, children perform these items easier with the prosthesis (median score 88.0; range 66.7–100) than without (p = 0.04).
Validity
Children wearing their prosthesis for more hours during the day had higher sum scores on the PUFI (rs = 0.70; p = 0.02) and the AHA (rs = 0.63; p = 0.048). The usefulness of the prosthesis correlated strongly with the ease of performance of activities (PUFI; rs = 0.82; p < 0.001). Children whose parents were more satisfied with functioning of the prosthesis had higher sum scores on the AHA (rs = 0.83; p = 0.01) and the PUFI (rs = 0.77; p = 0.01) (Table III).
| Table III. Construct and convergent validity of functional tests and questionnaires. |
| | Without prosthesis (n = 20) | | With prosthesis (n = 9) |
| UBET | AHA | PUFI | ABILHAND-Kids | | UBET | AHA | PUFI | ABILHAND-Kids |
| Construct validity | | | | | | | | | |
| 1. Correlation with prosthetic wearing time | | | | | | 0.07 | 0.63* | 0.70* | 0.43 |
| 2. Correlation with ratings on usefulness of prosthesis | | | | | | –0.28 | 0.55† | 0.82* | –0.43 |
| 3. Correlation with parent satisfaction with prosthetic functioning | | | | | | 0.24 | 0.83* | 0.77* | 0.39 |
| 4. No correlation with age of the patient | 0.51* | –0.02 | –0.13 | 0.30 | | 0.67* | 0.38 | 0.03 | 0.30 |
| 5. No difference between boys and girls (p-value) | 0.82 | 0.57 | 0.26 | 0.63 | | 1.0 | 0.88 | 1.0 | 0.63 |
| Convergent validity | | | | | | | | | |
| 6. Correlation with therapist’s global assessment | 0.00 | 0.01 | 0.09 | 0.19 | | 0.17 | 0.84* | 0.66* | 0.37 |
| 7. Sum scores on instruments are interrelated | | | | | | | | | |
| UBET | – | | | | | – | | | |
| AHA | 0.28 | – | | | | 0.28 | – | | |
| PUFI | 0.24 | 0.33† | – | | | –0.08 | 0.79* | – | |
| ABILHAND-Kids | 0.55* | 0.41* | 0.53* | – | | 0.49† | 0.32 | –0.12 | – |
| Expected relations are in bold. Values are Spearman rho coefficients (one-tailed). *p < 0.05; † 0.05 < p < 0.08. UBET: Unilateral Below Elbow Test; AHA: Assisting Hand Assessment; PUFI: Prosthetic Upper Extremity Functional Index. |
We found no correlations between age of the patients and sum scores for the AHA, PUFI and ABILHAND-Kids (≥ 6 years, n = 15) (Table IV). Age correlation was moderate to good with the sum scores of the UBET with or without a prosthesis (rs = 0.67 or 0.51). For all instruments, sum scores did not differ between boys and girls.
For arm functioning without a prosthesis, no correlations were found between the therapist’s global assessment of arm functioning and sum scores of instruments, and only moderate inter-relationships were found between instruments (Table III). Regarding arm functioning with a prosthesis, the therapist’s global assessment correlated strongly with the AHA (rs = 0.84; p = 0.005) and a good correlation was found with the PUFI (rs = 0.66; p = 0.03). A strong correlation was found between the AHA and the PUFI (rs = 0.79; p = 0.01; Table III).
Test-retest reliability
ICCs were excellent for each instrument, except for the PUFI with a prosthesis, which had a good ICC (Table IV). Due to small sample sizes, 95% confidence intervals were rather broad. SEMs ranged from 1.5 to 4.9 score points and 12.9 for the PUFI with a prosthesis. Corresponding SDD95 ranged from 4.1 to 24.5. The SDD95/range ratio ranged from 0.11 to 0.24 (Table IV), indicating the instruments can distinguish 4–9 steps on the total measurement range.
| Table IV. Test-retest reliability of functional tests and questionnaires. |
| Instrument | Test Mean (SD) | Retest Mean (SD) | ICC (95% CI) | SEM | SDD95 | SDD95/range |
| UBET without prosthesis (n = 10) with prosthesis (n = 5) | 29.6 (2.9) 23.4 (5.6) | 29.6 (3.7) 26.2 (6.3) | 0.80 (0.38–0.95) 0.79 (0.03–0.98) | 1.5 2.7 | 4.1 8.1 | 0.11 0.23 |
| AHA without prosthesis (n = 8) with prosthesis (n = 4) | 62.6 (19.1) 36.3 (10.9) | 57.3 (15.5) 34.0 (23.3) | 0.70 (0.36–0.94) 0.94 (0.35–0.996) | 7.4 5.4 | 20.6 14.9 | 0.21 0.15 |
| PUFI without prosthesis (n = 10) with prosthesis (n = 5) | 78.8 (11.8) 47.4 (22.0) | 78.3 (15.7) 50.8 (27.4) | 0.88 (0.58–0.97) 0.65 (0.34–0.96) | 4.9 12.9 | 13.5 24.5 | 0.13 0.24 |
| ABILHAND-Kids (n = 10) | 35.0 (5.51) | 36.2 (4.3) | 0.89 (0.66–0.94) | 1.7 | 6.7 | 0.16 |
| SD: standard deviation; ICC: intraclass correlation coefficient; CI: confidence interval; SEM: standard error of measurement; SDD95: smallest detectable difference at 95% confidence level; UBET: Unilateral Below Elbow Test; AHA: Assisting Hand Assessment; PUFI: Prosthetic Upper extremity Functional Index. |
DISCUSSION
Functional activities
Overall, children with ULRD, both users and non-users, performed well on bimanual activities, which can be concluded from the high sum scores on the instruments. In daily life, children with ULRD make relatively little use of the prosthesis, and use the device mostly for certain specific activities. Activity-specific use of the prosthesis has been reported previously (26), and this should be taken into account when judging prosthetic functioning. Therefore, to make a fair assessment of ease of performance with a prosthesis and the usefulness of the prosthesis, we presented additional scores of the PUFI addressing only those specific activities, in which the child actually used the prosthesis. Children performed these activities easily with the prosthesis and the prosthesis was found to be very useful.
Results showed a discrepancy between capacity and performance of activities. Children can perform most activities with a prosthesis, but in daily life they perform most activities with the residual limb. This discrepancy could possibly be caused by variations in motivation of a child, its prosthetic skills or technical aspects, such as weight and speed of a myoelectric device. Secondly, users of a prosthesis performed more activities unilaterally compared with non-users. When handling of the prosthesis is difficult, children may find it easier to perform the activity with one hand. They may get used to performing activities with one hand, and non-use of their residual limb.
These aspects should be taken into account in functional training with the prosthesis. The question arises as to whether limited capacity of functioning may cause the limited use of the prosthesis, or whether the prosthesis might be most useful for specific activities and therefore children perform well with it. Future studies on the effects of functional training on prosthetic functioning are warranted.
A comparison of normalized sum scores on different instruments showed that children with ULRD score relatively low on the AHA. This might be explained by a different focus of the AHA compared with the other instruments. Whereas other instruments primarily focus on difficulty of performance, the AHA addresses more detailed aspects of task performance, such as manipulation, grasping, readjusting or stabilizing grip. These aspects of task performance were originally identified for assessing children with CP or OBPP, and might be relatively difficult for children without a hand. Gripping, holding and releasing objects are known to be difficult tasks for children with a prosthesis (27). These aspects have therefore been incorporated in the Assessment of Capacity for Myoelectric Control (ACMC), a recently developed instrument for children with ULRD (27). Unfortunately, the ACMC was not available when this study was started and therefore was not included in the head-to-head comparison of outcome measures.
Limitations of the study
Some limitations of this study need to be addressed. First, sample sizes were small. Regarding validity, 3 expected relations apply only to children who wear a prosthesis (n = 9). The statistical power to detect actual correlations was therefore limited. However, detection of good (≥ 0.6) and strong (≥ 0.7) correlations was not hampered. Also, for assessing test-retest reliability sample sizes were very small since we limited the number of repeated measurements. This resulted in rather broad confidence intervals of the assessed ICCs. Thus, analyses on validity and test-retest reliability were of an explorative nature, and the results might at most be interpreted as additional support for psychometric quality that was known from previous studies.
Secondly, regarding the questionnaires, we focused on the parent-reported results. However, some parents, especially parents of older children (10), indicated that they experienced some difficulties in rating activity performance of their child, since they are not present when their children perform activities at school, or when playing with their friends. On the other hand, it is known that children are less discriminative than their parents (12).
Validity
Additional support for validity was found, especially for the AHA and the PUFI. Scores on the AHA and PUFI are correlated with each other, both for assessments with and without a prosthesis. Although the UBET might be a useful instrument in clinical practice, particularly because it is easy and quick to perform, we found only limited support for construct and convergent validity. This could be due to a drop in the total sum score of the UBET when a child is unable to perform one specific activity (out of 9 activities) even though the child may be a very good prosthetic user. Since we found that most children used their prosthesis mainly for specific activities, this effect might have been present. The UBET can, however, show the differences in capacity of activities with and without a prosthesis. The AHA is conducted as a semi-structured play session rather than asking the child to perform a specific activity (as is the case with the UBET), and thus assesses performance of activities. In fact, if a child does conduct a certain activity, it means that he or she can do that particular activity, which makes a functional test aiming at performance of activities very valuable.
For assessing hand functioning without a prosthesis, the therapist’s global assessment did not show any correlations with sum scores on the instruments. The therapist is probably more accustomed to evaluating prosthetic functioning than functioning of the residual limb.
To obtain further evidence for construct validity of the instruments for children with ULRD, insight into the hierarchy of the items for these children might be gained by means of Rasch analysis on a larger sample of patients.
Test-retest reliability
With some caution, due to the small sample size, we conclude that test-retest reliability was good for the AHA without a prosthesis and for the PUFI with a prosthesis. All other ICCs were excellent. One additional comment should be made. In the present study we calculated ICCs on raw sum scores of the AHA and ABILHAND-Kids instead of logarithmic transformed Rasch scores. However, from other instruments (such as the Gross Motor Function Measure) we know that ICCs calculated on raw scores do not differ from ICCs calculated on Rasch scores (28). Therefore, we assume that the ICCs calculated in the present study are a fair estimate of the instrument’s reliability.
Our assessments of reliability of the PUFI are comparable to those of Wright et al. (10) who reported ICCs for the PUFI parent version of 0.40–0.84 (10). For the other instruments, no ICCs for test-retest reliability in children with ULRD have been published yet.
In addition to ICC, we presented SEM and SDD95 to inform clinicians on the magnitude of measurement error which should be taken into account when judging whether a child’s arm or prosthetic functioning has really changed. Based on the calculated SDD/range ratios, we judged that the measurement error is small enough to make the instruments valuable in clinical practice, except for the UBET and PUFI with prosthesis. For a definite judgment about sensitivity to change, a longitudinal follow-up study in patients is required.
In conclusion, the results show that children with ULRD perform well on functional activities in daily life. The prosthesis has additional value for specific activities rather than for general activities of daily life. This study showed that assessing functional activities in children with ULRD using standardized instruments provides relevant information that can be useful for clinical judgment. We found additional support for validity and reliability of the selected instruments for children with ULRD, especially of the AHA and the PUFI.
Acknowledgements
We thank all the children and their parents who participated in the study. We thank the Johanna Children’s Fund (JKF) and the Children’s Fund Adriaanstichting (KFA) for their financial support.
REFERENCES
1. Tolsma MHJ, Meeuwisse-de Vries B, Pesch-Batenburg JMFB, Rol M, Arendzen JH, Roebroeck ME, et al. Level of functioning of adolescents and young adults with a congenital reduction deficiency of the upper limb. [Het niveau van functioneren van adolescenten en jongvolwassenen met een congenitaal reductiedefect van de arm]. Revalidata 2003; 25: 18–21 (in Dutch).
2. Kuyper MA, Breedijk M, Mulders AH, Post MW, Prevo AJ. Prosthetic management of children in The Netherlands with upper limb deficiencies. Prosthet Orthot Int 2001; 25: 228–234.
3. Silcox DH, 3rd, Rooks MD, Vogel RR, Fleming LL. Myoelectric prostheses. A long-term follow-up and a study of the use of alternate prostheses. J Bone Joint Surg Am 1993; 75: 1781–1789.
4. Pruitt SD, Varni JW, Seid M, Setoguchi Y. Functional status in limb deficiency: development of an outcome measure for preschool children. Arch Phys Med Rehabil 1998; 79: 405–411.
5. Pruitt SD, Varni JW, Setoguchi Y. Functional status in children with limb deficiency: development and initial validation of an outcome measure. Arch Phys Med Rehabil 1996; 77: 1233–1238.
6. WHO. International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization; 2001.
7. Buffart LM, Roebroeck ME, Pesch-Batenburg JM, Janssen WG, Stam HJ. Assessment of arm/hand functioning in children with a congenital transverse or longitudinal reduction deficiency of the upper limb. Disabil Rehabil 2006; 28: 85–95.
8. Bagley AM, Molitor F, Wagner LV, Tomhave W, James MA. The Unilateral Below Elbow Test: a function test for children with unilateral congenital below elbow deficiency. Dev Med Child Neurol 2006; 48: 569–575.
9. Krumlinde Sundholm L, Eliasson AC. Development of the Assisting Hand Assessment: a Rasch-built measure intended for children with unilateral upper limb impairments. Scand J Occup Ther 2003; 10: 16–26.
10. Wright FV, Hubbard S, Jutai J, Naumann S. The Prosthetic Upper Extremity Functional Index: development and reliability testing of a new functional status questionnaire for children who use upper extremity prostheses. J Hand Ther 2001; 14: 91–104.
11. Wright FV, Hubbard S, Naumann S, Jutai J. Evaluation of the validity of the prosthetic upper extremity functional index for children. Arch Phys Med Rehabil 2003; 84: 518–527.
12. Arnould C, Penta M, Renders A, Thonnard JL. ABILHAND-Kids: a measure of manual ability in children with cerebral palsy. Neurology 2004; 63: 1045–1052.
13. Pruitt SD, Varni JW, Seid M, Setoguchi Y. Prosthesis satisfaction outcome measurement in pediatric limb deficiency. Arch Phys Med Rehabil 1997; 78: 750–754.
14. Roorda LD, Roebroeck ME, Lankhorst GJ, van Tilburg T, Bouter LM. Measuring functional limitations in rising and sitting down: development of a questionnaire. Arch Phys Med Rehabil 1996; 77: 663–669.
15. Kirshner B, Guyatt G. A methodological framework for assessing health indices. J Chronic Dis 1985; 38: 27–36.
16. MacDermid JC, Stratford P. Applying evidence on outcome measures to hand therapy practice. J Hand Ther 2004; 17: 165–173.
17. Streiner DL, Norman GR. Health measurement scales: a practical guide to their development and use, 2nd edn. Oxford: Oxford University Press; 1995, p. 126–137.
18. Schreuders TA, Roebroeck ME, Jaquet JB, Hovius SE, Stam HJ. Measuring the strength of the intrinsic muscles of the hand in patients with ulnar and median nerve injuries: reliability of the Rotterdam Intrinsic Hand Myometer (RIHM). J Hand Surg [Am] 2004; 29: 318–324.
19. Roebroeck ME, Harlaar J, Lankhorst GJ. The application of generalizability theory to reliability assessment: an illustration using isometric force measurements. Phys Ther 1993; 73: 386–395.
20. Fleiss JL. Statistical methods for rates and proportions. New York: Wiley; 1981.
21. Schreuders TA, Roebroeck ME, Goumans J, van Nieuwenhuijzen JF, Stijnen TH, Stam HJ. Measurement error in grip and pinch force measurements in patients with hand injuries. Phys Ther 2003; 83: 806–815.
22. Van Baalen B, Odding E, van Woensel MPC, van Kessel MA, Roebroeck ME, Stam HJ. Reliability and sensitivity to change of measurement instruments used in a traumatic brain injury population. Clin Rehabil 2006; 20: 686–700.
23. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003; 41: 582–592.
24. Miller GA. The magical number seven plus or minus two: some limits on our capacity for processing information. Psychol Rev 1956; 63: 81–97.
25. Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. 1956. Psychol Rev 1994; 101: 343–352.
26. Davidson J. A survey of the satisfaction of upper limb amputees with their prostheses, their lifestyles, and their abilities. J Hand Ther 2002; 15: 62–70.
27. Hermansson LM, Fisher AG, Bernspang B, Eliasson AC. Assessment of capacity for myoelectric control: a new Rasch-built measure of prosthetic hand control. J Rehabil Med 2005; 37: 166–171.
28. Russell DJ, Avery LM, Rosenbaum PL, Raina PS, Walter SD, Palisano RJ. Improved scaling of the gross motor function measure for children with cerebral palsy: evidence of reliability and validity. Phys Ther 2000; 80: 873–885.
| Appendix |
| | Functional tests | Questionnaires |
| UBET | AHA | PUFI | ABILHAND-Kids |
| Publication | Bagley et al.(8) www.shrinershq.org/research | Krumlinde-Sundholm et al. (9) | Wright et al. (10, 11) | Arnould et al.(12) www.abilhand.org |
| Diagnosis | ULRD | CP, OBPP | ULRD | CP |
| Age | 2–12 years | 18 months – 5 years + 5–12 years | 3–18 years | 6–12 years |
| Purpose | To evaluate arm or prosthetic functioning in children with ULRD who do or do not wear a prosthesis (capacity) | To evaluate activity performance by assessing how effectively the child uses his/her assisting hand (affected hand) in bimanual play. | To evaluate the prosthetic use in daily activities, the ease of task performance with or without the prosthesis and the usefulness of a prosthesis | To measure the ability of children with CP to use their hands in daily activities |
| Items | 9 bimanual activities (4 age versions: 2–4, 5–7, 8–10 and 11–21 years) | 22 items on quality of task performance* | 26 (younger child); 38 (older child) bimanual activities | 21 (mostly) bimanual activities |
| Assessment time | 15 minutes | 10–15 minutes play session + 15 minutes scoring from video | 30 minutes | 10 minutes |
| Rating scale | Completion of task: 4 = no difficulty; 3 = minimal difficulty; 2 = moderate difficulty; 1 = maximal difficulty; 0 = unable to complete task. Method of use: A = active use; P = passive use; E = elbow or trunk grasp; N = no use. | Effectiveness of assisting hand: 4 = effective; 3 = somewhat effective; 2 = ineffective; 1 = does not do. | Ease of performance: 4 = no difficulty; 3 = some difficulty; 2 = great difficulty; 1 = with help; 0 = cannot do. Method of performance: bimanual; with use of forearm, elbow or trunk; one-handed; with help; cannot do. Usefulness of prosthesis: 2 = very useful; 1 = somewhat useful; 0 = not useful. | Difficulty: 2 = easy; 1 = difficult; 0 = impossible |
| Sum scores | 0–36 | 0–100 | 0–100 | 0–42 (raw sum scores) |
| Validity | | Rasch analysis confirmed unidimensional hierarchical scale | Correlation with UNB test and observational assessment | Rasch analysis confirmed unidimensional hierarchical scale Relation tested with age, gender, handedness, school education, type of CP and gross motor function |
| Test-retest reliability | Method of use: Kappa coefficient = 0.43–0.85 | | ICC: 0.40–0.84 | Pearson r = 0.91 |
| Inter-rater reliability | Completion of task: ICC = 0.58–0.97 Method of use: Kappa coefficient = 0.40–0.82 | ICC: 0.97 (AHA News, March 2005) | ICC: 0.30–0.77 | – |
| CP: cerebral palsy; OBPP: obstetric brachial plexus palsy; ULRD: upper limb reduction deficiency. *21 items for children with ULRD, in consultation with the authors, the item “moves fingers” was removed from the test. |