OBJECTIVES: To compare the efficacies of an intramuscular stimulation technique and 0.5% lidocaine injection to trigger points in myofascial pain syndrome.
PARTICIPANTS: Forty-three people with myofascial pain syndrome of the upper trapezius muscle.
Interventions: Twenty-two subjects were treated with intramuscular stimulation and another 21 with 0.5% lidocaine injection at all the trigger points on days 0, 7 and 14.
RESULTS: Intramuscular stimulation resulted in a significant reduction in Wong-Baker FACES pain scale scores at all visits and was more effective than trigger point injection. Intramuscular stimulation also resulted in significant improvement on the Geriatric Depression Scale – Short Form. Local twitch responses occurred in 97.7% (42/43) of patients. All the passive cervical ranges of motion were significantly increased. Post-treatment soreness was noted in 54.6% of patients in the intramuscular stimulation group and 38.1% in the trigger point injection group, respectively, and gross subcutaneous haemorrhage (> 4 cm2) was seen in only one patient in the trigger point injection group.
CONCLUSION: In managing myofascial pain syndrome, after one month intramuscular stimulation resulted in more significant improvements in pain intensity, cervical range of motion and depression scales than did 0.5% lidocaine injection of trigger points. Intramuscular stimulation is therefore recommended for myofascial pain syndrome.
Key words: myofascial pain syndrome, intramuscular stimulation, trigger point injection.
J Rehabil Med 2007; 39: 374–378
Correspondence address: Ji-Ho Choi MD, PhD, Inha University Hospital, 7-206, 3-GA, Sinheung-Dong, Jung-Gu, Incheon, 400-711, Korea. E-mail: wisdom@inha.ac.kr
Submitted August 22, 2006; accepted December 27, 2006
INTRODUCTION
Approximately 10% of the general population have one or more chronic disorders of the musculoskeletal system; these are among the leading causes of disability in the elderly population (1, 2). Myofascial pain syndrome (MPS) is characterized by trigger points (TP) in a taut band of muscle fibres, limited range of motion in joints, referred pain and local twitch response (LTR) during mechanical stimulation of TP (3).
Non-pharmacological treatment modalities include trigger point injection (TPI) with local anaesthetic, saline, or steroids, acupuncture, osteopathic manual medicine techniques, massage, acupressure, ultrasonography, application of heat or ice, transcutaneous electrical nerve stimulation, and ethyl chloride Spray and Stretch technique, but the long-term clinical efficacy of various therapies is not clear, because data that incorporates pre- and post-treatment assessments with control groups are not available (4). Some studies showed that TPI with lidocaine injection is better than dry needling in view of pain reduction and improvements of range of motion (ROM) (5, 6). In fact, lidocaine injection at TP, as recommended by Simons et al. (3), is a technique utilized primarily for MPS in current clinical environments (6).
There has recently been growing interest in the intramuscular stimulation (IMS) technique developed by Gunn (7), which regards nerve roots of associated segmental regions as causes and treatment targets of chronic pain. Based on “Canon’s Law of Denervation Supersensitivity”, Gunn emphasized that, when a portion from a chain of nerve units is destroyed, the receptor sensitivities to chemical stimuli in that point and the zones below it (muscles, skin, blood vessels, ligaments, periostea) become abnormally increased and that these effects are maximized at the directly damaged sites (8). Gunn also insisted that the most common sites of supersensitivity are skeletal muscles; this leads to the “muscle shortening” when a nerve unit is injured, and by which MPS is induced (7). The IMS technique is grounded upon neuroanatomy and neurophysiology of damaged segments in examining and managing various symptoms, so it is suggested that pain from denervation supersensitivies can be treated effectively only by IMS techniques, not by the established classic methods (9).
Several investigations have recently revealed that IMS is superior to dry needling at TP for MPS in the alleviation of pain and ROM improvements (10, 11); however, few studies were found that compared TPI effects with IMS and also few clinical trials have been published for populations of advanced age with MPS.
This randomized prospective, single-blind trial was designed to evaluate and compare the efficacies and adverse events of IMS and lidocaine injection to TP in MPS of a community-based elderly population.
METHODS
Participants
Forty-five subjects with chronic MPS of the upper trapezius were selected from a pool of 50 volunteers at 4 community-based facilities. Subjects were selected based on physical examinations and interviews after informed consent had been given. Except for 2 dropouts, 43 (5 males and 38 females) people were randomized into 2 groups: (i) the IMS group and (ii) the TPI group. Under the following circumstances, participants were excluded from this study: (i) having myofascial TPI or IMS within the 6 months immediately preceding this study; (ii) having neck and/or shoulder surgery within one year preceding this study; (iii) taking narcotic medicine within one month preceding this study; (iv) having symptoms and signs meeting the 1990 ACR (American College of Rheumatology) criteria for fibromyalgia; (v) having a diagnosis of cervical radiculopathy or myelopathy; (vi) having severe cardiovascular or respiratory diseases; (vii) allergy history for drugs or injections, per se, (viii) having evidence of a cognitive deficit or difficulty with communication; (ix) exhibiting inadequate co-operation. There was no significant difference between both groups concerning age, gender, body mass index (BMI) and or literacy rates (Table I).
| Table I. Characteristics of study subjects (mean (SD)). |
| Groups | n | Gender (M/F) | Age (years) | BMI (kg/m2) | Illiteracy |
| IMS | 22 | (3/19) | 76.27 (8.63) | 24.02 (3.34) | 1 |
| TPI | 21 | (2/19) | 75.90 (8.69) | 24.46 (2.46) | 2 |
| p-value | | 1.000* | 0.986† | 0.323† | 0.208† |
| Total | 43 | (5/38) | | | 3 |
| *Analysed by χ2 test. †Analysed by Student’s t-test. BMI: body mass index; Illiteracy: inability to read; IMS: intramuscular stimulation; TPI: trigger point injection with 0.5% lidocaine injection. |
Treatment protocols
TP needling was performed by the modified technique suggested by Simons et al. (3). The subjects were asked to lie in a prone position. The taut band, localized between the thumb and the index finger, was needled forward and backward repeatedly until all the TPs were inactivated (3, 12, 13). The patients were treated at weeks 0, 1 and 2 using the techniques described below.
• IMS group: TP needling was carried out by the method described above with stainless steel needles (diameter 0.30 mm, length 60 mm; Dong-Bang Korea, Korea) fixed by a plunger (Neo-Doctor, Korea); nerve root stimulation at the C3–5 level by the technique recommended by Gunn (7) was also performed. The stimulating methods we chose were "grasping and winding up."
• TPI group: TP needling was carried out by the method described above with 5 ml syringes (Bo-In Medica, Korea) with 25-gauge, 38 mm long needles, pre-filled with 0.5% lidocaine. Injections were made at 0.2 ml per TP.
All treatments were performed by the author, who completed the "Trigger Point Injection Training Course" held by the Korean Academy of Rehabilitation Medicine and the "Basic Course for Gunn's IMS" by the Korea Society of Interventional Muscle and Soft Tissue Stimulation Therapy.
The volunteers were instructed to continue self-stretching exercises (3) for the upper trapezius muscle 3 times per day until the next treatment.
Outcome measures
Patients described their current intensity of pain at the shoulder and neck and their headache based on a visual analogue scale (VAS) from 0 to 10, and Wong-Baker FACES pain scale (FACES) from 0 to 5. TP pain pressure threshold scores (PTS) were obtained by placing the thumb on the skin covering the muscle containing the TP in a perpendicular fashion and exerting pressure until there was whitening of the nail bed and then evaluating the pain intensity. Scoring was from 0 to 3 (0 = no report of pain and no visible reaction, 1 = report of pain, 2 = painful tenderness and visible reaction by face, 3 = severe pain and marked visible reaction or avoidance). All the results were obtained on days 0, 7, 14 and 28 just before each treatment.
A goniometer was used to measure passive ROM of the cervical spine during anterior flexion, extension (posterior flexion), lateral tilting to the right and left, and rotation to the right and left on days 0, 7, 14 and 28 just before each treatment.
Depression was evaluated using the Korean version of the Geriatric Depression Scale – Short Form (GDS-SF) on days 0 and 28.
We surveyed the number of cases and duration of post-treatment soreness at the second visit and the number of cases of haemorrhage greater than 4 cm2 and dizziness at every visit.
Interviews were carried out by the staff employed by each facility who had educational qualifications equal to or higher than those of high-school graduates. All physical examinations were performed by the author, a family physician and an authorized geriatrician, and were supervised by 2 residents of family medicine. All results were recorded after mutual agreement among these 3 doctors.
Blinding
The volunteers were not informed which group they were in, and were treated in a prone posture with the aim of not exposing volunteers to the differing methods. Also, when performing the physical examinations the author did not know to which group the subjects had been assigned.
Statistical analysis
Paired t-tests were used to compare VAS, Wong-Baker FACES scale, PTS and GDS-SF values between days 0 and 28, and slopes of changes according to time were compared by repeated measures ANOVA. Adverse events among the 2 groups were analysed by Student’s t-test and gender was analysed by χ2 test. Statistical significance was set at 0.05.
RESULTS
Pain
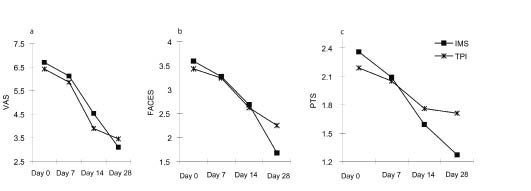
There was no significant pre- and post-treatment difference in VAS, Wong-Baker FACES scale and PTS between both groups at all visits (p > 0.05 by Student’s t-test). Significant improvements were observed in VAS and Wong-Baker FACES scale at the end of the first month after whole treatments in both groups (Table II). For the VAS, the TPI group results between days 14 and 28 did not show a significant difference (p = 0.084 by paired t-test) In Wong-Baker FACES scale, TPI group between days 0–7 and 14–28 did not show significant differences (p = 0.104 and p = 0.069, respectively, by paired t-test). In PTS, TPI group did not show significant improvement between days 0–7, 7–14, and 14–28 (p > 0.05 by paired t-test). The IMS group showed significant improvements in VAS, Wong-Baker FACES scale and PTS on every visit except PTS between days 0 and 7 (p = 0.110).
| Table II. Serial changes in values of pain and depression (mean (SD)). |
| Values | Day 0 (pre-treatment) | Day 7 | Day 14 | Day 28 | p-value* |
| IMS group |
| VAS | 6.71 (1.84) | 6.13 (1.85) | 4.54 (1.82) | 3.11 (2.01) | < 0.001 |
| FACES | 3.59 (0.73) | 3.27 (0.77) | 2.68 (0.65) | 1.68 (0.84) | < 0.001 |
| PTS | 2.36 (0.66) | 2.09 (0.75) | 1.59 (0.73) | 1.27 (0.88) | < 0.001 |
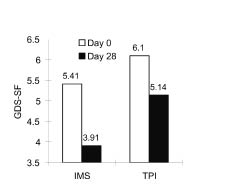
| GDS-SF | 5.41 (3.63) | | | 3.91 (3.19) | 0.024 |
| TPI group |
| VAS | 6.43 (2.08) | 5.87 (2.37) | 3.90 (2.12) | 3.46 (2.47) | < 0.001 |
| FACES | 3.43 (0.87) | 3.24 (0.94) | 2.62 (0.92) | 2.25 (1.16) | < 0.001 |
| PTS | 2.19 (0.60) | 2.05 (0.50) | 1.76 (0.77) | 1.71 (0.72) | 0.038 |
| GDS-SF | 6.10 (3.95) | | | 5.14 (4.35) | 0.086 |
| *Analysed by paired t-test between each value for days 0 and 28. VAS: visual analogue scales (0–10); FACES: Wong-Baker FACES pain scales (0–5); PTS: trigger point pain pressure threshold scores on thumb nail bed whitened; GDS-SF: Korean version of geriatric depression scales – short form, IMS: intramuscular stimulation, TPI: trigger point injection with 0.5% lidocaine injection. |
Depression
There was no difference between the 2 groups in values of GDS-SF (p = 0.515 by Student’s t-test) before treatment. Only the IMS group indicated significantly favourable changes at the end of the first month after treatment (p = 0.024) (Table II, Fig. 1).
Fig. 1. Changes in depression measured by Korean version of Geriatric Depression Scales – short form (GDS-SF) (p = 0.024 in IMS group and p = 0.086 in TPI group by paired t-test). IMS: intramuscular stimulation, TPI: 0.5% lidocaine injection.
Slopes of pain changes
There was significant interaction between time and type of treatment in Wong-Baker FACES scale, but no significant interactions were found in VAS and PTS (Fig. 2).
Fig. 2. (a) Serial changes in visual analogue scale (VAS) values (p = 0.285 for time × group interaction by repeated measures ANOVA). (b) Serial changes in Wong-Baker FACES Scale values (p = 0.013 for time × group interaction by repeated measures ANOVA). (c) Serial changes in Pain Pressure Threshold Scores (PTS) (p = 0.402 for time × group interaction by repeated measures ANOVA). IMS: intramuscular stimulation, TPI: trigger point with 0.5% lidocaine injection.
Local twitch response during injection or needling
LTRs were elicited in 81.4% (35/43) during the first treatments, and 97.7% (42/43) showed at least one LTR during the entire course of treatments. Only one subject from the TPI group never showed LTR.
Passive cervical range of motion
All the passive ROMs were improved in both groups on every visit (Table III). In a paired comparison between the IMS and TPI groups, there was significant interaction preferring the IMS group only in extension of the ROM.
| Table III. Serial changes in passive cervical ROM* (mean (SD)). |
| Values | Day 0 (pre-treatment) | Day 7 | Day 14 | Day 28 | p-values† |
| IMS group |
| Flexion | 49.09 (10.08) | 57.73 (11.72) | 67.05 (14.36) | 78.18 (7.80) | < 0.001 |
| Extension | 64.09 (16.08) | 61.36 (17.13) | 69.32 (15.14) | 72.50 (13.52) | 0.007 |
| Tilting | 58.86 (21.15) | 70.45 (19.39) | 79.77 (25.52) | 84.77 (22.60) | < 0.001 |
| Rotation | 138.18 (24.91) | 142.05 (21.75) | 152.50 (16.74) | 155.68 (20.31) | 0.002 |
| TPI group |
| Flexion | 41.67 (12.88) | 46.90 (14.45) | 59.29 (15.35) | 68.33 (14.78) | < 0.001 |
| Extension | 58.10 (17.14) | 65.95 (13.10) | 68.57 (11.95) | 65.00 (13.87) | 0.049 |
| Tilting | 59.29 (19.32) | 69.29 (19.58) | 76.43 (19.63) | 76.90 (21.24) | 0.001 |
| Rotation | 140.00 (25.50) | 148.10 (21.94) | 151.19 (21.67) | 155.48 (16.87) | 0.001 |
| *Passive range of cervical motion in degrees of an angle. †Analysed by paired t-test between each value for days 0 and 28. Tilting: right tilting + left tilting, Rotation: right rotation + left rotation; IMS: intramuscular stimulation, TPI: 0.5% lidocaine injection. |
Post-treatment soreness
There were no significant differences in numbers or the duration of post-needling or post-injection soreness. Visible subcutaneous haemorrhage (> 4 cm2) was examined in 1 subject in the TPI group, but not in IMS group (Table IV).
| Table IV. Adverse events after each treatment (mean (SD)). |
| Groups | Total | Cases with soreness n (%) | Duration of soreness (days) | Cases with haemorrhage* n (%) | Cases with dizziness n (%) |
| IMS | 22 | 12 (54.6) | 1.73 (2.05) | 0 (0) | 2 (9.1) |
| TPI | 21 | 8 (38.1) | 1.48 (2.32) | 1 (4.8) | 1 (4.8) |
| p-value† | | 0.379 | 0.535 | 0.037 | 0.274 |
| *Visible subcutaneous haemorrhage > 4 cm2. †Analysed by Student’s t-test. IMS: intramuscular stimulation, TPI: 0.5% lidocaine injection. |
Drop-outs
Two of the initial 45 participants dropped out. One from the IMS group discontinued treatment for fear of needling pain and 1 from the TPI group dropped out for unknown reasons.
DISCUSSION
Local twitch response
In this study, 97.7% of subjects showed LTR at least once, and this might have contributed to the favourable results. As the previous clinical studies (3, 5) demonstrated, dry needling per se is as effective as the injection of local anaesthetics in inactivating a TP, and LTR is a valuable factor for obtaining an optimal effect. It should be noted that, compared with 70.7% of LTR rate among younger subjects (in their early 40s) in the previous study (5), most of our elderly subjects (mean age 76 years) showed LTR.
Pain
Among the VAS, Wong-Baker FACES Scale and PTS, only the Wong-Baker FACES Scale showed significant time × group interaction. The mean age of the participants was 76 years. Incidentally, recent surveys suggest inconsistency in the VAS for pain scales among the elderly (14, 15), and several studies (16, 17) support the usefulness of Faces Pain Scales for people over 65 years. These results therefore indicate that IMS reduced pain more effectively than TPI.
Concerning serial changes in the VAS and Wong-Baker FACES Scale, IMS showed significant pain relief on every visit, but TPI did not after 2 weeks. This phenomenon suggests that the effect of IMS lasts longer than that of TPI and that the therapeutic effects of TPI decreased 2 weeks after treatment, which is similar to results determined in former studies (5).
Depression
Only the IMS group showed significant improvement in GDS-SF scores after 4 weeks. Many surveys (18–20) have revealed that depression in the elderly group has a positive correlation with pain intensity, so this favourable change in depression rates in the IMS group is thought to be associated with pain relief. More than half of the participants in this study were isolated from their family members; it is the author’s hypothesis that the fact that the greater time requirement for the IMS technique (when compared with the time needed for TPI) might have had a positive effect on pain and depression relief.
Passive cervical range of motion
Both the IMS and TPI methods showed increases at each examination for all kinds of ROM. However, IMS resulted in a better effect than TPI in extension ROM. Compared with the TPI method, which targeted only the upper trapezius, the IMS technique also released paravertebral nerve roots, and this might be one of the contributing factors for the difference. The hypothesis for this phenomenon is that, anatomically, the upper trapezius muscle is related with neck extension, tilting and rotation, so after release of the TP for the trapezius muscle, neck flexion, tilting and rotation ROM can be increased with relatively little effect on extension ROM.
Mechanisms of intramuscular stimulation on myofascial pain syndrome
Nerve roots are surrounded by nerve sheaths, cerebrospinal fluid and meninges, and adjacent networks of arterioles and venules are, therefore, loose. This structure makes nerve roots susceptible to mechanical injury or stimulation and leads to “neuropathy” (21). Neuropathic lesions stretch nerve roots and make vascular coil twist, ultimately increasing mechanosensitivity and inducing pain (22–24). This theory is associated with “Canon’s law of denervation supersensitivity” (8) and is the theoretical basis for IMS.
The recently emerged concept of “peripheral neuropathic pain” includes peripheral nerve or nerve root lesions and “nerve trunk pain,” a form of peripheral neuropathic pain that cannot easily be distinguished from pain resulting from MPS (25, 26). In addition, myofascial TP and/or motor points show spontaneous needle electro-myography activity (27), and a survey revealed that radiating pain and other sensory disorders originate from sites of nerve-entrapment where ectopic neural pacemaker nodules are made (25). Therefore, MPS can be explained as a form of nerve root dysfunction.
With the IMS technique, muscle stimulation is performed by the rotation of a needle, which is connected to muscle fibres and makes strong stimulation of muscle proprioceptors (9). A recent animal study (28) suggests that needle grasp is due to mechanical coupling between the needle and the connective tissue, rather than muscle, with the winding of tissue around the needle during rotation. It was also hypothesized that needle manipulation transmits a mechanical signal to the connective tissue cells via mechanotransduction. Such a mechanism may explain local, remote and long-term effects of needling.
The overall effects of IMS in our study can be explained by the above hypotheses.
Adverse events
Post-needling or post-injection soreness is due to local haemorrhage at the needling site and can be prevented by sufficient compression after treatment (3). In the previous study (5), the TPI group complained of less soreness than the IMS group after treatment, but in the current study there was no significant difference. There are 2 possible explanations for this result: (i) we used pointed-tipped needles for IMS and thicker syringe needles for TPI. Thick and wet needles (syringe needles) can induce more tissue injuries than thinner, pointed dry needles (7). The fact that visible haemorrhages were examined only in the TPI group supports this hypothesis; (ii) we asked the question about soreness one week later, which might have affected the memories of the subjects related to their soreness.
Limitations of this study
First, we measured the pain threshold with thumb pressure not using an algometer. However, some surveys (29, 30) indicate digital and algometer measures are equally reliable, and the examination was performed by a blinded experienced physician under strict monitoring by 2 other doctors on every visit.
Secondly, the staff administering the procedures were not completely blinded from measuring outcomes, except in the first visit, because we carried out every treatment just after each measurement. But all the procedures were performed by standard methods (3, 7, 12, 13).
In conclusion, intramuscular stimulation and 0.5% lidocaine injections into the TP effectively reduced pain intensity and cervical ROM among the elderly participants with myofascial pain syndrome of the upper trapezius muscle. Intramuscular stimulation resulted in significant improvements on geriatric depression scales after 4 weeks and on the extension of cervical ROM, with no visible haemorrhage at the needling site. Overall, IMS is suggested to be an optimal method for management of myofascial pain syndrome of the upper trapezius muscle.
acknowledgement
This work was supported by INHA UNIVERSITY Research Grants.
REFERENCES
1. Imamura ST, Fischer AA, Imamura M, Teixeira MJ, Tchia YL, Kaziyama HS, et al. Pain management using Myofascial approach when other treatment failed. Phys Med Rehabil Clin North Am 1997; 8: 179–196.
2. Cole TM, Edgerton VR. Musculoskeletal disorders. In: Cole TM, Edgerton VR, editors. Report of the Task Force on Medical Rehabilitation Research: June 28–29, 1990, Hunt Valley Inn, Hunt Valley, MD. Bethesda: National Institutes of Health; 1990, p. 61–70; 1999, p. 11–93.
3. Simons DG, Travell JG, Simons LS, editors. Travell & Simons’ myofascial pain and dysfunction: the trigger point manual, 2nd edn. Baltimore: Williams & Wilkins; 1999, p. 5.
4. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician 2002; 65: 653–660.
5. Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point. Importance of the local twitch response. Am J Phys Med Rehabil 1994; 73: 256–263.
6. Kamanil A, Kaya A, Ardicoglu O, Ozgocmen S, Ozkurt Zengin F, Bayik Y. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int 2005; 25: 604–611.
7. Gunn CC, editor. The Gunn approach to the treatment of chronic pain, New York: Churchill Livingstone; 1996, p. 1–19.
8. Canon WB, Rosenblueth A, editors. The supersensitivity of denervated structures, New York: Macmillan; 1949, p. 195–203.
9. Ahn K. What is IMS? What is pain? J Korean Acad Fam Med 1999; 20: 1496–1509 [in Korean].
10. Byeon HT, Park SH, Ko MH, Seo JH. Effects of intramuscular stimulation in myofascial pain syndrome of upper trapezius muscle. J Korean Acad Rehab Med 2003; 27: 753–756 [in Korean].
11. Karakurum B, Karaalin O, Coskun Ö, Dora B, Üçler S, Inan LE. The “dry-needle technique”: intramuscular stimulation in tension-type headache; Blackwell Science Ltd Cephalalgia 2001; 21: 813–817.
12. Yunus MB, Kalyan-Raman UP. Muscle biopsy findings in primary fibromyalgia and other forms of nonarticular rheumatism. Rheum Dis Clin North Am 1989; 15: 115–134.
13. Rosen NB. Physical medicine and rehabilitation approaches to the management of myofascial pain and fibromyalgia syndromes. Baillieres Clin Rheumatol 1994; 8: 881–916.
14. Gagliese L, Weizblit N, Ellis W, Chan VW. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain 2005; 117: 412–420.
15. Gloth FM 3rd, Scheve AA, Stober CV, Chow S, Prosser J. The Functional Pain Scale: reliability, validity, and responsiveness in an elderly population. J Am Med Dir Assoc 2001; 2: 110–114.
16. Herr KA, Mobily PR, Kohout FJ, Wagenaar D. Evaluation of the Faces Pain Scale for use with the elderly. Clin J Pain 1998; 14: 29–38.
17. Miro J, Huguet A, Nieto R, Paredes S, Baos J. Evaluation of reliability, validity, and preference for a pain intensity scale for use with the elderly. J Pain 2005; 6: 727–735.
18. Roy R. A psychosocial perspective on chronic pain and depression in the elderly. Soc Work Health Care 1986; 12: 27–36.
19. Williamson GM, Schulz R. Pain, activity restriction, and symptoms of depression among community-residing elderly adults. J Gerontol 1992; 47: 367–372.
20. Chou KL, Chi I. Reciprocal relationship between pain and depression in elderly Chinese primary care patients. Int J Geriatr Psychiatry 2005; 20: 945–952.
21. Olmarker K, Rydevik B, Hansson T, Holm S. Compression-induced changes of the nutritional supply to the porcine cauda equina. J Spinal Disord 1990; 3: 25–29.
22. Ogata K, Ngaito M. Blood flow of peripheral nerve effects of dissection, stretching and compression. J Hand Surg 1986; 11B: 10–14.
23. Lundborg G, Gelberman RH, Minteer-Convery M, Lee YF, Hargens AR. Median nerve compression in the carpal tunnel – functional response to experimentally induced controlled pressure. J Hand Surg 1982; 7: 252–259.
24. Shacklock MO, editor. Moving in on pain. Australia: Butterworth Heinemann; 1995, p. 125.
25. Devor M, Rappaport ZH. Pain and pathophysiology of damaged nerve. In: Fields HL, editor. Pain syndromes in neurology. Oxford: Butterworth Heinemann; 1990, p. 47–83.
26. Devor M. Neuropathic pain and injured nerve: peripheral mechanisms. Br Med Bull 1991; 47: 619–630.
27. Hubbard D, Berkoff. Myofascial trigger points show spontaneous needle EMG activity. Spine 1993; 18: 1803–1807.
28. Langevin HM, Churchill DL, Cipolla MJ. Mechanical signaling through connective tissue: a mechanism for the therapeutic effect of acupuncture. FASEB J 2001; 15: 2275–2282.
29. Cott A, Parkinson W, Bell MJ, Adachi J, Bedard M, Cividino A, et al. Interrater reliability of the tender point criterion for fibromyalgia. J Rheumatol 1992; 19: 1955–1959.
30. Tunks E, McCain GA, Hart LE, Teasell RW, Goldsmith CH, Rollman GB, et al. The reliability of examination for tenderness in patients with myofascial pain, chronic fibromyalgia and controls. J Rheumatol 1995; 22: 944–952.