OBJECTIVE: To examine goal attainment scaling for evaluation of treatment for upper limb post-stroke spasticity with botulinum toxin-A.
DESIGN: Secondary analysis of a multi-centre double-blind, placebo-controlled randomized clinical trial.
SETTING: Six outpatient clinics in Australia.
PARTICIPANTS: Patients (n = 90) completing per protocol 2 cycles of treatment/placebo. Mean age 54.5 (standard deviation 13.2) years. Mean time since stroke 5.9 (standard deviation 10.5) years.
Interventions: Intramuscular botulinum toxin-A (Dysport ® 500–1000U) or placebo given at 0 and 12 weeks. Measurement points were baseline, 8 and 20 weeks.
Main outcome measures: Individualized goal attainment and its relationship with spasticity and other person-centred measures – pain, mood, quality of life and global benefit.
RESULTS: A significant treatment effect was observed with respect to goal attainment (Mann-Whitney z = –2.33, p ≤ 0.02). Goal-attainment scaling outcome T-scores were highly correlated with reduction in spasticity (rho = 0.36, p = 0.001) and global benefit (rho = 0.45, p < 0.001), but not with other outcome measures. Goal-attainment scaling T-scores were lower than expected (median 32.4, interquartile range 29.6–40.6). Goals related to passive tasks were more often achieved than those reflecting active function. Qualitative analysis of goals nevertheless demonstrated change over a wide area of patient experience.
CONCLUSION: Goal-attainment scaling provided a responsive measure for evaluating focal intervention for upper limb spasticity, identifying outcomes of importance to the individual/carers, not otherwise identifiable using standardized measures.
Key words: goals; outcome assessment; muscle spasticity; botulinum toxin.
J Rehabil Med 2010; 42: 81–89
Correspondence address: Lynne Turner-Stokes, Regional Rehabilitation Unit, Northwick Park Hospital, Watford Road, Harrow, Middlesex, HA1 3UJ UK. E-mail: lynne.turner-stokes@dial.pipex.com
Submitted April 30, 2009; accepted September 1, 2009
Trial registration: ClinicalTrial.gov NCT00216411.
INTRODUCTION
There is now a well-established body of evidence demonstrating the effectiveness of botulinum toxin type A (BoNT-A) as a focal intervention for reduction in spasticity in the clinical setting (1). Controlled studies (1–9) have demonstrated the benefits of BoNT-A at the level of impairment. Functional gains are also demonstrated for both “active” and “passive” (ease of care) tasks (10), although impact on active function may be limited by underlying motor dysfunction. Meta-analysis demonstrates that there is often a time lag between maximum reduction in spasticity and functional gain, so that the latter may be missed if primary outcomes are measured only at a single early time-point (11).
It is also necessary, however, to demonstrate that the outcomes are meaningful to patients and those who care for them. Generic health or “quality of life” measures may fail to capture the effects of focal interventions which impact on just one or two items in the scale (12). Any improvement in these items may be lost in the overall “noise” of the unchanging items. Therefore current guidelines for the use of BoNT-A in management of spasticity advocate the application of more focused outcome evaluations, targeted on the attainment of priority goals that are important to the individual (13).
Goal attainment scaling (GAS) is a method of assimilating achievement in a number of individually-set goals into a single goal attainment score, originally described by Kiresuk & Sherman (14). It has been applied in various areas of complex intervention (15–19) including spasticity management (20, 21). In addition to providing a semi-quantitative (ordinal) assessment of goal attainment, GAS offers potentially useful qualitative information regarding the patient’s priority goals for treatment. Moreover, the process of goal-setting and rating itself offers an opportunity for dialogue and negotiation between the patient and their treating team, which may help to establish mutual agreement of expectations for outcome. However, clinicians require sufficient knowledge and experience to support patients to set realistic goals (22).
The use of GAS is still somewhat controversial. Whilst it is reported to be responsive and sensitive to patients’ values (23, 24), as well as flexible across the domains of impairment, disability and participation (18); concerns have been raised in some quarters about non-linearity of the scaling (25) and lack of uni-dimensionality (26). To overcome these problems, whilst still maintaining the recognized benefits of GAS, some authors have proposed the development of standardized goals or “item banks” (26, 27). As a first step towards this approach, it is necessary to understand the types of goals that are commonly set for a given intervention, and in particular those that are most likely to be achieved.
A multi-centre prospective Phase IV randomized-controlled placebo-controlled trial (RCT) (n = 96) of BoNT-A for treatment of upper limb spasticity following stroke was undertaken recently in Australia, with the primary intention of evaluating the impact of treatment on quality of life and other person-centred outcomes, including GAS (28). Although the study did not demonstrate impact on quality of life (as measured by the Assessment of Quality of life (AQoL) (29) through the reduction in spasticity, it did show a highly significant effect of BoNT-A with respect to goal attainment. This secondary analysis of data from those patients who completed the trial provides a more detailed evaluation of its application as a person-centred outcome in this context, with the following specific questions:
• Does GAS provide added value over standardized measures of impairment and disability as a responsive indicator of meaningful change in functional activity, following treatment of spasticity with BoNT-A?
• If so, how does it relate to other standardized measures of patient-centred outcomes such as pain, mood and quality of life?
• Which are the main priority goal areas for treatment from the patients’ perspective, and what types of goals are most often achieved?
METHODS
Trial design and participants
The study had ethics permission from independent research ethics committees at each investigational site. Full details of the study design, methods and intention-to-treat analysis are described elsewhere in accordance with the CONSORT (Consolidated Standards of Reporting Trials) guidelines (28), but an abbreviated description is included here for ease of reference. Randomization was achieved using a computer-generated list of allocation codes prepared centrally with a 1:1 ratio between treatment and placebo. Group assignment was concealed from the treating team by sequential allocation of identical treatment packs with pre-assigned numbers. All patients, treating teams and assessors were blinded to the group assignment.
All patients presenting at the 6 centres between November 2004 and January 2006 for treatment of upper limb spasticity following stroke were considered for the trial
Inclusion criteria were:
• stable adult patients after stroke (≥ 18 years old, at least 6 months after stroke) with a hemiparetic arm, and
• moderate to severe unwanted spasticity – scoring a minimum score of 2 on the Modified Ashworth Scale (MAS) (30) in at least 2 out of 3 of wrist, elbow and finger flexor tone, and a minimum of 1+ for the third area of tone).
Of 122 patients screened, 102 were eligible; 96 gave their fully informed consent and were randomized (for CONSORT diagram see primary article (28)). The 90 patients who completed per protocol and attended for final evaluation of outcome were included in this secondary analysis.
Intervention
The trial was designed to reflect routine clinical practice as closely as possible. Patients received 2 cycles of either BoNT-A (Dysport ® 750-1000U; Ipsen, Slough, UK) or placebo at week 0 and either BoNT-A (Dysport ® 500-1000U; Ipsen) or placebo at 12 weeks, injected according to clinical judgement into the dominant spastic muscles of the arm and/or forearm. Muscles were identified using electromyography or nerve stimulation according to the normal practice of the clinician. Patients were offered follow-up therapy/rehabilitation (including stretching, splinting, orthotics and/or exercise), again in accordance with routine practice for the treating centre. This was subject to the normal arrangements for funded healthcare which varied according to local provision and purchasing arrangements, but was anticipated to be similar for both randomized groups.
Outcome measures
Goal attainment scaling. GAS was applied by a method previously described (10) based on that of Kiresuk & Sherman (14). At baseline patients, with their treating team, identified up to 2 personal goals for treatment and one preferred functional outcome from the Patient Disability Scale. Goals were weighted by importance and difficulty each graded on a scale of 0–3 (10). In order to allow for deterioration, and in accordance with previous applications (15, 31), baseline scores for each goal were allocated as –1 unless no clinically plausible outcome was possible – in which case a score of –2 was given. Goal attainment was rated at weeks 8 and 20 on a 5-point scale, where 0 denotes the expected level of achievement: +1 and +2 are “a little” and “a lot” better than expected, respectively; whilst –1 and –2 are correspondingly a little and a lot less than the expected level. These attainment levels were combined in a single T-score by applying the formula recommended by Kiresuk & Sherman (14), which accounts for variable numbers of goals, inter-correlation of goal areas and variable weighting:
Total score = 50 +{(10Σ(wixi))/(0.7Σwi2 + 0.3(Σwi)2)½}
where wi =weight assigned to the ith goal and xi = the score of the ith goal. The originators argue (14) that, if goals are set in an unbiased fashion so that results exceed and fall short of expectations in roughly equal proportions, over a sufficiently large number of patients, one would expect a normal distribution of T-scores with a mean of 50 and standard deviation (SD) of 10. It should be understood, however, that the arithmetic operations in GAS are undertaken on what are effectively statements rather than real numbers and that the data generated are therefore of ordinal, rather than interval quality.
GAS outcome T-scores were compared with change from baseline in the following standardized outcome measures at 20 weeks:
• Impairment: MAS (30) – scores for spasticity at the elbow, wrist and fingers were summed to produce a composite spasticity score (11).
• Disability and carer burden: Patient Disability, Carer Burden (2).
• Pain: Pain (10 cm visual analogue scale).
• Mood: Hospital Anxiety and Depression Scale (HADS) (32).
• Assessment of Quality of Life (AQoL) (29).
A global assessment of benefit was made independently at the end of each cycle (weeks 12 and 24) by the investigator and the patient and/or carer. Both were asked to rate the overall benefit to the arm since the injection as: 1 = much worse; 2 = worse; 3 = same; 4 = some benefit; and 5 = great benefit. An index of overall “global benefit” for each patient at week 24 was taken as the mean of the 2 ratings, and subjects were categorized as responders (mean response ≥ 4) and non-responders (mean response < 4).
The World Health Organization (WHO) International Classification for Functioning Disability and Heath (ICF) (33) provides a common language for categorizing goals into different domains of personal experience. Goal categories were categorized retrospectively into the closest ICF domains, with reference to the linking rules published by Cieza et al. (34) and with the assistance of the ICF Illustration Library online (www.icfillustration.com). Second-level categories (3-digit codes) were used as they are considered to provide the best trade-off between breadth and depth of coding (35, 36). A descriptive analysis was undertaken to compare the treatment groups with respect to their rates of improvement towards goals, as well as actual achievement of goals within each category.
Statistical analysis
The intention-to-treat analysis used parametric statistical techniques, as standard diagnostic tests showed the distribution of the primary data to conform adequately to requirements for normality (28). One-sample Kolmogorov-Smirnov tests of GAS T-scores at 20 weeks were z 1.1 p = 0.20 (active treatment group) and z 1.0 p = 0.21 (placebo group). However, even though statistical tests did not show significant deviation from normality, the distribution was not entirely “normal” due to 3 high outliers who responded significantly above expectation (GAS T-scores > 60) in the active treatment group. In addition, some may argue that, as GAS and other tools used in this study generate ordinal data, non-parametric tests should be used in any event. In view of the above, and to complement our previous article (28), in this secondary per protocol analysis we applied non-parametric statistical tests, using SPSS version 15. Wilcoxon signed-rank tests were used to evaluate changes in GAS from baseline, and Mann-Whitney U tests were used for between group comparisons. Spearman rank correlations were used to examine the association between GAS and changes from baseline in other measures, and χ2 tests were used for group comparisons between dichotomous variables.
RESULTS
The mean age of the 90 patients included in this per protocol analysis was 59.5 (SD 13.2) years; the male: female ratio was 54:36 and mean time since onset of stroke was 5.9 (SD 10.5) years. Fifty-two patients received active BoNT-A treatment and 38 received placebo. The reason for this discrepancy is unknown and can be explained only by chance (28). Nevertheless the groups were not significantly different at baseline with respect to any of the key demographic variables included in this analysis.
Table I shows the median (interquartile range (IQR)) composite GAS T-scores for the 2 groups at weeks 0, 8 and 20. There was a significant change in GAS score between baseline and weeks 8 and 20 for the Dysport ® group only, with significant group interaction at week 20, both in terms of the T-score and the change from baseline. The T-score and GAS change score were strongly correlated (rho 0.72, p < 0.0001).
| Table I. Weighted goal attainment scaling (GAS) scores compared over time and between treatment groups |
| Time-point | Treatment group | Between-group differences: Mann-Whitney |
| Placebo Median (IQR) | Dysport ® Median (IQR) | Z value, p-value |
| First cycle | n = 38 | n = 52 | |
| GAS T-score, Week 0 | 27.2 (24.5–36.3) | 27.2 (22.8–32.5) | z –0.46, p = 0.64 |
| GAS T-score, Week 8 | 29.1 (25.0–32.4) | 30.0 (24.6–36.4) | z –0.87, p = 0.38 |
| GAS change from baseline | 0 (–3.9–3.9) | 4.0 (0–6.2) | z –1.87, p = 0.06 |
| Within-group significance*: Wilcoxon z p-value | z –0.28, p = 0.78 | z –2.7 p = 0.007 | |
| Second cycle | | | |
| GAS T-score, Week 20 | 29.1 (25.4–36.7) | 32.4 (29.6–40.6) | z –2.33, p = 0.02 |
| GAS change from baseline | 0 (–3.4–5.3) | 5.4 (0–9.1) | z –3.24, p = 0.001 |
| Within-group significance*: Wilcoxon z p = value | z –0.94, p = 0.35 | z –4.7 p < 0.001 | |
| *Within-group significance of change from baseline. ICR: interquartile range. |
Relationship with other person-centred parameters
Table II summarizes the results of between group comparisons for the other measures at final outcome evaluation, and compares the relationship between changes from baseline and GAS outcome T-score for the whole study population. There were significant group interactions for change in spasticity (composite MAS score) and global benefit, but none of the other measures showed significant between- or within-group differences in change from baseline (27). Pain and depression, however, were not prominent symptoms in this group. Median scores for depression and anxiety at baseline were both 5 (normal 0–7), and median pain scores were only 3/100 (IQR 0–25) at rest and 17.5 (IQR 1–40) on movement.
| Table II. Group comparisons of other measures and relationship of goal attainment scaling (GAS) with other outcome measures at end-point evaluation for whole study population |
| | Other measures | Relationship between change scores and GAS outcome T-score |
| Between-group differences in change from baseline | Whole population, Spearman correlation |
| Mann-Whitney Z | p-value | rho | p-value |
| Composite spasticity score (MAS) | –4.9 | < 0.001 | 0.35 | 0.001 |
| Global benefit: patient-report | –3.05 | 0.002 | 0.46 | < 0.001 |
| Global benefit: investigator-report | –3.61 | < 0.001 | 0.41 | < 0.001 |
| HADS (anxiety) | –0.15 | 0.88 | 0.05 | 0.64 |
| HADS (depression) | –0.45 | 0.65 | 0.06 | 0.61 |
| Pain at rest | –0.43 | 0.66 | 0.03 | 0.77 |
| Pain on movement | –0.59 | 0.55 | –0.03 | 0.78 |
| AQoL | –1.13 | 0.18 | 0.07 | 0.52 |
| Patient disability score | –0.65 | 0.51 | 0.19 | 0.08 |
| Carer burden score | –1.17 | 0.24 | 0.14 | 0.26 |
| AQoL: Assessment of Quality of Life; HADS: Hospital Anxiety and Depression Scale; MAS: Modified Ashworth Scale. |
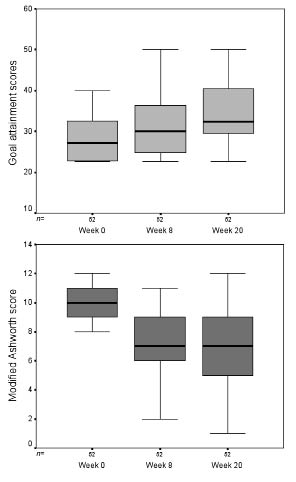
There was a strong correlation between the GAS T-score and reduction in spasticity, as measured by the composite MAS (Spearman rho 0.35 p = 0.001) and with global benefit assessed by both patients and investigators at the end of the second cycle (week 24). There were no significant associations, however, with changes in pain, mood, or quality of life, nor with overall patient disability or carer burden. When examined within the 2 treatment arms there was a significant relationship between change from baseline in GAS score and reduction in spasticity in the BoNT-A treatment arm (rho 0.28 p = 0.04), but not in the placebo group (rho 0.04, p = 0.80). Fig. 1 shows the progression of change in GAS and MAS scores for the active treatment group, over the 2 cycles. GAS scores continued to improve between weeks 8 and 12, whilst spasticity scores remained constant overall.
Fig. 1. Goal attainment and composite spasticity scores for the active treatment group for weeks 0, 8 and 20. The figure shows the progression of change in goal attainment scaling (GAS) and Modified Ashworth Scale scores for the active treatment group, over the 2 cycles. GAS scores continued to improve between weeks 8 and 12, whilst spasticity scores improved from week 0 to week 8, but then remained constant overall to week 20.
Subjects were divided into “responder” and “non-responder” categories on the basis of their mean global benefit at the end of the study. In the active treatment group there were 36/52 (70%) responders, compared with 11/37 (30%) (one case had missing data) in the placebo group (χ2 = 13.5, df = 1, p < 0.001). Across the whole sample, a change in GAS score from baseline of 6 predicted a positive response, with 52% sensitivity, 85% specificity, 81% positive predictive value and 60% negative predictive value.
The provision of concomitant therapies (physio- or occupational therapy) varied widely: median 6 sessions (IQR 0–17, range 0–91) over the 6-month study period, with nearly one-third (26 (28.9%)) receiving no follow-up therapy at all. Unfortunately, apart from the total number of treatment sessions, no detailed information was recorded concerning the actual content or type of therapy intervention. In this study there was no significant difference in goal attainment scores between those who did and did not receive any follow-up therapy sessions. Neither was there any difference between the active/placebo arms with respect to the number of therapy sessions attended. It is possible that the low levels of therapy provision overall contributed to the relatively disappointing outcomes of this study. However, the data available did not permit sufficiently detailed exploration to be able to draw any firm conclusions either for or against the contribution of concomitant therapies to goal attainment.
Qualitative aspects of personal goals set and achieved
A total of 165 personal goals were set by the 90 patients included in this analysis. Of these, 133 (81%) were rated as “very” important, 28 (17%) “moderately”, and only 4 (2%) “a little” important. Similarly, 135 (82%) were rated as “very” difficult, 22 (13%) “moderately”, 6 (4%) “a little”, and 2 (1%) “not at all” difficult. At baseline, 104 goals (63%) were rated at –2 (i.e. could not be any worse with respect to that goal) and 61 (37%) at –1.
Over the course of the study, there was improvement of at least 1 grade in just over one-third of the goals (58 (35%)), of which only 32 (19%) were actually achieved. Only 6 goals (4%) were achieved beyond expectation; 5 of these were in the active treatment group. There was a trend towards lower attainment scores for the very difficult goals, but this could not be tested statistically because of the small number of goals with low difficulty ratings.
Although the improvement rates were generally higher for the BoNT-A group (42/94 compared with 16/71 (χ2 = 8.7, df = 1, p = 0.003)), goal achievement was disappointing overall. We therefore undertook further analysis to examine the goal areas in which progress was more frequently made.
The 165 personal goals were categorized and mapped on to the closest matching domains of the WHO ICF (Table III). Approximately 28% of the goals (n = 46) were set within domains relating to impairment or “Body functions” reflecting pain, passive movement and maintaining joint range, reducing unwanted involuntary reactions, and simple active movements of the hand/arm. The remaining 119 goals related to domains of Activities and Participation, and were broadly divided into:
| Table III. Mapping of main goal categories onto the relevant World Health Organization ICF codes |
| Domain and goal area | Chapter | Primary ICF Code | Associated ICF codes |
| Body functions |
| Pain | 2 – Sensory & Pain | b280 – Pain | b735 |
| Passive movement/range | 7 – Neuro-musculoskeletal | b735 – Muscle tone | b710 |
| Reducing associated reactions | 7 – Neuro-musculoskeletal | b755 – Involuntary movement reactions to position/balance | b735, d415, d450 |
| Simple hand/arm movements | 7 – Neuro-musculoskeletal | b760 – Control of movements | b735, b710 |
| Activities and participation |
| Upper limb activities |
| Lifting and carrying objects | 4 – Mobility | d430 – Lifting and carrying | d445 |
| Fine finger use/dexterity | 4 – Mobility | d440 – Fine hand use | d445 |
| Holding, grasping objects | 4 – Mobility | d445 – Hand/arm use | d430 – lifting /carrying, d415, d450, d475, d550 |
| Mobility |
| Using upper limbs for support/balance | 4 – Mobility | d415 – Maintaining body position | d445 |
| Improved walking/gait pattern | 4 – Mobility | d450 – Walking | d620 – shopping |
| Self care |
| General Independence | 5 – Self care | d500 – General Independence | b510 – washing |
| Hygiene/skin integrity | 5 – Self care | d520 – Caring for body parts | b735, b710, b510 |
| Dressing | 5 – Self care | d540 – Dressing | d440, b735, d710 |
| Eating/drinking | 5 – Self care | d550 – Eating | d560 – drinking, |
| Domestic |
| Meal preparation/cooking | 6 – Domestic Life | d630 – Preparation of meals | d440, d445 |
| Household tasks | 6 – Domestic Life | d640 – Doing housework | d440, d445 |
| Community |
| Recreation/leisure/hobbies | 9 – Community/social | d920 – Recreation/leisure | d440, d445, d455 – swimming, d570 – exercise, d475 – driving |
| ICF: International Classification of Functioning Disability and Health. |
• Upper limb activities, such as lifting and carrying, holding objects still (n = 30).
• Mobility, e.g. maintaining balance or improving gait (n = 11).
• Self-care tasks such a hygiene, dressing or feeding (n = 57).
• Domestic and community tasks, such as housework or recreational activities (n = 21).
Table IV summarizes the performance within these different goal categories for both the treatment and placebo groups, and this is illustrated in percentage terms in Fig. 2.
| Table IV. Performance against the personally set goals within the different World Health Organization (WHO) ICF domains |
| WHO ICF | | Total goals set, n | Dysport ® | Placebo |
| Domain and goal area | Primary Code | Total n | Improved* n (%) | Achieved** n (%) | Total n | Improved n (%) | Achieved n (%) |
| Body functions |
| Pain | b280 | 3 | 2 | 2 | 0 | 1 | 0 | 0 |
| Passive movement/range | b735/710 | 12 | 7 | 6 | 3 | 5 | 3 | 2 |
| Reducing associated reactions | b755 | 20 | 14 | 10 | 7 | 6 | 0 | 0 |
| Simple hand/arm movements | b760 | 11 | 7 | 2 | 2 | 4 | 1 | 1 |
| | | 46 | 30 | 20 (67) | 12 (26) | 16 | 4 (25) | 3 (19) |
| Activities and participation |
| Upper limb activities | | | | | | | | |
| Lifting and carrying objects | d430 | 2 | 1 | 1 | 0 | 1 | 0 | 0 |
| Fine finger use/dexterity | d440 | 5 | 2 | 1 | 0 | 3 | 0 | 0 |
| Holding, grasping objects | d445 | 23 | 16 | 4 | 1 | 7 | 2 | 1 |
| | | 30 | 19 | 6 (32) | 1 (3) | 11 | 2 (18) | 1 (9) |
| Mobility | | | | | | | | |
| Using upper limb for support/ balance | d415/d445 | 4 | 1 | 0 | 0 | 3 | 0 | 0 |
| Improved walking/gait pattern | d450 | 7 | 3 | 1 | 1 | 4 | 2 | 2 |
| | | 11 | 4 | 1 (25) | 1 (9) | 7 | 2 (29) | 2 (29) |
| Self care | | | | | | | | |
| General Independence | d500 | 6 | 2 | 1 | 0 | 4 | 1 | 1 |
| Hygiene /skin integrity | d520/d510 | 9 | 8 | 4 | 4 | 1 | 0 | 0 |
| Dressing | d540 | 27 | 14 | 4 | 3 | 13 | 5 | 2 |
| Eating/drinking | d550/560 | 15 | 5 | 2 | 0 | 10 | 1 | 1 |
| | | 57 | 29 | 11 (38) | 7 (12) | 28 | 7 (25) | 4 (14) |
| Domestic | | | | | | | | |
| Meal preparation/ cooking | d630 | 4 | 2 | 0 | 0 | 2 | 0 | 0 |
| Household tasks | d640 | 7 | 3 | 2 | 0 | 4 | 1 | 0 |
| Community | | | | | | | | |
| Recreation/leisure/hobbies | d920 | 10 | 7 | 2 | 1 | 3 | 0 | 0 |
| | | 21 | 12 | 4 (33) | 1 (5) | 9 | 1 (11) | 0 (0) |
| Total | | 165 | 94 | 42 (45) | 22 (23) | 71 | 16 (23) | 10 (14) |
| *Number of goals showing improvement (i.e. were either partially or fully achieved). **Subset of these goals that were fully achieved. ICF: International Classification of Functioning Disability and Health. |
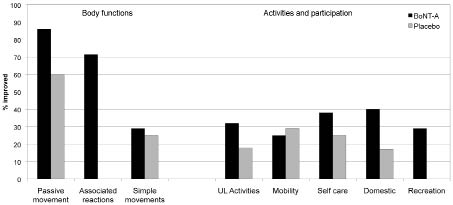
Fig. 2. Relative proportions of goals improving in the principal International classification of functioning disability and health (ICF) domains of “Body functions” and “Activities and participation” within the 2 treatment groups (botulinum toxin type A (BoNT-A) and placebo). The figure illustrates in percentage terms the relative proportions of goals showing improvement within the principal ICF domains. The highest rates of improvement were seen in relation to Body functions, particularly those reflecting passive movement and reduction in associated reactions. Although goal improvement rates were lower within the domains of Activities and Participation, they were generally higher for the active treatment group than in the controls. (N.B. Pain was excluded due to the small numbers.)
As may be expected, improvements in the collective areas of Body Functions are well demonstrated, with two-thirds of the Dysport ® treated patients showing improvement, compared with only a quarter of the placebo group (χ2 = 7.3, df = 1, p = 0.007). However, whilst overall performance was lower in the goal areas representing Activities and Participation, rates of goal improvement were still significantly greater in the context of BoNT-A treatment than placebo (34% vs 22%: χ2 = 4.6, df = 1, p = 0.03).
DISCUSSION
In this secondary analysis we explored the contribution of GAS in comparison with standardized measures as a responsive indicator of change in areas that are important to patients in the treatment of upper limb spasticity following stroke. We were also interested to understand main priority goal areas for treatment from the patients’ perspective, and to learn what types of goals are most often achieved.
Our analysis showed significantly higher levels of goal attainment in the BoNT-A treatment group compared with controls and a cumulative effect over 2 cycles of treatment. Whilst association does not prove causation, correlation between changes in goal attainment and MAS for the active treatment group, but not those on placebo, provides at least some support for the hypothesis that goal attainment was related to the reduction in spasticity by the botulinum toxin. Continued improvement in goal attainment between weeks 8 and 20 after maximum change in spasticity mirrors previous reports that maximum functional gains may take time to achieve, as patients are likely to need to learn how to use any reduced muscle tone (11). The second injection of BoNT-A may have been important in this respect to prevent the return of spasticity and allow functional gain to continue. Further study is needed to explore the relative contributions of: (i) repeat injection; (ii) concomitant therapies; and (iii) self-directed practice/passive stretching to the longer term gains from BoNT-A injection. In the meantime, this finding highlights the importance of applying measures at appropriate time intervals, which may vary for different outcomes.
The lack of relationship of GAS with pain, or with quality of life as measured by the AQoL, is not altogether surprising. The AQoL is a global measure comprising 12 items in 4 main domains (Independent living, Social relationships, Physical senses and Psychological well being). Our qualitative analysis revealed that patients’ priority goals for treatment overlapped with just 4 of the 12 items (personal care, household tasks, mobility and pain). Of these, spasticity-related pain was not a prominent feature in the study group, with only 3 patients setting pain reduction as one of their goals. Moreover, both the AQoL and the HADS showed considerable inter- and intra-patient variability through the course of the study, presumably in response to external factors other than arm spasticity (28). This finding illustrates the inherent problems in applying global measures to evaluate outcome from a focal intervention, and emphasizes the need to focus assessment in the areas where change is anticipated.
In this study, GAS appeared to be more responsive to the effects of BoNT-A intervention than other standardized person-centred measures. Nevertheless overall levels of goal attainment were much lower than expected. If goals are predicted without bias, under- and over-achievement should occur approximately equally, and the GAS T-score should be distributed around a mean of 50. In this study, a median score of just 32.5, even in the active treatment group, suggests that treating teams had a tendency to incorporate over-ambitious goals within the GAS. This could reflect a number of factors including: (i) inexperience in negotiation to agree realistic goals; (ii) difficulty in predicting the outcome of BoNT treatment; or (iii) sub-optimal treatment. The high proportion of “–2” scores at baseline, together with a low mean GAS score of 27.2 in comparison with other series (10), suggests that clinicians were aware that patients were starting from a low level. Moreover, the large majority of goals were rated as “very difficult”. Team reflections after the end of the study have identified the need for further training in GAS, particularly in the area of goal negotiation.
Goal analysis showed that patients tended to choose active tasks when setting their personal goals for treatment. Nearly two-thirds (63%) of the goals related to activities involving active movement of the affected upper limb, such as dressing, eating, housework, hobbies, or being able to grasp and hold objects for bimanual activities. In a group of patients with long-standing spasticity, these goals were highly ambitious, and hence the relatively low rates of achievement are not unexpected. However, other goals areas fared better. Passive tasks, such as ease of maintaining range of movement, hygiene or reducing care needs, were chosen less often by patients (16% of goals), but were more often achieved. The reduction in “associated reactions” (involuntary movements induced by position/balance) was a further successful goal area, with potential importance for safe mobility. Finally, for the few who did set pain reduction as a goal for treatment, this was a further successful area of goal attainment in the active treatment group.
These findings underline an important aspect of using GAS as an outcome measure. Ultimately goal attainment depends on more than just the ability of the patient to meet the goal; it also depends on the accuracy with which clinicians can predict expected outcomes, and their ability to negotiate realistic goals. Many clinicians find this challenging, and feel inherently more comfortable with standardized measuring tools, which at least provide a clear yardstick for comparison.
The clinical application of GAS therefore provides both opportunities and challenges. The goal-setting process itself supports co-ordination of team effort as well as communication with the patients and their relatives. Importantly, it offers the opportunity to negotiate and agree realistic goals as part of “a priori” goal-setting. However, the treating team needs to be experienced in the area of management in order to predict outcome accurately. Team members also require high-level negotiating skills to establish realistic expectations and set achievable goals.
The development of standardized goals, as proposed by Tennant 2007 (26), may assist the application of GAS in a number of respects. His reasoning was to satisfy psychometric requirements of uni-dimensionality by building up item banks of goals, which can then be calibrated onto a metric uni-dimensional scale, thus allowing for an individualized yet generalized approach. As he acknowledges, the extent to which some higher order constructs, such as health status, can be constructed remains an empirical question, and there is a balance to be found between standardization for the sake of satisfying mathematical principles while still retaining the flexible, person-centred goal-setting that underlies the principle of GAS. However, there are also some practical aspects of goal setting that would be assisted by standardized goal sets. Clinicians report that the setting and wording of SMART goals can be very time-consuming. A set of pre-worded goal statements that could be chosen or adapted for the individual in order to save starting anew each time, would help to streamline the process making it more feasible for application in routine clinical practice. In addition, a menu of common goal items may assist the patient and clinicians in choosing realistic goals and attainment levels to match their particular circumstances.
As a first step towards the development of standardized goal sets, we performed this analysis to identify the types of goals that are commonly set and achieved in this context, and we also attempted to categorize these based on the WHO ICF as a common language framework. This retrospective mapping of goals onto the ICF domains was not entirely straightforward. By inference, all goals are assumed to relate in some sense to Body Structures s730 (upper extremity) and Body Functions b735 (muscle tone) to qualify for the study, although some goals explicitly mentioned reduction in muscle tone or “spasms”. For the majority of goal categories a primary ICF domain could be identified. However, most goals covered several ICF domains, as shown in Table III. For example, a return to swimming could reflect any or all of the following domains: d445 (hand/arm use), d455 (moving around), d570 (fitness and exercise) and/or d920 (recreation/leisure). The reduction in associated reactions was also difficulty to classify. For some patients, management of involuntary movement appeared to be the goal itself. In others it was specified as a goal to improve walking or standing ability.
As noted in the introduction, the fact that GAS may combine goals across the range of impairment, activity and participation into a single measure is a feature that attracts many clinicians. However, to satisfy psychometric requirements it may be necessary, in future, to separate GAS scores from these different ICF levels. For future development of the goal sets, ICF mapping will need to be undertaken prospectively as part of the goal setting, in order to define the relevant domain(s) more accurately.
Limitations of the study
The authors recognized some clear limitations to this study:
• There were a number of faults in the main study design that are discussed in more detail in the main paper (28), but which impact on this analysis. The study was designed to reflect real-life current practice in Australia. Use of electromyography and/or nerve stimulation to guide injection was left up to the discretion of the injector, and injection accuracy is therefore uncertain. Moreover, follow-up therapy was only provided according to routine practice and approximately one-third of patients did not receive significant follow-up therapy. It is possible that both of these factors contributed to the relatively low attainment scores. Unfortunately, apart from the number of therapy sessions attended, trial records did not detail either injection technique or concurrent therapeutic interventions, so it was not possible to explore the differential impact of either of these factors on outcome in this analysis.
• Although the study itself was of a reasonable size, the goal areas were diverse. In the less common goal areas, such as pain and symptom management, goal numbers were very small. These response rates to individual goal areas within our analysis should therefore be interpreted with caution.
• As noted above, goals were retrospectively allocated to the ICF categories. Goal wording was not always clear and this may have led to mis-categorization of some goals. Further work with prospective allocation is required.
• The assessment of global benefit is widely used in evaluations of complex intervention, because it captures a range of different aspects of the response, which may not be reflected in formal measures. Although the evaluation of global benefit usually encompasses experience well beyond the 2–3 selected goals that are included in GAS, there is some potential for confound with GAS, since the goal-setting and evaluation of goal attainment is an integral part of management. The association between GAS and global benefit should therefore also be interpreted with caution.
In summary, accepting the limitations of this study, some clear trends emerged. GAS did appear to be a responsive measure for evaluating outcomes of importance to the individual patient that were not picked up by the standardized measures. Although patients tended to chose active tasks as their goals for treatment, the principal effects of spasticity management impacted predominantly on goal areas where relieving spasticity was itself the desired effect – such as improved movement/range and ease of caring for the affected limb (passive function). This is expected as BoNT-A reduces excessive muscle tone and the return of active selective movement occurs only in the minority if patients in whom the unwanted spasticity masks such activity. This group of patients had had “substantial spasticity” for a mean of 6 years post-stroke. The opportunities for achieving return of active function that long after injury are clearly limited for many patients after this length of time, and should be taken into account when goal setting. Nevertheless, some significant gains were made in important areas of function including the ability to engage in hobbies and household tasks, and in mobility which have significant implications for quality of life. These findings may help to inform the future development of standardized goal sets. In the meantime, further research with a priori categorization of goals in large prospective cohort studies is required to describe the full value of BoNT-A in management of upper limb spasticity.
ACKNOWLEDGEMENTS
The primary study was funded by Ipsen Pty Ltd, who had no influence on the interpretation of data and the final conclusions drawn. Financial support was also provided by the Luff Foundation and Dunhill Medical Trust for this secondary analysis and for preparation of the manuscript.
We would like to thank all the clinical staff and patients who took part in this study. We would like to acknowledge the contribution of the late Dr J. Sandanam of St Joseph’s Hospital, Sydney, who was a contributor to the study but sadly passed away before this article could be presented. Special thanks are to Gavin Assauw, and to Melinda Munns for assistance with data handling. Statistical support was provided by Peter Mullins of Sage Consulting, New Zealand.
Conflict of interest
Ipsen Pty produces Dysport and has an interest in demonstrating benefit. Most of the authors have a financial relationship with Ipsen in the form of honoraria, sponsorship to attend meetings and/or consultancy fees. However, none of the authors has any personal financial interest in Dysport, or in any of the methods used in this research.
REFERENCES