FACILITATING RECOVERY: EVIDENCE FOR ORGANIZED STROKE CARE*
Lalit Kalra1 and Peter Langhorne2
From the 1Department of Stroke Medicine, King’s College London School of Medicine, London and 2Academic Section of Geriatric Medicine, Glasgow, UK
FACILITATING RECOVERY: EVIDENCE FOR ORGANIZED STROKE CARE*
Lalit Kalra1 and Peter Langhorne2
From the 1Department of Stroke Medicine, King’s College London School of Medicine, London and 2Academic Section of Geriatric Medicine, Glasgow, UK
Despite dramatic advances in the management of thrombolysis and acute stroke, organized rehabilitation remains the cornerstone of recovery from stroke. The importance of organized stroke care in facilitating recovery has been recognized for the last 10 years, but it is still unclear how organized rehabilitation contributes to improved outcomes. This paper presents a synthesis of evidence of the benefits of organized care, especially with respect to stroke severity and different types of organized stroke care. It presents an overview of possible processes within organized rehabilitation that may contribute to good outcomes. The role of integrated care pathways within rehabilitation settings is discussed, highlighting the limitations of current evidence and uncertainty about their benefits. Finally, some key challenges have been identified for stroke units in improving rehabilitation outcomes over the next decade and for healthcare planners in investing adequately in organized stroke services.
J Rehabil Med 2007; 39: 97–102
Correspondence address: Lalit Kalra, Department of Stroke Medicine, King’s College London School of Medicine, Denmark Hill Campus, Bessemer Road, London SE5 9PJ, UK. E-mail: lalit.kalra@kcl.ac.uk
Submitted September 5, 2006; accepted November 20, 2006
*This paper is based partly on a lecture given at the international symposium ”Evidence for stroke rehabilitation – bridging into the future”, in Göteborg, Sweden, 26–28 April, 2006.
Introduction
Stroke is a major health problem and a leading cause of death and adult disability worldwide (1). Population statistics suggest that there are 5 million stroke deaths and that 15 million people suffer non-fatal strokes each year worldwide. It is estimated that there may be as many as 50 million stroke survivors worldwide, with significant physical and cognitive consequences of their stroke (1). Studies show that as many as 12–18% of survivors are dysphasic, 22% may be unable to walk, 32% are clinically depressed and 24–53% remain dependent on caregivers for activities of daily living (2). The economic costs of stroke are also considerable; a recent National Audit Office report shows that stroke care costs the National Health Service (NHS) in the UK about £2.8 billion a year in direct care costs and another £2.4 billion in informal care costs (3).
The introduction of thrombolysis for patients after acute stroke in clinical practice has revolutionized the management of patients after stroke. There are no doubts that thrombolysis is indeed a powerful intervention that significantly reduces death or dependency (odds ratio (OR) 0.66, 95% confidence interval (CI) 0.53–0.83) with no significant increase in adverse effects (OR 1.13, 95% CI 0.86–1.48) for patients treated within 3 hours of ischaemic stroke onset (4). This equates to one extra patient being alive and independent for every 7 patients treated. Unfortunately, the trial benefits of thrombolysis do not translate in clinical effectiveness in mainstream practice. The proportion of patients with ischaemic stroke varies between settings, ranging from 80–85% in western settings to 60–65% in East European and Asian settings, where the prevalence of untreated hypertension may be higher (1). In addition, the 3-hour time window for thrombolysis severely limits the benefits of an otherwise powerful intervention. Many studies have shown that only 25–33% of patients present to hospitals within 3 hours of stroke onset (5, 6) and that only a small proportion, 5–11% of incident ischaemic stroke, actually end up being thrombolysed (7, 8). Taking into account the number of patients with ischaemic stroke, the number eligible for thrombolysis and the numbers likely to benefit following thrombolysis, only 1–2 in every 100 patients after stroke are likely to derive benefit from this treatment, emphasizing the need for other proven interventions, such as organized stroke care, to complement thrombolysis for improving outcomes (9).
CONCEPTUAL RATIONALE FOR ORGANIZED STROKE CARE
Evidence suggests that organized care, such as that provided in stroke units, both facilitates neurological recovery and expedites discharges (Fig. 1) (10). An important concept in rehabilitation is that of “brain plasticity”, which implies that it is possible to modulate or facilitate reorganization of cerebral processes by external inputs. This is supported by positron emission tomography and functional magnetic resonance imaging studies showing patterns of increased activation in the uninjured ipsilateral and intact contralateral areas of the brain after stroke, which correlate with the level of recovery (11). The paradigm for function has shifted from strict cerebral localization to that of interactive functioning multiple motor circuits activated by the constantly changing balance of inhibitory and excitatory impulses. Disruption of major pathways in stroke reduces the inhibition normally exerted by these pathways and allows activation of alternate pathways, which take over the function of the damaged circuits (12). Furthermore, neuroimaging studies have shown that increased intensity of therapy results in greater activation of areas associated with the function towards which this therapy is directed (13). Hence, evidence suggests that the human brain is capable of significant recovery after stroke, provided that the appropriate treatments and stimuli are applied in adequate amounts and at the right time. It is likely that this is achieved better with organized stroke care, where the intensity and timing of interventions can be managed proactively.
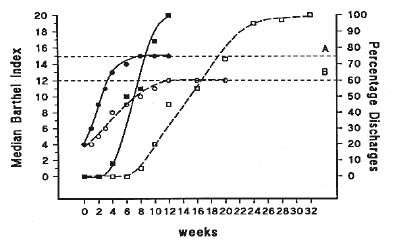
Fig. 1. Effect of stroke units on functional recovery and discharge (10). A = Barthel Index of patients managed on the stroke rehabilitation unit; B = Barthel Index of patients managed on general medical wards.
p-value A vs B = 0.001.
= stroke rehabilitation unit; = general medical ward.
The other conceptual rationale for organized stroke care is the awareness that stroke affects several domains of human performance and results in multiple impairments, many of which have significant interactions in determining the level of disability (14). It is also clear that no single discipline has all the skills, resources and expertise required to manage all aspects of recovery from stroke. Facilitation of recovery is further compounded by the different speeds at which impairments recover, demanding a staged approach to interventions and therapy inputs. Rehabilitation goals are also shaped by personal needs of stroke patients, the environment they will return to and the personal support available after discharge. Hence, the complex interdisciplinary process of stroke rehabilitation requires a multidisciplinary approach and collaborative policy of co-ordinated delivery of treatments based on comprehensive assessments and delivered by staff trained in stroke management in consultation with patients and their caregivers. This level of co-ordination of care is another argument to support the development of organized stroke services (15).
EVIDENCE FOR ORGANIZED CARE
During the 1980s and 1990s a number of randomized controlled trials (RCTs) suggested that organized care offered advantages to patients with stroke. However, many of these studies were too small to demonstrate a robust statistical benefit. Hence the Stroke Unit Trialists’ Collaboration (SUTC) was set up to pool data from these and other ongoing studies from Australia, North America and Europe (16). These studies were undertaken in different settings using different methods of organized care and patients after stroke at varying duration from stroke onset. Interventions ranged from acute dedicated units to teams providing co-ordinated care in community settings and the patients included in the study ranged from those within a few hours of stroke onset to those included only when they were neurologically and medically stable. Despite these variations, the meta-analysis of pooled data from 29 trials, which include 6536 patients, shows odds reductions in mortality of 0.86 (95% CI 0.71–0.94), death or dependence of 0.78 (95% CI 0.68–0.89), and death or institutionalisation of 0.80 (95% CI 0.71–0.90) at one year associated with organized care, which are independent of age and gender (17). More importantly, and in contrast with thrombolysis for acute stroke, these benefits are seen for all patients after stroke regardless of stroke aetiology or the duration between stroke onset and intervention, suggesting that most, rather than only a small proportion of, patients after stroke will benefit from this intervention (9). This expectation of the translation of trial efficacy into clinical effectiveness in mainstream practice has been demonstrated in longitudinal studies (18, 19).
ISSUES IN THE ORGANIZATION OF STROKE CARE
Although evidence strongly supports a role for organized care in improving mortality, dependence and institutionalization in patients after stroke, there is considerable debate about the different methods of organizing stroke care, the type of patients after stroke who may benefit most from organized care, the sustainability of the benefits seen in various studies and mechanisms that contribute towards the good outcomes with organized stroke care.
Strategies for organizing stroke care
One of the difficulties faced in the interpretation of the evidence is that organized stroke care, especially stroke units, may mean different things to different people (16). Definitions vary from “a team of specialists who are knowledgeable about the care of patients after stroke and who consult throughout a hospital or the community wherever a patient may be” to “a geographic location within the hospital designated for stroke and stroke-like patients who are in need of medical and rehabilitation services and the skilled professional care that such a unit can provide.” There is also considerable controversy about the number and diversity of disciplines that need to be involved in stroke care, and differences in staff composition between different settings have limited the generalization of findings in individual settings. The prevalent strategies for providing specialist stroke care are summarized in Table I.
| Table I. Different types of stroke care organizations (21) | |||
| Type | Admission | Discharge | Features |
| Acute, intensive | Acute (hours) | Days | High nurse staffing Life support facilities |
| Acute, semi-intensive | Acute (hours) | Days | Close physiological monitoring |
| Comprehensive | Acute (hours) | Days – weeks | Acute care/rehabilitation Conventional staffing |
| Rehabilitation | Delayed | Weeks | Rehabilitation |
| Mobile team | Variable | Days – weeks | Medical/rehabilitation advice |
| Mixed rehabilitation | Variable | Weeks | Mixed patient group Rehabilitation |
There are several problems in assessing the independent benefits of different types of organization of stroke care, mainly because the comparators for organized care in different studies range from general medical wards to different types of organized care. This heterogeneity of comparisons makes it difficult to determine whether one type of stroke care organization is superior to others, as there is no common yardstick against which the benefits of different strategies of stroke care can be measured. This difficulty may be overcome by using the indirect comparisons method, which estimates intervention effects against a common control using mathematical adjustments for the different comparators used in different studies (20). Using this methodology on the SUTC database, Langhorne et al. (21) have shown that there is a definite benefit associated with comprehensive and rehabilitation stroke units and mixed rehabilitation units, all of which show an OR of 0.85 to 0.89 in favour of organized care. There is also a possible benefit with acute (semi-intensive) units, although this just fails to achieve statistical significance (OR 0.88; 95% CI 0.76–1.01). Mobile stroke teams were associated with no benefit in this analysis (OR 0.98; 95% CI 0.95–1.05). There are no trials of acute intensive care, so this strategy of organizing stroke care remains untested. However, a review of the data suggests that emphasis on acute intensive care alone may not be adequate to change overall outcomes and that continuity of care is needed to realize the full potential of organized stroke unit care.
Effect of stroke severity on benefits from organized care
A limitation of most RCTs on organized care is that they include patients with moderate stroke severity and exclude those with mild or very severe strokes. There are several individual studies that have suggested that the benefits of organized care may be limited in patients with less severe strokes and that general care may have outcomes similar to those seen in stroke units (22, 23). On the other hand, patients with severe stroke may benefit more with stroke unit care (23, 24). An analysis of pooled RCT data for patients stratified according to stroke severity at the time of inclusion showed that organized stroke care prevented 1 death/100 patients (95% CI –2 to 3 deaths) in patients with mild stroke (Barthel Index 0–20), 3 deaths/100 patients (95% CI 1 to 6 deaths; p < 0.05) in the those with moderate (Barthel Index 3–9) and 9 deaths/100 patients (95% CI 4 to 14 deaths; p < 0.005) in those with severe strokes (Barthel Index 0–2) (25). This suggests that the benefits of organized inpatient care for mortality increase with stroke severity and that absolute reduction in deaths is greater for patients with more severe stroke. The analysis also showed that most of the deaths prevented were those that would have occurred at 1–4 weeks after stroke onset and would be attributable to stroke-related complications. The heterogeneity in data prevented the assessment of the interaction between organized stroke care and functional recovery, which would be particularly important for patients after stroke, where organized care, understandably, has no effect on mortality.
When are the benefits of organized care greatest?
A variable length of follow-up has been used in various RCTs on organized stroke care, making it difficult to assess the period during which maximum benefits are incurred. Studies suggest that these are during the earlier phases of stroke management with Kaplan–Meier curves showing separation in the first few weeks after stroke (26). However, there are studies that show that the beneficial effect can be sustained as long as 5 or even 10 years, with patients managed in stroke units showing better mortality, institutionalization and functional outcome at these time-points (27, 28). An analysis of the SUTC data for timing of deaths showed that not only was there a significant decrease in mortality in the first 4 weeks after stroke, but there was also a significant decrease in the number of deaths at 5 years after stroke (Table II) (25). Therefore, the survival benefits of stroke unit care appear to occur early and can be long lasting. However, these conclusions are qualified by the decreasing number of patients at the 5-year time-point.
| Table II. Timing of death in the Stroke Unit Trialists’ Collaboration pooled data analysis (19 trials, 3823 patients) (21) | |||
| Time | Stroke unit (%) | Control (%) | Risk difference (95% CI) |
| 4 days | 3 | 4 | –1 (–2, 1) |
| 1 week | 6 | 9 | –3 (–4, –1)* |
| 4 weeks | 13 | 18 | –5 (–7, –3)** |
| 3 months (3823 patients) | 16 | 20 | –4 (–6, –1)* |
| 6 months (3067 patients) | 19 | 24 | –4 (–6, –1)* |
| 1 year (3728 patients) | 28 | 32 | –3 (–6, 0)* |
| 5 years (1139 patients) | 52 | 60 | –7 (–13, –2)* |
| *p < 0.05; **p < 0.005. CI: confidence interval. | |||
Processes that contribute to a good outcome
Organized stroke unit care is considered a “black box” intervention, and several studies have been designed to identify processes that may be associated with good outcomes. Different studies have chosen different foci for investigation, some have compared differences in processes between intervention and control groups, whilst others have investigated the frequency of complications or intensities of therapy input between organized and conventional care. A major problem in the generalizability of the findings of these studies is the fact that most stroke units have evolved in response to local patient needs, priorities and service arrangements, which may not be replicated in other settings (19). Hence, the same process may have a different impact on outcomes on different units, depending upon case mix, the type of unit and the environment in which the unit functions. However, there are some generalizations that can be extended to most units. Research has consistently shown that better outcomes are associated with comprehensive and early processes of stroke-specific assessments, particularly assessments for swallowing and aspiration risk, early detection and management of infections, maintenance of hydration and nutrition, early mobilization, clear goals for function, and communication with patients and their families (Table III) (29, 30). In other words, stroke units appear to improve outcome by greater attention to stroke-specific medical, nursing and therapy processes, greater involvement of caregivers and fewer stroke-related complications.
| Table III. Processes associated with good outcomes in organized care (29) | |||
| SU (%) | Non-SU (%) | OR | |
| Swallow assessment | 89 | 71 | 3.1 (1.7–5.7) |
| O2 therapy | 69 | 52 | 2.0 (1.3–3.3) |
| Rx pyrexia | 82 | 41 | 6.4 (1.5–27.4) |
| Rx aspiration | 85 | 48 | 6.0 (2.3–15.5) |
| Early feeding | 88 | 35 | 14.4 (5.1–40.9) |
| Early mobilization | 82 | 67 | 6.4 (3.3–10.9) |
| OT in 7 days | 40 | 21 | 2.4 (1.5–4.1) |
| SW in 7 days | 15 | 5 | 2.8 (1.1–7.0) |
| Goals defined | 92 | 78 | 3.2 (1.6–6.5) |
| Higher function | 49 | 36 | 1.7 (1.1–2.8) |
| Carer involvement | 77 | 21 | 12.4 (7.2–21.4) |
| OT: occupational therapy; SW: social worker; SU: stroke unit; OR: odds ratio; Rx: treatment. | |||
INTEGRATED CARE PATHWAYS IN IMPROVING ORGANIZED CARE
In addition to improving outcome, organized stroke care has also been responsible for increasing the efficiency of stroke management, with the SUTC meta-analysis showing a 6-day reduction in the overall length of hospital stay (17). The question that arises is whether there are other management techniques that can further improve the co-ordination of processes in stroke care, enhancing the gains achieved with stroke unit type care. Integrated care pathways (ICP) is one of the methods suggested for improving stroke management and is defined as a technique that can facilitate the co-ordination of complex interdisciplinary processes by promoting organized and efficient care, based on best available evidence and guidelines (31).
There are very few studies on the application of ICP methodology to stroke care; a recent meta-analysis was able to identify only 14 studies, of which 3 were RCT and 11 “before and after” studies (32). Two of the 3 RCT and 4 of the 11 other studies were in stroke rehabilitation. Individually, none of the RCT showed any benefits of ICP on stroke outcomes. On the other hand, “before and after” studies suggested some improvements in outcomes using ICP methodology. Despite the heterogeneity of studies, design limitations of the “before and after” studies and inadequate data, all 14 studies were included in a Cochrane type meta-analysis, the validity of which can be debated (32). This meta-analysis showed a positive effect on imaging and vascular studies in acute settings, but no significant effect on patient outcomes, rehabilitation processes or length of stay. In addition, ICP-determined care was associated with less satisfaction and poorer quality of life at the time of discharge.
The role of ICP in rehabilitation on specialist units may be limited, mainly because they fail to capture the full spectrum of physical, psychological, emotional and social needs of patients and are not flexible enough to accommodate the variable and unpredictable course of recovery. The main objective of ICP is to promote collaboration, co-ordination and team functioning in clinical settings; these elements already exist on specialist units and there is limited potential for further improvement. The limitations of ICP in such setting is that they do not have the same flexibility as the multidisciplinary process to accommodate individual patient needs and community-based issues, nor can they enforce team working and shared values, which are a central component of successful units (31).
FUTURE CHALLENGES FOR STROKE UNITS
There is no doubt that considerable progress has been made in the organization of acute and co-ordination of rehabilitation care, which has improved stroke outcomes dramatically. However, it is of concern that a recent paper reported that patients spend more than 50% of their time in bed, 28% sitting out of bed, 13% in therapeutic activities and are alone for 60% of the time during the therapeutic day, even on a stroke unit (33). The first challenge, therefore, is not only to focus on organizing stroke care, but also on strategies to use the time patients spend in stroke units more productively by introducing new processes that increase the intensity of therapeutic activities and interaction with patients.
Recent years have seen the development of new and sophisticated technologies to assist therapists in improving the quantity and quality of rehabilitation for patients after stroke (34). These include techniques such as the use of impairment specific therapy techniques, robotic assisted rehabilitation, virtual reality and motor imagery techniques, all of which have proven effective in small studies. Stroke units offer an excellent environment, not only for testing such techniques, but also the standardized optimal conditions for their implementation in clinical practice. The “added value” of effective treatment of specific impairments will contribute further to improving neurological recovery, activity and participation.
The establishment of stroke units has successfully reduced severe disability and institutionalization, which has increased the number of disabled patients living at home and being supported by caregivers. It is estimated that 25–74% of stroke survivors require assistance with activities of daily living from informal caregivers, often family members. Although the physical, psychological, emotional and social consequences of caregiving and its economic benefit to society are well-recognized, caregivers’ needs are often given low priority in stroke management and many caregivers feel inadequately trained, poorly informed and dissatisfied with the level of support provided after discharge (35). An important challenge for stroke units is a conceptual shift in the philosophy of stroke care from being predominantly engaged with patient-oriented interventions to a strategy in which the patient and the caregiver are seen as a combined focus for intervention, with the objective of empowering and equipping caregivers to be competent facilitators of activities of daily living when caring for disabled patients after stroke (36).
THE COSTS OF ORGANIZED STROKE CARE
There are many studies on clinical outcomes of organized stroke care, but very few data on cost issues and the cost effectiveness of such care. Limited cost data from earlier studies suggested that organized care for stroke is less expensive than general medical care, principally because of a reduction in the hospital length of stay for patients managed in stroke units (37). However, cost analyses in these studies were simplistic, did not take a societal perspective, and were not according to the established principles of health economics analyses (38). One study, which included a comprehensive cost evaluation, showed that stroke unit management was more expensive than care in other settings, but was still associated with significant reductions in mortality and institutionalization (Table IV) (39). In other words, stroke units improve outcomes, but at a higher cost than other strategies of organized stroke care. These costs need to be acknowledged and met by service providers in order to deliver effective stroke care. There will always be difficult choices for commissioners of health services; for example, if the health services are willing to pay only £30,000 per additional quality adjusted life year (QALY) (the implicit current threshold value per QALY in the UK), the probability that they will choose a dedicated stroke unit to provide stroke care for patients after moderate stroke is 29%, but that of choosing supported rehabilitation at home by a specialist team is 42% (39). A strategy that restricts the admission of patients after acute stroke to hospital would be unacceptable in the face of current evidence and clinical guidelines for the management of stroke patients. This suggests that clinical imperatives may dictate more expensive solutions that require real investment into services. However, there may be other innovative strategies for organizing stroke care, such as acute management in hospital with early supported discharge and rehabilitation at home, which may help to bridge the gap between desirable clinical practice and affordability.
| Table IV. Incremental cost-effectiveness ratios (ICERs) for stroke units (36) | ||
| Cost perspective | Additional cost per additional 1% of deaths/institutions avoided | Additional cost per additional QALY gained |
| Immediate care costs | £534 | £67,323 |
| QALY | £496 | £64,097 |
| Total cost including informal care costs based on minimum wage rate | £682 | £89,132 |
| QALY: quality adjusted life year | ||
Conclusion
Rehabilitation remains the cornerstone of stroke management, and organized rehabilitation expedites recovery, prevents complications, decreases mortality and reduces institutionalization. Organized stroke care, such as that provided by stroke units, improves outcome for patients regardless of stroke severity, but those with more severe strokes have more to gain from such management. Despite the advances made in organized stroke care in the last decade, there remains considerable scope for improvement and continued evolution in response to patient and caregiver needs, new therapies and the changing environment of healthcare provision. Good stroke care is expensive and adequate investment is required both within hospitals and in the community in order to achieve the full potential of organized stroke care. Further research is required to investigate new strategies that provide better outcomes at lower costs.
ACKNOWLEDGEMENTS
This paper is based partly on a lecture given at the international symposium “Evidence for stroke rehabilitation – bridging into the future”, in Göteborg, Sweden, 26–28 April, 2006.
The authors acknowledge the use of data from the Stroke Unit Trialists Collaboration, presented at the European Stroke Conference in Bologna, Italy, 2005, and are grateful for permission to reproduce Tables I and II from the presentation at the European Stroke Conference.
References
1. Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, Yatsu F. The global stroke initiative. Lancet Neurol 2004; 3: 391–393.
2. Anderson CS, Linto J, Stewart-Wynne EG. A population based assessment of the impact and burden of care-giving for long-term stroke survivors. Stroke 1995; 26: 843–849.
3. National Audit Office. Reducing brain damage: faster access to better stroke care. London: The Stationery Office; 2006.
4. Wardlaw JM, Zoppo G, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2003; CD000213.
5. Morris DL, Rosamond W, Madden K, Schultz C, Hamilton S. Pre-hospital and emergency department delays after acute stroke. The Genentech Stroke Presentation Survey. Stroke 2000; 31: 2585.
6. Harraf F, Sharma AK, Brown MM, Lees KR, Vass RI, Kalra L. A multicentre observational study of presentation and early assessment of acute stroke. BMJ 2002; 325: 17–21.
7. California Acute Stroke Pilot Registry (CASPR) Investigators. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology 2005; 64: 654–659.
8. Weir NU, Buchan AM. A study of the workload and effectiveness of a comprehensive acute stroke service. J Neurol Neurosurg Psychiatry 2005; 76: 863–865.
9. Diez-Tejedor E, Fuentes B. Acute care in stroke: the importance of early intervention to achieve better brain protection. Cerebrovasc Dis 2004; 17 Suppl 1: 130–137.
10. Kalra L. The influence of stroke unit rehabilitation on functional recovery from stroke. Stroke 1994; 25: 821–825.
11. Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Functional MRI detects posterior shifts in primary sensorimotor cortex activation after stroke: evidence of local adaptive reorganization? Stroke 2001; 32: 1134–1139.
12. Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL.Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke 2000; 31: 656–661.
13. Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 2002; 125: 2731–2742.
14. Wade DT. de Jong BA. Recent advances in rehabilitation. BMJ 2000; 320: 1385–1388.
15. Langhorne P, Cadilhac D, Feigin V, Grieve R, Liu M. How should stroke services be organised? Lancet Neurol 2002; 1: 62–68.
16. Stroke Unit Trialists Collaboration. Collaborative systemic review of the randomised trials of organised inpatient (stroke unit) care after stroke. BMJ 1997; 314: 1151–1158.
17. Organised inpatient (stroke unit) care for stroke. Stroke Unit Trialists’ Collaboration.Cochrane Database Syst Rev 2002; CD000197.
18. Irwin P, Hoffman A, Lowe D, Pearson M, Rudd AG. Improving clinical practice in stroke through audit: results of three rounds of National Stroke Audit. J Eval Clin Pract 2005; 11: 306–314.
19. Stegmayr B, Asplund K, Hulter-Asberg K, Norrving B, Peltonen M, Terent A, et al. Stroke units in their natural habitat. Can results of randomised trials be reproduced in routine clinical practice? Stroke 1999; 30: 709–714.
20. Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 2003; 326: 472.
21. Langhorne P for Stroke Unit Trialists Collaboration. The effect of different types of organised inpatient (stroke unit) care. Cerebrovasc Dis 2005; 19 Suppl 2.
22. Evans A, Harraf F, Donaldson N, Kalra L. Randomized controlled study of stroke unit care versus stroke team care in different stroke subtypes. Stroke 2002; 33: 449–455.
23. Briggs DE, Felberg RA, Malkoff MD, Bratina P, Grotta JC. Should mild or moderate stroke patients be admitted to an intensive care unit? Stroke 2001; 32: 871–876.
24. Kalra L, Eade J. Role of stroke rehabilitation units in managing severe disability after stroke. Stroke 1995; 26: 2031–2034.
25. Langhorne P for Stroke Unit Trialists Collaboration. The effect of organised inpatient (stroke unit) care on death after stroke. Cerebrovasc Dis 2005; 19 Suppl. 2.
26. Kalra L, Evans A, Perez I, Knapp M, Donaldson N, Swift CG. Alternative strategies for stroke care: a prospective randomised controlled trial. Lancet 2000; 356: 894–899.
27. Fjaertoft H, Indredavik B, Lydersen S. Stroke unit care combined with early supported discharge: long-term follow-up of a randomized controlled trial. Stroke 2003; 34: 2687–2691.
28. Indredavik B, Bakke F, Slordahl SA, Rokseth R, Haheim LL. Stroke unit treatment. 10-year follow-up. Stroke 1999; 30: 1524–1527.
29. Indredavik B, Bakke F, Slordahl SA, Rokseth R, Haheim LL. Treatment in a combined acute and rehabilitation stroke unit: which aspects are most important? Stroke 1999; 30: 917–923.
30. Evans A, Perez I, Harraf F, Melbourn A, Steadman J, Donaldson N, Kalra L. Can differences in management processes explain different outcomes between stroke unit and stroke-team care? Lancet 2001; 358: 1586–1592.
31. Sulch D, Kalra L. Integrated care pathways in stroke management. Age Ageing 2000; 29: 349–352.
32. Kwan J, Sandercock P. In-hospital care pathways for stroke. Cochrane Database Syst Rev 2004; CD002924.
33. Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke 2004; 35: 1005–1009.
34. Teasell RW, Kalra L. What’s new in stroke rehabilitation. Stroke 2004; 35: 383–385.
35. Kalra L, Evans A, Perez I, Melbourn A, Patel A, Knapp M, Donaldson N. Training care givers of stroke patients: randomised controlled trial. BMJ 2004; 328: 1099–1101.
36. McCullagh E, Brigstocke G, Donaldson N, Kalra L. Determinants of caregiving burden and quality of life in caregivers of stroke patients. Stroke 2005; 36: 2181–2186.
37. Hankey GJ, Warlow CP. Treatment and secondary prevention of stroke: evidence, costs, and effects on individuals and populations. Lancet 1999; 354: 1457–1463.
38. Drummond M, Manca A, Sculpher M. Increasing the generalizabil ity of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int J Technol Assess Health Care 2005; 21: 165–171.
39. Patel A, Knapp M, Perez I, Evans A, Kalra L. Alternative strategies for stroke care: cost-effectiveness and cost-utility analyses from a prospective randomized controlled trial. Stroke 2004; 35: 196–203.
