ARE CLINICAL CHARACTERISTICS ASSOCIATED WITH UPPER-EXTREMITY HYPERTONIA IN SEVERE ISCHAEMIC SUPRATENTORIAL STROKE?
Annette A. van Kuijk, MD1,4, Henk T. Hendricks, MD, PhD1, Jaco W. Pasman, MD, PhD2, Berry H. Kremer, MD, PhD3 and Alexander C. Geurts, MD, PhD1
From the Departments of 1Rehabilitation Medicine, 2Clinical Neurophysiology and 3Neurology, Radboud University Medical Centre, Nijmegen and 4Rehabilitation Centre Tolbrug, ‘s-Hertogenbosch, The Netherlands
OBJECTIVE: The primary goal of this study was to identify clinical risk factors, in addition to muscle weakness, for upper-extremity hypertonia in patients with severe ischaemic supratentorial stroke. The secondary goal was to investigate the time course of upper-extremity hypertonia in these patients during the first 26 weeks post-stroke.
DESIGN: Inception cohort.
Patients: Forty-three consecutive patients with an acute ischaemic supratentorial stroke and an initial upper-extremity paralysis admitted to an academic hospital.
Main outcome measures: Primary outcome: hypertonia assessed by the Ashworth scale at week 26 post-stroke. Potential risks factors: motor functions assessed by the upper-extremity subscore of the Fugl-Meyer motor assessment, Barthel Index at week 1, consciousness, sensory disturbances, apraxia, neglect, and hyper-reflexia. Secondary outcome: time course of upper-extremity hypertonia by assessing its prevalence at 6 consecutive moments post-stroke during a follow-up period of 26 weeks.
RESULTS: Twenty-five patients (63%) developed hypertonia during the follow-up period of 26 weeks. During this period, the prevalence of hypertonia followed a rather dynamic course, with cases of early, transient and late hypertonia. Univariate analyses yielded none of the selected clinical characteristics as significantly associated with hypertonia.
CONCLUSION: Despite the high incidence of hypertonia (63%) observed, none of the selected clinical characteristics could be identified as a risk factor for hypertonia.
Key words: stroke, upper extremity, hypertonia, incidence, risk-factors.
J Rehabil Med 2007; 39: 33–37
Correspondence address: A. A. van Kuijk, Rehabilitation Centre Tolbrug, PO Box 90153, NL-5200 ME ‘s-Hertogenbosch, The Netherlands. E-mail: a.v.kuijk@tolbrug.nl
Submitted January 20, 2006; accepted July 11, 2006.
Introduction
Spasticity is a characteristic component of the upper motor neurone syndrome in the post-acute and chronic phases of stroke. Spasticity develops in about 20–40% of all stroke survivors, usually within 3 months post-stroke (1–5). In the upper extremity, spasticity may cause difficulty with basic arm and hand abilities, such as reaching and grasping, as well as with many more complex activities of daily living (ADL) (6–8). In the acute and post-acute phases after stroke, recurrent negative reinforcement in attempts to use the affected arm can lead to so-called “learned disuse”, in particular when spastic antagonists counteract selective voluntary muscle activity (9). Moreover, in the long-term, untreated spasticity can lead to secondary complications, such as changes in the visco-elastic properties of the musculo-tendinous apparatus (stiffness), loss of muscle length (contractures), and pain, which may further impede the functional use of the upper extremity (3, 10).
The precise relationship between spasticity elicited by passive tendon or muscle stretch and active movement capacity, however, remains unclear. As a consequence, it is not clear whether reduction of spasticity will always improve active motor functions and dexterity. A systematic review of the treatment of post-stroke upper-extremity spasticity by focal neuronal or neuro-muscular blockade revealed the potential efficacy of such treatments in reducing hyper-reflexia, muscle tone, and in improving passive range of joint motions (11), yet, functional benefits could not be convincingly demonstrated. Generally, functional effects of spasticity treatment seem to depend highly on a critical selection of subjects, individualized goal setting, and appropriate selection of outcome measures (12, 13). Patients after severe stroke with a low potential for motor recovery in particular may profit from a pro-active treatment approach to prevent disabling spasticity and the functional consequences of secondary complications, such as muscles stiffness, contractures and pain. Benefits can be achieved with regard to activities such as dressing, bathing and grooming, as well as with regard to limb positioning, cosmetic appearance and comfort (12, 13). Against this background, it seems clinically relevant to assess, besides the probability of motor recovery, the risk of developing spasticity in the individual patient early after stroke.
However, although motor recovery can be predicted reasonably well by clinical assessment (14), early recognition of the risk of developing spasticity based on clinical characteristics is much more difficult and the literature does not really support clinical reasoning. Because the clinical assessment of spasticity measures resistance against passive stretch (hypertonia), it cannot distinguish between the neural (reflexive) mechanisms, and the secondary (intrinsic) changes in muscle properties. Studies on the risk factors for post-stroke hypertonia are also scarce. Besides a recently published cohort study by Leathly et al. (4), no other studies are available in (sub-)acute stroke patients. In stroke survivors, Leathly et al. (4) found a moderate association of both muscle weakness and a low Barthel Index (BI) (15) on day 7 post-stroke with hypertonia at 12 months post-stroke. Based on clinical experience, some authors have suggested the importance of sensory impairments, visuospatial deficits, and apraxia for developing hypertonia (16, 17). However, these disorders were not identified as risk factors in the study by Leathly et al. (4), which might have been due to the relatively low number of cases with hypertonia (36%). Against this background, we conducted a cohort study including only patients with an initial paralysis of the upper extremity after supratentorial stroke to maximize the likelihood of observing hypertonia and, thus, to optimize the chance of identifying additional risk factors for post-stroke upper-extremity hypertonia. As a secondary goal, we studied the time course of upper-extremity hypertonia by assessing its prevalence at 6 consecutive moments post-stroke during a follow-up period of 26 weeks.
Material and Methods
Patients
As part of a larger study on the value of motor-evoked potentials (MEP) in predicting motor and functional outcome after stroke (18), 43 consecutive acute patients with an ischaemic supratentorial stroke were recruited during a 1.5-year period. These patients were admitted to the Department of Neurology at the Radboud University Medical Centre Nijmegen. The diagnosis of stroke was made clinically by a neurologist according to the World Health Organization (WHO) clinical criteria (19) and confirmed by computerized tomography (CT) scan.
Only patients presenting with stage I of the upper extremity according to Brunnstrom (20) (i.e. no tone and no voluntary muscle activity at the elbow, wrist or finger flexors) at day 1 were included within 7 days post-stroke. Patients with a poor prognosis for survival (loss of consciousness, severe CT abnormalities, and severe co-morbidity) as well as patients with severe pre-existing impairments of the upper extremity of any type (e.g. rheumatic deformities, contractures) were excluded. Because all patients had to undergo transcranial magnetic stimulation to record MEPs, those with a history of craniotomy, epilepsy, cardiac prosthetic valve or pacemaker implantation, or severe polyneuropathy were also excluded. The local ethics committee approved the study protocol and written informed consent was obtained from all patients before study entry.
Each patient received “best medical treatment” according to the guidelines of the Netherlands Society of Neurology, including a multidisciplinary initial rehabilitation approach. This approach ensured that each patient received physiotherapy to maintain optimal passive and active range of motion of all upper-extremity joints from day 1 post-stroke. However, for the first 3 weeks post-stroke, no specific therapy was initiated aimed at facilitation of arm-hand function recovery.
Potential risk factors
Clinical assessment. Within the first 24 hours after stroke the treating neurologist assessed all patients with regard to initial motor functions, muscle tone and level of consciousness. All consecutive clinical assessments of motor, sensory, and cognitive functions were performed at weeks 1, 2, 3, 6, 12 and 26 post-stroke by a rehabilitation specialist (HH). Motor functions were assessed according to the upper limb subset of the Fugl-Meyer Motor Assessment (FMA) (20). A selected hand motor score was derived from the FMA, which consisted of the 7 original hand items of the FMA with a maximum score of 14 points. Early hand motor function recovery was defined as any change in the FMA hand score within the first 3 weeks post-stroke.
Sensory deficits were assessed by clinical examination of light touch, pinprick, and vibration sense of the hemiplegic arm. Proprioception was assessed by the “thumb-finding test” (21). Sensory deficit was ultimately recorded on a binary scale as either “absent” or “present” based on reproducible differences in at least 2 sensory modalities compared with the non-paretic arm. Biceps and triceps tendon reflexes were quantified according to the Mayo Clinic scale for tendon reflex assessment (range –4 to +4) (22). Hyper-reflexia was considered present if the tendon reflex on the paretic side was ≥+1.
Neglect was defined as the inability to detect, attend to, or respond to stimuli located on the contra-lesional side of body or action space (23). Apraxia was defined as the inability to perform previously learned skilled acts, despite sufficient comprehension, motor capacity, and sensation. Both the existence of neglect and apraxia was based on clinical observations of the patient’s ADL performances by the nursing staff, the treating neurologist, and the consulting rehabilitation physician within the first 3 weeks post-stroke. Neglect and apraxia were considered present if symptoms were witnessed by at least 2 of these 3 observers.
Finally, ADL performances were assessed with the BI (15). Consistent with Leathly et al. (4), the BI score at the first week post-stroke was considered as a potential risk factor.
Outcome assessment. Hypertonia was clinically assessed by grading muscle tone through the Ashworth scale (AS) (24). Muscle tone was assessed within the first 24 hours post-stroke and consecutively at weeks 1, 2, 3, 6, 12 and 26 post-stroke under standardized test conditions by a rehabilitation specialist (HH). The patients lay supine or sat in a comfortable sitting position with their forearms supinated and resting on a horizontal plane. Patients were instructed to completely relax while their affected elbows and wrists were passively moved throughout the maximal range in both flexion and extension directions. Passive extension of the patient’s elbow was performed during approximately one second by counting “one thousand and one” while the forearm was held just proximal to the wrist. When the elbow was extended, the upper arm was stabilized just proximal to the elbow. Passive extension of the patient’s wrist was also performed during approximately one second while the hand was held just proximal to the metacarpo-phalangeal joints and the forearm just proximal to the wrist. Muscle tone was quantified according to the criteria outlined by Ashworth (24) (grades 0–4). Clinically relevant hypertonia was operationally defined as an AS score equal to or greater than 2 in at least one joint.
Data analysis
From 2×2 contingency tables, positive and negative predictive values for each of the potential risk factors with their 95% confidence interval were calculated. In addition, to test the association of gender with hypertonia, Pearson’s χ2 analysis was performed. The required 2-tailed significance level was set at 0.05. For the ordinal outcome measure BI at week 1 post-stroke, the Mann-Whitney U test was performed to assess the association between the BI and hypertonia. Again, the required 2-tailed significance level was set at 0.05.
In case of positive outcome of the univariate analysis, multiple backwards logistic regression was planned to determine the explained variance by each characteristic with regard to hypertonia, independent of its possible association with other characteristics.
All analyses were performed using the Statistical Package for the Social Sciences (SPSS version 11).
Results
Patient characteristics and time course of post-stroke hypertonia
Two patients died within the first 2 weeks post-stroke. Both continued to have a flaccid paralysis of the upper extremity from clinical presentation. Another patient was excluded from the study at week 13 because of poor prognosis for survival after he had a recurrent stroke. Thus, 40 patients, 20 women and 20 men, completed the study. The clinical characteristics of these 40 patients are shown in Table I. Sixteen (40%) patients had had a previous stroke, whereas 24 (60%) patients had had a first-ever stroke.
| Table I. Characteristics of the 40 patients | ||
| Characteristics | n | |
| Gender | Female | 20 |
| Male | 20 | |
| Stroke history | First ever | 24 |
| Previous | 16 | |
| Lesion side | Left | 22 |
| Right | 18 | |
| Median age, years (interquartile range) | 68 (59–77) | |
| Median Barthel Index on admission (IQ range) | 0 (0) | |
The highest estimated AS scores in our patients were 0 (n=9), 1 (n = 6), 2 (n = 8), 3 (n = 12), and 4 (n = 5). If clinical hypertonia was defined as AS ≥2, 25 (63%) patients developed hypertonia at any time post-stroke. In 20 patients hypertonia developed within the first 6 weeks post-stroke, and in 10 (25%) patients even within the first 3 weeks post-stroke. This “early” (≤3 wks) hypertonia was not correlated with previous stroke (Fisher's exact p = 0.48). We identified 16 hypertonic patients at 6 months who were not initially hypertonic, as well as 3 patients with normal muscle tone at 6 months who were initially hypertonic. Fig. 1 illustrates how the prevalence of hypertonia evolved in our study sample.
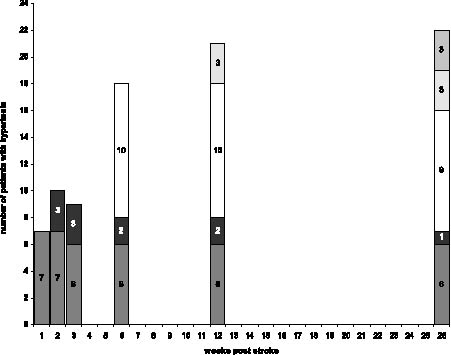
Fig. 1. Prevalence of post-stroke hypertonia during the first 26 weeks post-stroke. Patients developing hypertonia at week 1 ( ), week 2 ( ), week 6 ( ), week 12 ( ), and week 26 post stroke ( ).
In 12 patients, recovery of hand motor function was present at week 26 post-stroke. In the group with persistent hypertonia, 7 (33%) patients showed any recovery of hand motor function, whereas in the group without hypertonia 5 patients showed such recovery (26%). Fig. 2 illustrates the FMA-hand scores at week 26 post-stroke in both the patients with and those without persistent hypertonia.
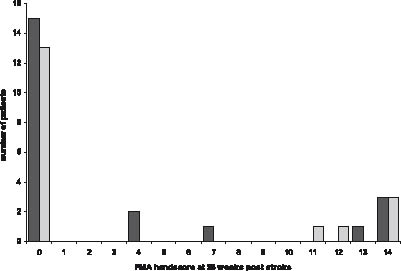
Fig. 2. Fugl-Meyer Motor Assessment (FMA) hand score at 26 weeks post-stroke in patients with ( ) and without ( ) persistent hypertonia.
Potential risk-factors for post-stroke hypertonia
Because, from a clinical point of view, we were most interested in predicting persistent hypertonia, we considered hypertonia at week 26 post-stroke as the primary outcome. In univariate analyses, all associations between hypertonia at week 26 and the potential risk factors were low and statistically not significant. Table II shows the positive and negative predictive values with their 95% confidence intervals for all factors. Positive predictive values varied from 0.52 to 0.68 and the negative values from 0.33 to 0.62.
| Table II. Association between potential risk factors and hypertonia (HT) at 26 weeks post-stroke | |||
| With HT (n) | Without HT (n) | PPV and NPV (95% CI) | |
| Unconsciousness at stroke | |||
| Present | 18 | 14 | PPV 0.56 (0.38–0.74) |
| Absent | 4 | 4 | NPV 0.50 (0.35–0.97) |
| Sensory disturbances | |||
| Present | 16 | 15 | PPV 0.52 (0.34–0.70) |
| Absent | 6 | 3 | NPV 0.33 (0.07–0.59) |
| Hyper-reflexia | |||
| Present | 17 | 13 | PPV 0.57 (0.40–0.74) |
| Absent | 5 | 5 | NPV 0.50 (0.19–0.81) |
| Apraxia | |||
| Present | 11 | 7 | PPV 0.68 (0.49–0.87) |
| Absent | 11 | 11 | NPV 0.50 (0.28–0.72) |
| Neglect | |||
| Present | 19 | 13 | PPV 0.58 (0.41–0.75) |
| Absent | 3 | 5 | NPV 0.62 (0.33–0.91) |
| Early hand motor recovery | |||
| Absent | 19 | 16 | PPV 0.54 (0.37–0.71) |
| Present | 3 | 2 | NPV 0.40 (0.05–0.75) |
| PPV: Positive predictive values; NPV: Negative predictive values; CI: confidence intervals. | |||
In addition, 10 females and 12 men suffered from hypertonia at 26 weeks post-stroke. In both patients with and those without hypertonia the median BI at week 1 post-stroke was 2 (interquartile range 0–4). There was no association between gender and hypertonia (χ2 0.404, p = 0.525) and neither between the BI at week 1 and hypertonia (p = 0.93). Changing the definition of persistent hypertonia into AS ≥2 at week 12 or week 6 post-stroke yielded similar results. Even if clinically relevant hypertonia was defined as AS ≥1, or if hypertonia at any time post-stroke was used as the primary outcome, no association with the potential risk factors could be demonstrated.
Discussion
In this study a sample of patients with initial paralysis of the upper extremity caused by ischaemic supratentorial stroke was included to optimize the chance of identifying risk factors, additional to muscle weakness, for early or persistent hypertonia. The observed overall incidence of hypertonia within 6 months post-stroke in this group was 63%. In comparison, Sommerfeld et al. (5) found a 24% overall incidence of hypertonia within 3 months in a hospital-based cohort of patients with acute stroke a first-ever ischaemic stroke. Other studies on stroke-related hypertonia mainly reported prevalences instead of incidences, varying from 19% to 39% of the hospitalized stroke population at large (4–6). Hence, our patient sample was clearly different from those in previous studies. It was restricted to a homogeneous subgroup of the most severely affected survivors from ischaemic supratentorial stroke, i.e. those with an initial paralysis of the upper extremity. The severity of stroke is also evident from the finding that all patients had an initial BI of 0. Based on the observed incidence in this study, this subgroup of stroke patients with initial paralysis of the upper extremity apparently has a substantial chance of developing hypertonia within 6 months post-stroke.
Yet, even in this severely affected group, we could not identify any of the selected clinical characteristics as a significant risk factor for early or persistent hypertonia. Even hyper-reflexia, which is regarded by many clinicians as a sign of “spasticity”, was not significantly associated with the AS in this study. This finding is corroborated by several electrophysiological studies that have shown dissociations between hyper-reflexia and hypertonia, indicating that phasic and tonic stretch reflexes are controlled differently by the central nervous system (2, 5). However, it might also reflect the fact that the AS is unable to distinguish between neural mechanisms (hyper-reflexia) and secondary intrinsic changes in muscle properties (contracture). As for sensory or cognitive deficits, our data do not underscore the clinical notion that loss of sensibility, neglect, or apraxia may increase the risk of developing post-stroke upper-extremity hypertonia. These findings are consistent with those of Patano et al. (25), who could not find any differences in sensory disturbances, aphasia, or visuospatial neglect between patients with prolonged muscular flaccidity and those with hypertonia in the subacute phase (2–6 months) post-stroke. Hence, as yet, only a modest association between early muscle weakness and chronic hypertonia has been found by Leathly et al. (4).
The secondary goal of this study was to explore the time course of upper-extremity hypertonia during the first 26 weeks post-stroke in patients with initial paralysis. Although it is generally assumed that muscle tone increases from flaccidity in the acute phase of stroke to various degrees of hypertonia in the long term (1, 2, 26), the patients with early (25% within 3 weeks post-stroke), transient (10%), or late (15%) hypertonia in our cohort did not fit within this general idea. Sommerfeld et al. (5) reported hypertonia within the first week post-stroke in 21% of their acute hospitalized patients as well. In contrast to the opinion of others (27), in our study early hypertonia was not correlated with previous stroke. In 3 patients who developed hypertonia within 6 weeks post-stroke, muscle tone had normalized at 26 weeks, perhaps related to neural plasticity. At 26 weeks we identified another 6 patients in which hypertonia developed only after the third month post-stroke. It seems likely that secondary intrinsic changes in muscle properties contributed to this late hypertonia (28).
At 26 weeks post-stroke, we did not find any difference in motor or functional recovery between patients with and those without hypertonia, which may be due to the fact that the majority of our patients ended up with a non-functional paralytic arm and hand. The unique influence of hypertonia on motor impairments and ADL performances is, no doubt, difficult to assess in a subgroup of patients with initial paralysis with such a poor chance of motor recovery. In addition, specifically patients with severe stroke may suffer from various other problems of, for example, mood, cognition, vision, and sensation, which may contribute to their overall disability. Lastly, the BI is probably not the best measure to assess ADL performances related to arm and hand function. When, for instance, hypertonia causes pain or discomfort or problems with arm positioning, dressing, or hygiene control, treatment that reduces hypertonia and prevents secondary complications may greatly benefit the patient on a functional level (12, 13), although such benefit does not need to be reflected in a change in the BI score.
One of the possible limitations of this study is that upper-extremity hypertonia was clinically assessed using the AS, whereas instrumented analysis of electromyographic (EMG) and force signals from the upper-extremity muscles on passive stretching might have allowed better discrimination between active (i.e. contractions) and passive (i.e. stiffness) contributions to muscular resistance (3, 29). However, instrumented tests are not yet available for routine clinical application, which is the reason that the Ashworth and modified AS are still the most commonly used measures of adult spasticity in clinical practice. The AS are most frequently used as primary outcome measures in intervention studies as well, and other scales, such as the tone assessment scale, can be regarded as modifications of the AS. All these measures are equally reliable regarding muscle tone assessment of the upper extremity (30). Overall, there is a reasonable association between the (modified) AS and EMG responses in patients with hemiparetic stroke (31), which does not preclude a significant contribution of passive muscle properties to (particularly late) hypertonia.
Another limitation of our study may have been a lack of power to identify risk factors for upper-extremity hypertonia due to the still limited number of patients. We included merely patients with initial paralysis to optimize the risk of post-stroke hypertonia. This subgroup comprises only 19–30% of the stroke population at large and has a high risk of post-stroke death (62%) (32). As a result, the inclusion rate was relatively low in just one academic hospital. However, 40 patients would have been sufficient to discriminate a moderate from an “absent” association between hypertonia and any clinical determinant (setting a at 0.05 and 1-b at 0.80). Hence, larger studies of high-risk patients will be needed to include the number of subjects to identify “weak” but significant associations.
In conclusion, the present study showed that a subgroup of patients with initial paralysis of the upper extremity apparently has a substantial chance of developing hypertonia at any time post-stroke. The observed incidence over the 26-week follow- up period was 63%. The prevalence of upper-extremity hypertonia during the first 26 weeks post-stroke followed a rather dynamic course, with cases of early, transient and late hypertonia. Even in this selected study sample with a high incidence of hypertonia, we could not identify any of the selected clinical characteristics as a risk factor for transient or persistent hypertonia. Unlike the stroke population in general, in patients with severe stroke and initial upper-extremity paralysis, hypertonia appeared not to be associated with motor or functional recovery of the affected arm.
References
1. Thilmann AF, Fellows SJ, Garms E. The mechanism of spastic muscle hypertonus. Variation in reflex gain over time course of spasticity. Brain 1991; 114: 233–244.
2. Fellows SJ, Ross HF, Thilmann AF. The limitations of the tendon jerk as a marker of pathological stretch reflex activity in human spasticity. Neurol Neurosurg Psychiatry 1993; 56: 531–537.
3. O’Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain 1996; 119: 1737–1749.
4. Leathly MJ, Gregson JM, Moore AP, Smith TL, Sharma AK, Watkins CL. Predicting spasticity after stroke in those surviving to 12 months. Clin Rehabil 2004; 18: 438–443.
5. Sommerfeld DK, Eek EU, Svensson AK, Holmqvist LW, von Arbin MH. Spasticity after stroke. Its occurrence and association with motor impairments and activity limitations. Stroke 2004; 35: 134–139.
6. Watkins CL, Leathley MJ, Gregson MJ, Moore AP, Smith TL, Sharma AK. Prevalence of spasticity post-stroke. Clin Rehabil 2002; 16: 515–522.
7. Katz RT, Rovai GP, Brait C, Rymer WZ. Objective quantification of spastic hypertonia: correlation with clinical findings. Arch Phys Med Rehabil 1992; 73: 339–347.
8. Lin FM, Sabbahi M. Correlation of spasticity with hyperactive stretch reflexes and motor dysfunction in hemiplegia. Arch Phys Med Rehabil 1999; 80: 526–530.
9. Taub E, Miller NE, Novack TA, Cook EW 3rd, Fleming WC, Nepomuceno CS, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil 1993; 74: 347–354.
10. Pizzi A, Carlucci G, Falsini C, Verdesca S, Grippo A. Evaluation of upper-limb spasticity after stroke: a clinical and neurophysiologic study. Arch Phys Med Rehabil 2005; 86: 410–415.
11. van Kuijk AA, Geurts ACH, Bevaart BJW, van Limbeek J. Treatment of upper extremity spasticity in stroke patients by focal neural or neuromuscular blockade: a systematic review. J Rehabil Med 2002; 34: 51–61.
12. Brashear A, Gordon MF, Elovic E, Kassicieh D, Marciniak C, Do M, et al. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after stroke. N Engl J Med 2002; 347: 395–400.
13. Francis HP, Wade DT, Turner-Stokes L, Kingswell RS, Coxon EA. Does reducing spasticity translate into functional benefit? An exploratory meta-analysis. J Neurol Neurosurg Psychiatry 2004; 75: 1547–1551.
14. Kwakkel G, Kollen BJ, van der Grond J, Prevo AJH. Probability of regaining dexterity in the flaccid upper limb. Impact of severity of paresis and time since onset in acute stroke. Stroke 2003; 34: 2181–2186.
15. Wade D, Collin C. The Barthel Index: a standard measure of physical disability? Int Disabil Stud 1988; 10: 64–67.
16. Bobath B. Adult hemiplegia: evaluation and treatment. London: Heinemann Medical; 1990.
17. Ward AB. Long-term modification of spasticity. J Rehabil Med 2003; 35 Suppl 41: S60–S65.
18. Hendricks HT, Pasman JW, van Limbeek J, Zwarts MJ. Motor evoked potentials in predicting recovery from upper extremity paralysis after acute stroke. Cerebrovasc Dis 2003; 16: 265–271
19. WHO task force on stroke and other cerebrovascular disorders. Stroke – 1989. Recommendations on stroke prevention, diagnosis, and therapy. Stroke 1989; 20: 1407–1431.
20. Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehab Med 1975; 7: 13–31.
21. Prescott RJ, Garraway WM, Akhtar AJ. Predicting functional outcome following acute stroke using a standard clinical examination. Stroke 1982; 13: 641–647.
22. Manschot S, van Passel L, Buskens E, Algra A, van Gijn J. Mayo and NINDS scales for assessment of tendon reflexes: between observer agreement and implications for communication. J Neurol Neurosurg Psychiatry 1998; 64: 2553–2555.
23. Heilman KM, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical neurophysiology. New York: Oxford University Press; 1993, p. 279–336.
24. Ashworth B. Preliminary trial of carisoprodal in multiple sclerosis. Practioner 1964; 192: 540–542.
25. Patano P, Formisano R, Ricci M, Di Piero V, Sabatini U, Barbanti P, et al. Prolonged muscular flaccidity after stroke. Morphological and functional brain alterations. Brain 1995; 118: 1329–1338.
26. Twitchell TE. The restoration of motor function following hemiplegia in man. Brain 1951; 74: 443–480.
27. Steiner I, Argov Z, Gomori J, Gottlieb D, Melamed E. Immediate spasticity with acute hemiplegia is a sign of basal ganglia hemorrhage. Acta Neurol Scand 1985: 71: 168–170.
28. Lieber RL, Steinman S, Barash IA, Chambers H. Structural and functional changes in spastic skeletal muscle. Muscle Nerve 2004; 29: 615–627.
29. Burridge JH, Wood DE, Hermens HJ, Voerman GE, Johnson GR, van Wijck F, et al. Theoretical and methodological considerations in the measurement of spasticity. Disabil Rehabil 2005; 27: 69–80.
30. Platz T, Eickhof C, Nuyens G, Vuadens P. Clinical scales for the assessment of spasticity, associated phenomena, and function: a systematic review of the literature. Disabil Rehabil 2005; 27: 7–18.
31. Cooper A, Musa IM, van Deursen R, Wiles CM. Electromyography characterization of stretch responses in hemiparetic stroke patients and their relationship with the Modified Ashworth scale. Clin Rehabil 2005; 19: 760–766.
32. Jørgensen HS, Reith J, Nakayama H, Kammersgaard LP, Raaschou HO, Olsen TS. What determines good recovery in patients with the most severe strokes? The Copenhagen Stroke Study. Stroke 1999; 30: 2008–2012.
