Sarah Prenton, BSc (Hons), PGCert, Kristen L. Hollands, PhD and Laurence P. J. Kenney, PhD
From the 1University of Huddersfield, School of Human and Health Sciences, Department of Health Sciences, Health and Rehabilitation Division, Huddersfield and 2University of Salford, School of Health Sciences, Salford, UK
OBJECTIVE: To compare the effects on walking of functional electrical stimulation (FES) and ankle foot orthoses for foot-drop of central neurological origin, assessed in terms of unassisted walking behaviours compared with assisted walking following a period of use (combined-orthotic effects).
DATA SOURCES: MEDLINE, AMED, CINAHL, Cochrane Central Register of Controlled Trials, Scopus, REHABDATA, PEDro, NIHR Centre for Reviews and Dissemination and clinicaltrials.gov, plus reference list, journal, author and citation searches.
STUDY SELECTION: English language comparative randomized controlled trials (RCTs).
DATA SYNTHESIS: Seven RCTs were eligible for inclusion. Two of these reported different results from the same trial and another 2 reported results from different follow-up periods and were therefore combined, resulting in 5 synthesized trials with 815 stroke participants. Meta-analyses of data from the final assessment in each study and 3 overlapping time-points showed comparable improvements in walking speed over 10 m (p = 0.04–0.79), functional exercise capacity (p = 0.10–0.31), timed up-and-go (p = 0.812 and p = 0.539) and perceived mobility (p = 0.80) for both interventions.
CONCLUSION: Data suggest that, in contrast to assumptions that predict FES superiority, ankle foot orthoses have equally positive combined-orthotic effects as FES on key walking measures for foot-drop caused by stroke. However, further long-term, high-quality RCTs are required. These should focus on measuring the mechanisms-of-action; whether there is translation of improvements in impairment to function, plus detailed reporting of the devices used across diagnoses. Only then can robust clinical recommendations be made.
Key words: electrical stimulation therapy; nervous system diseases; stroke; walking; foot drop; systematic review; meta-analysis.
J Rehabil Med 2016; 48: 646–656
Correspondence address: Sarah Prenton, Room RG/23, Ramsden Building University of Huddersfield, Queensgate, Huddersfield, West Yorkshire, HD1 3DH, UK. E-mail: S.Prenton@hud.ac.uk
Accepted Jul 15, 2016; Epub ahead of print Aug 22, 2016

*Some of this material was presented as a poster on 8th and 9th May 2015 at iFESSUKI at the University of Sheffield.
INTRODUCTION
Conditions such as stroke, brain injury (BI), multiple sclerosis (MS), spinal cord injury (SCI) and cerebral palsy (CP) affect upper motor neuronal pathways (1) and are collectively referred to as pathologies of central neurological origin (CNO) (2). In the UK there are approximately 1.2 million people living with stroke (3), 100,000 MS and 40,000 SCI (4), there are 160,000 BI admissions per year (5) and 1 in 400 people have CP (6). Foot-drop is a common impairment seen across these conditions (7) and although prevalence data in some of the CNO conditions is very limited, a commonly cited figure suggests that it is seen in 20–30% of people with stroke (7, 8).
Foot-drop is categorized as an inability to dorsiflex the foot, with or without excessive inversion and is most commonly caused by weakness in the dorsiflexor (and evertor) and/or overactivity in the plantarflexor (and invertor) muscle groups. Foot-drop results in walking being slower, less efficient and potentially unsafe (7); as foot clearance during swing and initial foot contact at the start of the stance phase are compromised. These factors have been associated with an increased risk of falls (7), reduced quality of life (7, 9) and increased levels of mortality (10).
Current practice in the treatment of foot-drop normally involves a form of ankle foot orthosis (AFO) (11). Functional electrical stimulation (FES) is also used but less frequently (9).
AFOs stabilize the foot and ankle and lift the toes when stepping (12). Meta-analyses have shown them to have positive effects on some aspects of walking (12, 13), but these analyses are primarily based on non-randomized control trial (RCT) evidence. AFOs have been criticized for detrimental effects on the adaptability of walking, propulsion, aesthetics and comfort (14–16), which can impact on compliance and satisfaction.
Foot-drop FES uses electrical pulse trains to stimulate the common peroneal nerve over key phases of the gait cycle to correct the foot-drop impairment (17). This phasic stimulation can be delivered via surface or implanted electrodes. Foot-drop FES has been shown to have positive effects on walking speed (18, 19), but meta-analyses have also, in part, been based on non-RCT evidence. For surface systems, limitations have been cited in relation to issues with effort of setup, skin irritation and pain (20), which again affects compliance and satisfaction. Implanted systems address some of these limitations, but are more costly (21).
Despite their limitations both are endorsed in the management of foot-drop, with clinical guidelines existing for AFO as a result of stroke (22, 23) MS (24), CP (25) and BI (26) and FES guidelines promoting use across all CNO diagnoses (2). However, these guidelines have had to rely on some non-RCT sources of evidence and as intervention specific guidelines, comparing with no treatment or physiotherapy, do not consider evidence from direct comparisons between these interventions. As a result, current guidelines do not provide clinicians with a clear patient pathway. Recently a number of RCTs providing direct comparisons have been published. Furthermore, these studies have advanced our understanding of the effects these interventions may produce:
The suggested mechanism-of-action for AFO is that the device remedies the loss of dorsiflexion/eversion by holding the foot in a neutral position, but this can result in negative effects on neuromuscular control and muscle biomechanics with long-term use (29–31). Therefore, it has been assumed that they only provide immediate-orthotic effects (12), a notion supported by the only known long-term AFO-specific RCT in the field (32).
In contrast, there are many reports of long-term neuromuscular control improvements with FES (19, 33), which are attributed to changes in neural plasticity, muscular strength and cardiovascular efficiency (31, 34, 35). The mechanism for these improvements has been hypothesized as being due to the coinciding of antidromic electrical stimulation-generated action potentials with volitional activity, leading to strengthening of modifiable Hebb-synapses at a segmental level (34, 36, 37).
Given these proposed mechanisms-of-action it could be assumed that FES will provide a distinct advantage over AFO with long-term use.
Two recent reviews (9, 38) have explored the long-term effects evidence for AFOs vs FES in stroke survivors; both concluding that there was a preference for FES but insufficient evidence to recommend one over the other. However, the first was not systematic (39) and included non-RCT studies (9) and the other did not meta-analyse; possibly due to the breadth of question posed (38). This review (38) reported that FES was superior at conserving energy but included a paper where FES was combined with botulinum toxin (40) and another that compared FES with therapy as opposed to AFO (41).
In order to provide improved clinical guidelines, which will help clinicians determine which of these interventions to prescribe and what the directly comparable effects are over a period of use, gold standard meta-analysis of RCT level evidence is required (42). Given that both interventions are most commonly prescribed as long-term orthotics (9, 30) and the assumption that studying long-term use will highlight any differences in walking behaviours resulting from the different mechanisms-of-action, we sought to perform a systematic examination of the evidence base to address the question: Are the combined-orthotic effects on walking for foot-drop of CNO greater for FES than AFO?
METHODS
This review was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (43). The full review protocol can be found at: http://www.crd.york.ac.uk/PROSPERO/register_new_review.asp?RecordID=9892&UserID=6114.
Nine electronic databases were searched: MEDLINE (Ovid), AMED (Ovid), CINAHL (EBSCO), Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, REHABDATA, PEDro, NIHR Centre for Reviews and Dissemination and clinicaltrials.gov. A search strategy including controlled vocabularies related to “electric stimulation”, “walking” and “nervous system diseases” and terms such as “foot drop” and “electric* stimulat*” were used with no date limits (full search strategy available on request from the corresponding author). Reference list, citation, key author and journal searches were also completed and all searches were limited to the English language.
Once duplicates were removed 1 reviewer (SP) screened titles and abstracts, categorizing each as “possibly” or “clearly not” relevant against the inclusion criteria (Table I). Full-length articles were retrieved for “possibly relevant” studies and 2 unmasked reviewers (SP and KH) independently assessed their eligibility (Table I), classing them as “relevant”, “definitely irrelevant” or “unsure”. Different outcome measurements from the same trial reported in separate publications were treated as a single publication; as were separate publications that reported different data collection time-points within the same trial. Any disagreements or “unsure” publications were discussed (between SP and KH). A third reviewer was available to resolve any disagreements (LK).
|
Table I. Inclusion criteria |
|
Design
Participants
Intervention
Comparator
Outcomes
|
SP extracted data using a predesigned pro forma; trial details extracted related to the characteristics of the included studies, participant and intervention details. Missing data and/or aspects that required clarification were requested from trial authors (14, 16, 44, 45), by SP (Appendix I). KH reviewed the extracted data for accuracy.
As an RCT-based review, and to avoid the limitations of scaled quality assessment tools (42, 46), the Cochrane risk of bias assessment tool (42) was used independently by 2 reviewers (SP and KH) with a third reviewer (LK) available if necessary. To ensure impartiality, risk of bias was based on published work only. Performance bias was not considered as the interventions precluded blinding of participants and measures were primarily objective (46).
Outcomes across the World Health Organization’s (WHO) International Classification of Functioning, Disability and Health (ICF) (47) were extracted. This helped to identify if there was any comparative evidence to support the assumed mechanisms-of-action and whether they translated into function. Therefore, all measurements were categorized as either being within the body functions and structures (BFS), activity or participation domain (47) by SP, using supporting literature (47–50). All post-intervention data collection point assisted-walking means and standard deviations (SD) were extracted with final-assessment data pooled for data analysis. Given the hypothesized mechanisms-of-action suggesting that FES would have greater benefits than AFO with longer-term use; broadly overlapping time-point data was also grouped for meta-analysis where possible. Standard errors were converted to SDs (14, 42, 51) and functional exercise capacity (an activity domain measurement (52)) was considered as metres walked, and was converted as necessary (15).
Meta-analyses were performed using RevMan 5.3® software. Where the same measurement was used across more than 2 trials, outcomes were combined using mean difference (MD) with 95% confidence intervals (95% CIs). Where an outcome was measured using different approaches, such as functional exercise capacity (distance walked in m measured over 2, 3 or 6 min), standardized mean difference (SMD) with 95% CIs was used. For crossover trials only pre-crossover data was extracted (15). Where there was more than one arm looking at the same intervention the similarity at baseline to the other intervention and size were used to decide which to use, and the data from the most comparable group was extracted (15).
Heterogeneity was examined using visual inspection of forest plot, χ2 test and I² statistic. If the χ2 test showed heterogeneity that the I² statistic identified as being moderate to low (< 50% (42)) a fixed-effects model was used. A random-effects model was used for heterogeneity > 50%.
RESULTS
A total of 1,836 citations were found, of which 7 were eligible for inclusion. Two of these reported outcomes from the same participants (44, 53) and were therefore grouped, and subsequently referred to by the first publication date (44). One trial published results up to 6 months (14) and had another publication reporting results at 12 months (51); and were therefore also grouped. For meta-analysis the relevant publication was used with the source identified by the date of the publication on the corresponding forest plot. Thus a total of 5 RCTs, published between 2007 and 2015 with 815 participants, were available for meta-analysis (Fig. 1).
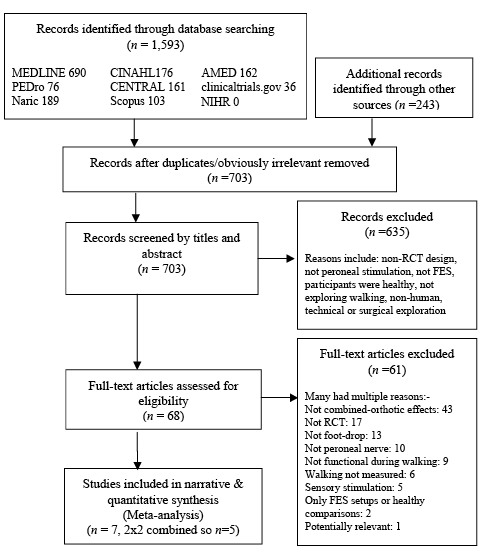
Fig. 1. Trial selection.
Characteristics of included trials
One trial used a multiple-site crossover design (15) with 2 AFO arms. Data from arm 2 (AFO-FES) was used as it was larger and similar to the FES group at baseline. The remaining 4 trials used 2-arm parallel RCT design, 2 single-site (44, 45) and 2 multiple-site (14, 16) (Table II).
Participant details
All the participants were over the age of 18 years and had suffered a stroke. Mean time since diagnosis ranged from 51.7 days (45) up to 6.9 years (14, 51). Of those trials that reported hemiplegic side (16, 44, 45) there was a relatively even distribution (116: 47.9% right, 126: 52.1% left). Two of the trials recruited current AFO users (16, 44), whereas the remaining 3 introduced the interventions to both groups for the first time (Table II).
|
Table II. Characteristics of included trials, participant and intervention details |
|||||||||||
|
|
Trial design |
N Diagnosis (R):(L) |
Men: Women |
Age (years) |
Time since diagnosis Mean (SD) |
Current or new AFO users |
AFO |
Mechanical properties reported |
FES |
Setup for measurement done by |
Use |
|
Bethoux et al. (14, 51) a |
2-arm parallel Multiple sites |
495 (242 FES: 253 AFO) CVA Not specified |
FES = 147:95 AFO = 157:96 |
FES = 63.87 (11.33) AFO = 64.3 (12.01) |
FES = 6.9 years (6.43) AFO = 6.86 years (6.64) |
New |
Customized |
No |
Surface Walkaide |
Not specified |
Home 2-week progressive wearing schedule then all day |
|
Everaert et al. (15)b |
3-arm crossover Multiple sites |
78 (43 FES: 35 AFO) CVA Not specified |
FES = 32:6e AFO = 19:12e |
FES = 57.1 (12.9)e AFO = 55.6 (11.9)e |
FES = 6.4 months (3.8)e |
New |
Customized |
No |
Surface Walkaide |
Not specified |
Home All day |
|
Kluding et al. (16)a |
2-arm parallel Multiple sites |
197 (99 FES: 98 AFO) CVA 93:104 |
FES = 51:48 AFO = 67:31 |
FES = 60.71 (12.24) AFO = 61.58 (10.98) |
FES = 4.77 years (5.29) AFO = 4.34 years (4.1) |
Current |
Customizedf plus TENS for 2 weeks |
No |
Surface NESS L300 |
Not specified |
Both Bioness clinical protocols followed 15min-all day Training: 15 min × 2 day 1 week then 20 min 2 × day next 2 weeks |
|
Kottink et al. (44)c |
2-arm parallel Single site |
29 (14 FES: 15 AFO) CVA 13:16 |
FES = 10:04 AFO = 10:05 |
FES = 55.2 (11.36) AFO = 52.87 (9.87) |
FES = 9.07 years (9.29) AFO = 5.67 years (4.64) |
Current |
Combinationf |
No |
Implanted 2-channel implant |
Not specified |
Home Gradual increase over 2 weeks, then all day |
|
Salisbury et al. (45)d |
2-arm parallel Single site |
16 (9 FES: 7 AFO) CVA 10:6 |
FES = 03:06 AFO = 03:04 |
FES = 55.8 (11.3) AFO = 52.6 (17.2) |
FES = 51.7 days (34.6) |
New |
Off the shelff |
No |
Surface ODFS |
Clinician for FES |
Supervised Part of physiotherapy 20 min, 5 × week with supervised/independent walking as appropriate |
|
aITT completed; bPost-intervention/dropout characteristics; cbased on 2007 not 2012 data. dPre-intervention/dropout characteristics; CVA: cerebrovascular accident/stroke. ePost-intervention/ dropout characteristics at later time-point than is included in this review (12 weeks); customized: custom-made/modified AFO; Combination: different AFOs used by different participants; off the shelf: prefabricated/unmodified AFO;. fBoth groups continued with physical therapy alongside intervention. FES: functional electrical stimulation; AFO: ankle-foot orthosis; TENS: transcutaneous electrical nerve stimulation with no motor response; NESS L300: Bioness model; ODFS: Odstock foot-drop system; BFS: body functions and structures. |
|||||||||||
Intervention details
Three of the trials (14–16, 51) reported providing “customized” AFOs prescribed by an orthotist; plus a physiotherapist for Kluding et al. (16). One used off-the-shelf AFOs (45) which is appropriate practice with their, sub-acute, population (54) and 1 used a combination (44). No trial reported any further details of the AFOs or how prescription decisions were made; none were hinged. All but one trial used surface FES systems (44), one trial highlighted that “clinicians” set up FES for measurement (45), but no trial reported details of set-up parameters, such as electrode placement, ramping, amplitude or frequency. The setting where interventions were used varied, with participants from 3 of the trials using the devices within their own environment (14, 15, 44, 51). One trial used them in both the participant’s own environment and under supervision (16) and 1 used them only under supervision (45). All-day-use was encouraged in all but one of the trials (45), some with a gradual introduction, although whether this was adhered to was not reported. Three trials provided concurrent therapy for both groups (16, 44, 45) (Table II).
Methodological quality
Table III summarizes the quality assessment, Kluding et al. (16) alone had no identified areas of high risk of bias.
|
Table III. Risk of bias |
||||||
|
Random sequence generation (selection bias) |
Allocation concealment (selection bias) |
Blinding of outcome assessment (detection bias) |
Incomplete outcome data (attrition bias) |
Selective reporting (reporting bias) |
Other bias |
|
|
Bethoux et al. (14, 51) |
Unclear |
High |
High |
Low |
Low |
Low |
|
Everaert et al. (15) |
Unclear |
Unclear |
Unclear |
High |
Low |
Low |
|
Kluding et al. (16) |
Low |
Low |
Unclear |
Low |
Unclear |
Low |
|
Kottink et al. (44) |
High |
Unclear |
High |
Unclear |
Low |
Low |
|
Salisbury et al. (45) |
High |
Low |
High |
Unclear |
Low |
Low |
Outcome measurements
All trials utilized ICF activity domain measurements; most commonly the 10-metre (m) walk test (Table IV). However, one did not collect any BFS domain measurements (14, 51) and another lacked participation domain measurements (15). The intervention period studied ranged from 6 weeks (15) to 12 months (51).
To allow direct comparison of the assumed mechanisms-of-action and functional translation, the following results are presented according to ICF domains. The narrative comparison found in Table IV is summarized below. Final-assessment meta-analyses are presented first. There were 3 overlapping data time-points, at 4–6 weeks, 12–13 weeks and 26–30 weeks, for activity domain measurements. These are categorized as short, medium and longer-term respectively (Table IV); meta-analyses at these time-points are then presented.
Body functions and structures
Physiological cost index (PCI) (15), cadence (45), spatiotemporal/kinematics (44) and lower limb Fugl-Meyer (16) were reported by single trials; therefore pooled-analysis was not possible. All the trials found within-group improvements, but no significant statistical differences were reported for any of these measures by the primary authors except Kottink et al. (44), who found some spatiotemporal and kinematic differences in favour of FES (p < 0.05) (Table IV).
Activity
Final-assessment outcomes of 10-m walking speed (all 5 trials, n = 789) and functional exercise capacity (3 trials, n = 761) were pooled. Meta-analysis showed between-group comparable improvement (MD = 0.01, [–0.04, 0.05]; I2 = 0%; p = 0.79, Fig. 2a); and SMD –0.07 [0.22, 0.07], I2 = 0%; p = 0.31, Fig. 3a), respectively.
The timed up-and-go test was used in 2 trials (16, 51), both reported between-group comparable improvement (p = 0.812 and p = 0.539), therefore meta-analysis was not required (Table IV).
All other final-assessment activity measures were used in single trials with between-group comparable improvement in all cases (Table IV).
Meta-analysis was possible for the 10-m walk test using data at short (4 trials, n = 771), medium (3 trials, n = 699) and longer-term (3 trials, n = 713) time-points (Fig. 2b–d). This revealed comparable improvement in the short-term (MD = 0.02 [–0.05, 0.10]; I2=66%; p = 0.54, Fig. 2b)) and longer-term (MD = –0.02 [–0.06, 0.03]; I2 = 50%; p = 0.43, Fig. 2d)). In the medium-term there was a marginal, but significant, difference in favour of AFO (MD = –0.04 [–0.09,–0.00]; I2 = 0%; p=0.04, Fig. 2c)).
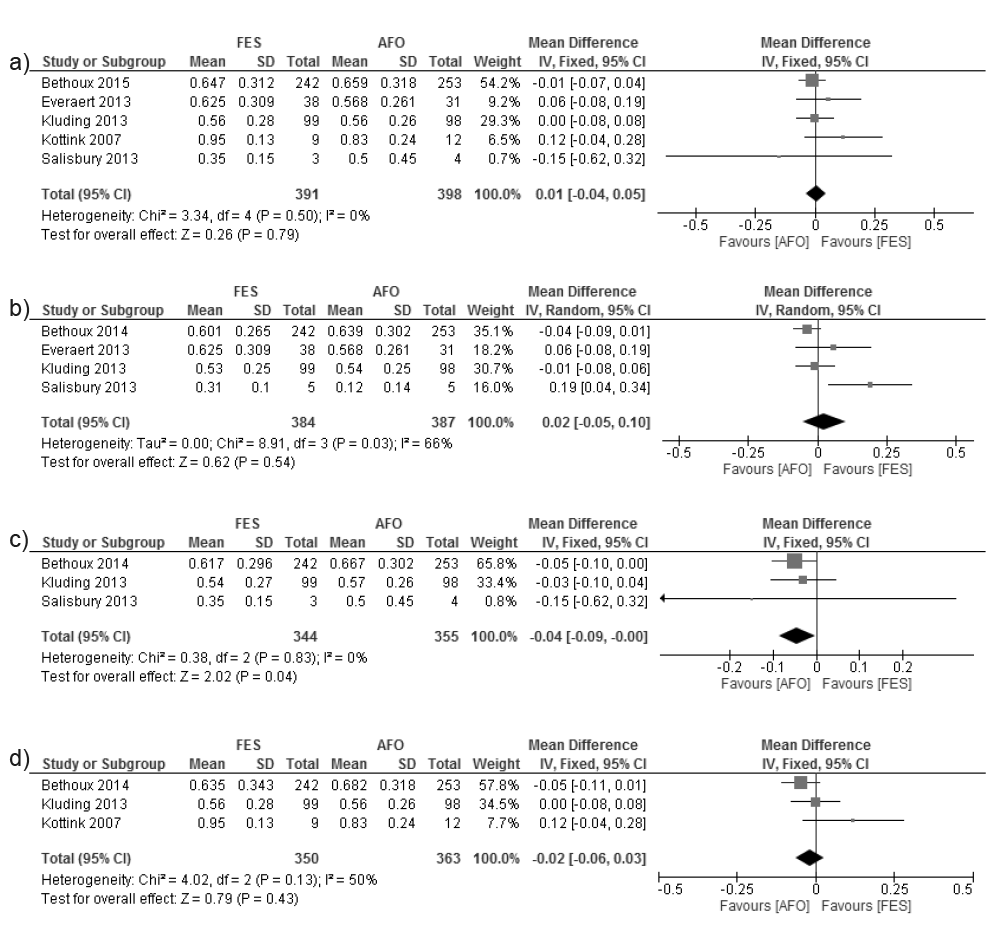
Fig. 2. Activity measure: 10-metre (m) walk test (metre/second). (a) Final assessment. (b) Short-term. Bethoux et al. (14) and Kluding et al. (16) data obtained via correspondence with authors. (c) Medium-term. Bethoux et al. (14) and Kluding et al. (16) data obtained via correspondence with authors. (d) Longer-term. Kluding et al. (16) data from correspondence with authors.
Functional exercise capacity meta-analyses were performed for short (3 trials, n = 761) and medium-term (2 trials, n = 692) time-points (Fig. 3b and c). Meta-analyses revealed between-group comparable improvement (SMD = –0.12 [–0.26–0.02]; I2 = 0%; p = 0.10, Fig. 3b) and SMD = –0.10 [–0.25, 0.05]; I2 = 0%; p = 0.19, Fig. 3c)).
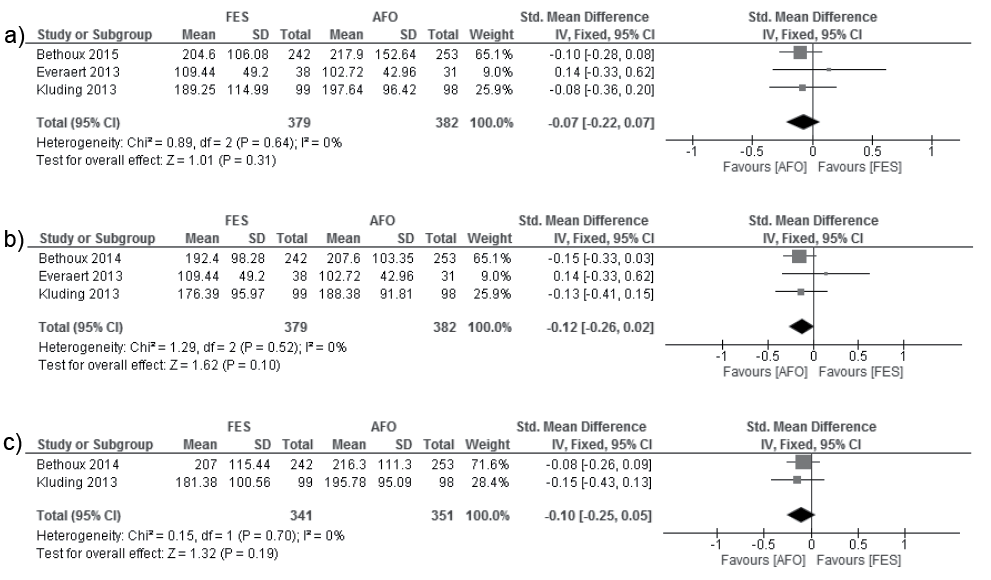
Fig. 3. Activity measure: functional exercise capacity metres (m). (a) Final-assessment. Kluding et al. (16) data obtained via correspondence with authors. (b) Short-term. Bethoux et al. (14) and Kluding et al. (16) data obtained via correspondence with authors. (c) Medium-term. Data obtained via correspondence with authors.
Participation
The mobility domain of the Stroke Impact Scale (SIS) was collected by 3 trials (n = 701) (14, 16, 45). Meta-analysis showed between-group comparable improvement (MD 0.31 [–2.06, 2.68]; I2 = 41%; p = 0.80, Fig. 4).

Fig. 4. Participation measure: Stroke Impact Scale (mobility sub-scale).
Activity monitoring was used by 2 trials (16, 44) (Table IV), but their data collection methods varied too significantly (steps taken compared with time spent in different positions) to pool results. Kluding et al. (16) found no significant differences in the number of steps taken and Kottink et al. (44) found the FES group spent significantly more time in sitting/lying than the AFO group (p = 0.04).
All other final-assessment participation measurements were used by a single trial (14) with between-group comparable improvements found (Table IV).
|
Table IV. Outcome measurements and intervention effects |
|||
|
|
Walking outcome measures used & ICF level |
Outcome collection points |
Combined-orthotic effects |
|
Bethoux et al. (14, 51) |
Activity:
Participationb:
|
0 Short: 1 month (not published) Medium: 3 month (not published) Longer: 6 month 12 monthb |
|
|
Everaert et al. (15) |
BFS:
Activity:
|
0, 3 weeks Short: 6 weeks |
|
|
Kluding et al. (16) |
BFS:
Activity:
Participation:
|
0 Short: 6 weeks (not published) Medium: 12 weeks (not published) Longer: 30 weeks (only change data published) |
|
|
Kottink et al. (44) |
BFS:
Activity:
Participation:
|
0 Longer: 26 weeks |
|
|
Salisbury et al. (45) |
BFS:
Activity:
Participation:
|
0 Short: 6 weeks Medium: 12 weeks |
|
|
aidentified as primary outcome measure by authors; bnot reported in Bethoux et al. (51) 12-month follow-up publication; cFrom Kottink et al. (53). mEFAP: modified Emory Functional Ambulation Profile; TUG: Timed Up and Go; QoL: Quality of Life; SIS: Stroke Impact Scale; ADL/IADL: Activities of Daily Living/Instrumental Activities of Daily Living; 10MWT: 10-metre walk test; PCI: Physiological Cost Index; RMI: Rivermead Mobility Index; BBS: Berg Balance Scale. FAC: Functional Ambulation categories; increase; >greater than; = equal to; < less than; BFS: body functions and structures. |
|||
DISCUSSION
This is the first systematic review, including meta-analysis, of studies comparing AFO with FES as interventions for people with CNO foot-drop, which focuses on the clinically relevant combined-orthotic effects on walking. As a RCT-based review with meta-analysis guided by the PRISMA statement (55) the results provide the highest level of evidence currently available to support clinical decision-making (42).
The RCTs were deemed to be of medium-methodological quality, which provides some confidence in our results that both interventions demonstrate equal combined-orthotic improvements in 10-m walking speed, functional exercise capacity, timed-up-and-go and the mobility sub-scale of the SIS; regardless of the length of time used.
Given the different hypothesized mechanisms-of-action detailed in the introduction it is somewhat surprising that there was no differentiation between the 2 interventions for any of the pooled measurements. To explore this result we examined outcome measurements within the BFS domain (which directly reflect mechanisms-of-action (48)) and whether or not these changes in BFS coincide with changes in activity and participation differentially between the interventions and over different time-points of use.
Body functions and structures
The majority of measurements used in the reviewed trials suggest that there are no differences between the 2 interventions. However, given the suggestions of a negative influence of AFO and a positive influence of FES on volitional muscle activation it was surprising that none of the included trials reported electromyography (EMG) or strength data. Throughout our systematic search of the literature we found only one RCT (which explored therapeutic as opposed to combined-orthotic effects) that compared EMG activity between FES and AFO treatments. This trial reported that EMG activity was greater following a period of FES than AFO use (37).
Kottink et al. (53) was the only reviewed trial to measure gait features and found differences between a FES group and an AFO group. Despite these findings, which are supported by results of non-RCT studies (57–61), no further inferences can be drawn at this time. Future trials should capture such measurements to determine whether restorative, as opposed to compensatory, changes are made (62) in order to more accurately understand the mechanisms-of-action.
Activity and participation
Meta-analysis of 3 validated measures of the activity domain (49, 52) and one mobility-specific participation domain measurement (49, 52) indicate that AFOs and FES produce equivalent functional improvements to walking for people with foot-drop as a result of stroke; regardless of length of use. The equivalency of effects between these interventions is supported by non-RCT studies, which have found no significant changes in activity domain measurements when FES is provided to AFO users (59, 60, 63).
Given the difference in hypothesized mechanisms-of-action between FES and AFO and the lack of BFS measurements, the question remains as to how these comparable effects on activity/participation are achieved. One explanation is that both simply correct the mechanical problem of foot-drop; as is suggested for AFO. However, this does not fully explain the differences between immediate-orthotic effect and orthotic effect after a period of use. The activity monitoring results from 1 trial highlight another potential explanation. Kluding et al. (16) found that the number of steps taken per day increased with use of either intervention (1,891–2,069, AFO and 2,092–2,369, FES at 6 and 30 weeks). This increase in repetition of walking in both FES and AFO intervention groups (facilitated by the correction of foot-drop) could explain the observed comparable improvements. Indeed intensity of task-specific repetition is widely accepted as critical for effective improvements of motor-impairments (64–66). This hypothesis is consistent with Kluding et al.’s suggestion that both interventions achieve combined-orthotic effects through immediate-orthotic and training effects (16).
A final hypothesis is that RCTs to date have not been long enough to detect differences given the predominantly chronic populations investigated (67). Bethoux et al. (51) did not find differences at 12 months, which may suggest even longer-term follow up is required (68). To facilitate comparisons, all future trials should ensure that data collection time-points are justified against physiological processes underlying treatment effects.
This review had some limitations. Firstly, it has revealed that, until 2007, research has been limited to examinations of a single intervention for a single diagnosis precluding comparisons between interventions that might usefully inform clinicians which intervention may be most suitable. Since 2007 comparative RCTs have been undertaken, making this review timely. Whilst future FES- (9, 69) and AFO-specific studies (13, 70, 71) are necessary for intervention development, where possible, research should be impairment focused in order to facilitate more discerning prescription.
Secondly, despite the literature search encompassing all CNO diagnoses, the reviewed trials only included participants who had experienced a stroke and who were over the age of 18 years, so our results can only be applied to this population. Trials using different CNO populations are necessary, given that current clinical guidelines encompass them. Similarly, in order to form clinical guidelines indicating which subgroups of patients with any given CNO diagnosis (e.g. time points post-stroke, severity of foot-drop impairment) might benefit most from either intervention future studies with carefully defined inclusion/exclusion criteria are needed. This approach is of critical importance in subsequent trials so that potentially important clinical effects are not diluted in heterogeneous study groups. Until such a time as sufficient high-quality RCTs in specific groups of patients become available any meta-analyses will also suffer similar limitations.
Thirdly, risk of bias was present in the reviewed studies with detection bias (assessor blinding) the most common area. While this might impact our results this area of bias is common within rehabilitation research. Indeed, previous FES (28) and AFO (12) reviews have chosen to discount it, suggesting it is impractical to address in studies of medical devices. It can also be argued that objective measures minimize the risk of this source of bias. However, 2 trials (15, 16) attempted to control for this, suggesting that it is feasible to blind assessors and should at least be considered in future trials (72). We based the quality assessment on published material alone; so as not to advantage trial authors who respond to requests for additional data. Therefore a lack of reported methodological detail might account for some of the other unclear and high areas of bias found.
Finally, the reader should note that a range of different AFO and FES devices were used in the included trials and our analysis combined these. While combining data from different types of AFO/FES does not allow a detailed look at the possible different effects of each individual sub-type, assuming the prescription of devices within each trial was provided on the basis of clinical judgement and complies with current guidelines, this allows for a clinically relevant comparison. Furthermore, limited reports of the details of AFO and FES interventions preclude reliable sub-group analyses. The traditional description of AFOs on the basis of the material used (carbon fibre, plastic, metal) or mode of manufacture (customized vs off-the-shelf (54) as with our included trials) should be discontinued. The mechanical properties (stiffness, mass) of an AFO determine its behaviour (73) so it is these that should be measured and reported (73–75). Similarly, differences in outcome between therapist and patient FES set-up have been found (76, 77) so this should also be reported. None of the included trials reported details of FES setup parameters and it remains unclear which set of parameters would be most useful when comparing across trials; further work is required in this area.
In conclusion, despite very different hypothesized mechanisms-of-action for AFO and FES this RCT, state-of-the-art review, with meta-analysis (39) conservatively indicates that AFOs have positive combined-orthotic effects on walking that are equivalent to FES for foot-drop caused by stroke. Methodological and reporting limitations within the current RCT pool preclude clinical recommendations regarding which type of AFO or FES set-up to use for particular patient groups from being made; as they do in guiding clinicians as to which intervention to prescribe for a specific patient. However, crucially, and for the first time, barriers to achieving such clinical recommendations within research design and reporting have been identified to progress future research. Furthermore long-term, high-quality RCTs are required across CNO diagnoses. These should focus on measuring the mechanisms-of-action, whether there is translation of improved impairment to function and reporting the correct device details; only then will discerning prescription be possible.
ACKNOWLEDGEMENTS
The authors would like to thank the corresponding authors from Bethoux et al. (Francois Bethoux/Helen Rogers), Kluding et al. (Kari Dunning) and Salisbury et al. (Lisa Salisbury) for generously providing their unpublished results. We would also like to thank John Stephenson, from the University of Huddersfield, for his support with the meta-analyses.
The authors declare no conflicts of interest.
|
Appendix I. Unpublished data |
|
|
|
|
|
|
REFERENCES
