Carla F. J. Nooijen, PhD1, Henk J. Stam, MD, PhD1, Imte Schoenmakers2,
Tebbe Sluis, MD3, Marcel Post, PhD4,5, Jos Twisk, PhD6,7, Act-Active Research Group
and Rita J. G. van den Berg-Emons, PhD1
From the 1Department of Rehabilitation Medicine, Erasmus MC University Medical Center Rotterdam,
2InMotion, Eindhoven, 3Rijndam Rehabilitation Institute, Rotterdam, 4Brain Center Rudolf Magnus and Center of
Excellence in Rehabilitation Medicine, University Medical Center and De Hoogstraat, Utrecht, 5Department of
Rehabilitation Medicine, Center for Rehabilitation, University of Groningen, University Medical Center Groningen,
Groningen, 6Department of Epidemiology & Biostatistics, VU University Medical Center and 7Department of Health
Sciences, VU University, Amsterdam, The Netherlands
OBJECTIVE: In order to unravel the working mechanisms that underlie the effectiveness of a behavioural intervention promoting physical activity in persons with subacute spinal cord injury, the aim of this study was to assess the mediating effects of physical and psychosocial factors on the intervention effect on physical activity.
DESIGN: Randomized controlled trial.
SETTING: Four rehabilitation centres in the Netherlands.
SUBJECTS: Thirty-nine persons with subacute spinal cord injury.
INTERVENTION: Behavioural intervention promoting an active lifestyle, based on motivational interviewing. The intervention involved a total of 13 individual sessions beginning 2 months before and ending 6 months after discharge from initial inpatient rehabilitation.
Main measures: The potential mediating effects of fatigue, pain, depression, illness cognition, exercise self-efficacy, coping and social support on the effect of the behavioural intervention on objectively measured physical activity (B = 0.35 h, p < 0.01) were studied. Measurements were performed at baseline, discharge, 6 months and 1 year after discharge.
RESULTS: No single factor was found that strongly mediated the effect of the behavioural intervention on physical activity; however, multiple factors could partly explain the effect. Mediating effects greater than 10% were found for proactive coping (17.6%), exercise self-efficacy (15.9%), pain disability (15.3%) and helplessness (12.5%).
Discussion: Proactive coping (the ability to anticipate and deal with potential threats before they occur), exercise self-efficacy (self-confidence with respect to performing exercise and daily physical activities), pain disability (interference by pain of daily activities) and helplessness (emphasizing the aversive meaning of the disease) are important concepts in interventions promoting physical activity in persons with subacute spinal cord injury.
Key words: spinal cord injuries; physical activity; behavioural intervention; working mechanisms.
J Rehabil Med 2016; 48: 583–588
Correspondence address: Carla F. J. Nooijen, Department of Rehabilitation Medicine, Erasmus MC University Medical Center, PO Box 2040, NL-3000 CA Rotterdam, The Netherlands. E-mail: carla.nooijen@ki.se
Accepted Apr 14, 2016; Epub ahead of print Jun 20, 2016
INTRODUCTION
After discharge from inpatient rehabilitation, physical activity levels of persons with spinal cord injury (SCI) decline to a level that is severely low compared with the general population, and low compared with persons with other chronic diseases (1, 2). These low physical activity levels are associated with more secondary health problems in persons with SCI (3). Physical factors, such as fatigue and pain (4), and psychosocial factors, such as depression, illness cognition, exercise self-efficacy, coping and social support, have all been linked to (changes in) physical activity (5, 6).
In a previous study (7), the addition of a behavioural intervention to regular care, including handcycle training, was found to be effective in promoting a more physically active lifestyle in persons with subacute SCI, resulting in 50% more physical activity 6 months after discharge from inpatient rehabilitation as well as continuation of the more active lifestyle up to one year after discharge.
It is important to understand the working mechanisms that underlie the effectiveness of a behavioural intervention promoting physical activity in persons with subacute SCI. Therefore, the goal of the current study was to assess the mediating effects of both physical and psychosocial factors on the intervention effect on physical activity. The physical factors assessed were fatigue and pain. The psychosocial factors were depression, illness cognition, exercise self-efficacy, coping and social support. These factors were expected to be influenced by the behavioural intervention, but not as a direct intervention effect, leading to significant between-group differences. We hypothesized that these factors would mediate the intervention effect on physical activity, and thus partly explain the effect of the intervention on physical activity.
METHODS
Study design
This study is part of Act-Active, a single-blind multi-centre randomized controlled trial (RCT). Research assistants performing the measurements were blinded for group allocation. Participating rehabilitation centres were: Rijndam Rehabilitation Institute in Rotterdam, Adelante in Hoensbroek, Heliomare in Wijk aan Zee, and Hoogstraat Rehabilitation in Utrecht, all in The Netherlands. The RCT was prospectively registered at the Dutch trial register: NTR2424.
Participants
Participants were included during inpatient rehabilitation if they satisfied the following criteria: diagnosed with SCI, initial inpatient rehabilitation, dependent on a manual wheelchair for their daily mobility, able to handcycle, and between 18 and 65 years old. Exclusion criteria were: insufficient comprehension of the Dutch language to understand the purpose of the study and its testing methods; a psychiatric condition; or a progressive disease that could interfere with participation. The medical ethics committee of Erasmus Medical Center Rotterdam, The Netherlands, approved the protocol of this study, and all participating centres granted local approval. All participants provided written informed consent.
Randomization and intervention
Randomization into the intervention or control group was performed in 4 strata based on lesion level (tetraplegia-paraplegia) and completeness (motor complete-motor incomplete). Randomization was performed by the first author of this manuscript by a concealed allocation procedure. A lesion at or above the Th1 segment was defined as tetraplegia, and a lesion below Th1 as paraplegia. A motor complete lesion was defined as American Spinal Injury Association Impairment Scale (AIS) grade A or B, a motor incomplete lesion as AIS grade C or D (8). An investigator with no clinical involvement in the trial prepared a computer-generated random list for the block randomization. Random group allocation was performed for each rehabilitation centre and within each stratum. Participants were assigned in chronological order of enrolment.
All participants in the control and intervention groups participated in regular rehabilitation and performed a structured handcycle training programme (9) during the last 8 weeks of inpatient rehabilitation.
Only persons in the intervention group participated in a behavioural intervention. The aim of this intervention was to promote a more physically active lifestyle after discharge from inpatient rehabilitation. Thirteen individual face-to-face sessions with a coach were planned, each with a maximum duration of 1 h. For practical reasons, a few sessions after discharge were performed via the telephone. From 2 months before until 3 months after discharge, 2 sessions were planned every month, and in the following 3 months there was 1 session a month. The coach was a physical therapist or occupational therapist. The transtheoretical model of change was used as a basis for the behavioural intervention (10). This model describes the change of behaviour as a process that runs through several stages. The coach helped to facilitate movement along the stages by using motivational interviewing. Motivational interviewing has been shown to be an effective approach for altering behaviour (11), and there is evidence to support the clinical utility of this method to increase physical activity in persons with chronic health conditions (12). Each session began with the participant setting the agenda. Both physical and psychosocial factors were included in the intervention. The 4 main components of the intervention were: (i) feedback on daily wheelchair activity using bicycle odometers. The participant was instructed to keep track of the distance travelled per day with the wheelchair and to set increasing distance goals; (ii) setting action plans on how and when to be physically active and on coping strategies for dealing with barriers that could hinder the performance of an action plan (13); (iii) home visit by the coach in the first month after discharge to help optimize the home and environment of the participant to undertake physical activity; (iv) providing additional information on request by the participant on relevant topics related to physical activity, e.g. possible health benefits.
Outcomes and follow-up
The start of the study was determined based on the discharge date set by the rehabilitation physician. Four measurements were performed: T1, prior to the start of the interventions at 2 months before discharge from inpatient rehabilitation; T2, before discharge from inpatient rehabilitation (< 2 weeks before); T3, after completion of the behavioural intervention at 6 months after discharge from inpatient rehabilitation; and T4, 1 year after discharge from inpatient rehabilitation.
Physical factors
Fatigue was measured using the Fatigue Severity Scale (FSS), a questionnaire assessing the severity of fatigue and the perceived impact of fatigue on an individual’s daily functioning (14, 15). Pain was measured with the Chronic Pain Grade questionnaire (16, 17). The Pain intensity score was used to determine the severity of pain (17). At the 2 measurements after discharge (T3 and T4), participants also completed items of the Chronic Pain Grade questionnaire on pain disability, including the interference of pain, and change in daily work/housework, and recreational/social activities due to pain.
Psychosocial factors
The Center for Epidemiological Studies – Depression scale (CES-D) (18) was used to measure symptoms of depression, a higher score indicates more symptoms of depression. The scale consists of 4 domains: somatic-retarded activity, depressed affect, positive affect and interpersonal affect.
Illness cognition was assessed with the Illness Cognition Questionnaire (19), to gain an indication of both unfavourable and favourable ways of adjusting to an uncontrollable long-term stressor, in this case the SCI. The questionnaire assesses 3 domains: helplessness, acceptance and disease benefits. Helplessness refers to emphasizing the aversive meaning of the disease, acceptance to diminishing the aversive meaning, and perceived benefits to adding a positive meaning to the disease.
Exercise self-efficacy was assessed using the Exercise Self-Efficacy Scale (20, 21). Self-efficacy is defined as the beliefs in one’s capabilities to organize and execute the courses of action required for producing given attainments (22). The Exercise Self-Efficacy Scale contains items about self-confidence with respect to performing exercise and daily physical activities.
Proactive coping was measured with the Utrecht Proactive Coping Competence Scale (23, 24). Proactive coping is the ability to anticipate and deal with potential threats before they occur. This scale assesses the individual’s competency with regard to the various skills associated with proactive coping.
Social support was measured with the Social Support for Exercise Behavior Scale (25). We reported on the domains of family support (participation and involvement) and friends support (exercising together). All questionnaires used in the current study were validated (14–21, 23–25).
Statistical analyses
A power calculation was based on the primary outcome of the trial, objectively measured physical activity, and the aim was to recruit 60 participants (7). Generalized Estimating Equation (GEE) analyses with exchangeable correlation structures were used for the analyses. To assess the mediating effects of the physical and psychosocial factors, these were added separately to the overall model for the effect of the behavioural intervention on objectively measured wheeled physical activity (primary outcome measure of the RCT). This overall model on physical activity showed a significant intervention effect of B = 0.35 h per 24-h period, p < 0.01 (confidence interval: 0.13–0.58) (26). B represents the overall between-group difference, adjusted for baseline levels, rehabilitation centre, sex and age. Thus, overall the intervention group was 0.35 h (i.e. 21 min) per 24-h period more physically active compared with the control group. Mediation was expressed as the percentage of change of the overall between-group difference after adding each of the potential mediators separately to the model on physical activity. For pain disability (only available at T3 and T4) the mediating effect was assessed in a model without the T2 measurement (B = 0.47 h). Furthermore, we made overall models, correcting for baseline values, age, sex and rehabilitation centre, for each outcome variable to verify our hypothesis that the outcome measures did not have direct intervention effects. Independent t-tests and χ2 tests were used to test for differences in personal and lesion characteristics between the drop-outs of both groups. SPSS version 21 was used for all analyses.
RESULTS
A flow diagram of inclusion is shown in Fig. 1. A total of 45 participants were included between January 2011 and August 2013. In August 2013 we stopped recruiting participants since we were restricted to a time-frame and budget. Three persons in each group (n = 6) were excluded from further analyses because they dropped out of the study before the second measurement. Table I shows baseline characteristics of the remaining 39 participants. Participants completed a mean of 73% of the behavioural intervention sessions. Drop-outs at T3 or T4 in the intervention group (n = 12) and control group (n = 11) were not significantly different in terms of personal characteristic and lesion characteristics. Table II shows observed data for the physical and psychosocial factors. None of the outcome measures showed a direct significant intervention effect: Fatigue: B = 0.03, p = 0.93; Pain intensity: B = 3.71, p = 0.57; Pain disability: B = 0.43, p = 0.24; Depression: B = 0.96, p = 0.72; Helplessness: B = –0.11, p = 0.57; Acceptance: B = 0.04, p = 0.81; Disease benefits: B = –0.09, p = 0.55; Exercise self-efficacy: B = –0.30, p = 0.81; Proactive coping: B = –0.10, p = 0.34; Social support family: B = –0.55, p = 0.71; Social support Friends: B = 0.07, p = 0.94). Results on mediating effects are shown in Table III. The intervention effect on physical activity was mediated separately by > 10% by pain disability, helplessness, exercise self-efficacy and proactive coping.
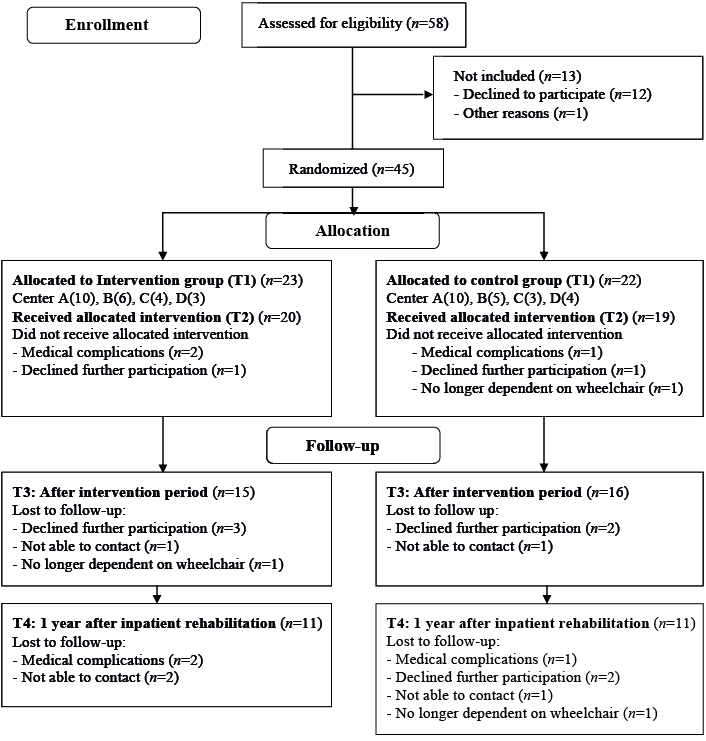
Fig. 1. Consolidated Standards of Reporting Trials (CONSORT) flow diagram of study participation.


|
Table I. Participant baseline characteristics |
||
|
|
Intervention group (n = 20) |
Control group (n = 19) |
|
Personal characteristics |
|
|
|
Age in years, mean (SD) |
44 (15) |
44 (15) |
|
Sex, men, n (%) |
17 (85) |
16 (84) |
|
Lesion characteristics |
|
|
|
Lesion level, tetraplegia, n (%) |
7 (35) |
6 (32) |
|
Completeness, motor complete, n (%) |
13 (65) |
11 (58) |
|
Days since injury, mean (SD) |
139 (67) |
161 (81) |
|
Days since admission, mean (SD) |
104 (64) |
108 (60) |
|
Cause, traumatic, n (%) |
14 (70) |
12 (63) |
|
Table II. Observed data over time, specified per allocated group. Reported as mean (SD) |
|||||||||
|
|
Intervention group |
|
Control group |
||||||
|
T1 Mean (SD) |
T2 Mean (SD) |
T3 Mean (SD) |
T4 Mean (SD) |
|
T1 Mean (SD) |
T2 Mean (SD) |
T3 Mean (SD) |
T4 Mean (SD) |
|
|
Fatigue (1–7) |
3.05 (1.31) |
3.24 (1.51) |
2.79 (1.30) |
3.39 (1.58) |
|
3.71 (1.41) |
3.35 (1.37) |
3.86 (1.37) |
3.46 (1.59) |
|
Pain |
|
|
|
|
|
|
|
|
|
|
Pain intensity (0–100) |
52.46 (18.39) |
50.74 (25.32) |
50.72 (24.98) |
45.76 (32.25) |
|
54.62 (21.84) |
47.78 (30.69) |
49.49 (29.72) |
49.67 (26.36) |
|
Disability score (0–6) |
– |
– |
0.86 (1.46) |
1.55 (1.57) |
|
– |
– |
0.92 (1.66) |
0.40 (0.70) |
|
Depression (0–60) |
11.61 (8.71) |
14.94 (10.28) |
15.93 (14.36) |
11.91 (12.16) |
|
13.00 (9.17) |
12.00 (7.30) |
16.62 (9.73) |
13.30 (8.60) |
|
Illness cognition |
|
|
|
|
|
|
|
|
|
|
Helplessness (1–4) |
2.73 (0.64) |
2.71 (0.67) |
2.62 (0.68) |
2.21 (0.98) |
|
2.83 (0.63) |
2.60 (0.77) |
2.79 (0.82) |
2.60 (0.68) |
|
Acceptance (1–4) |
2.26 (0.64) |
2.37 (0.79) |
2.39 (0.85) |
2.52 (0.91) |
|
2.45 (0.82) |
2.53 (0.94) |
2.46 (0.87) |
2.60 (0.74) |
|
Disease benefits (1–4) |
2.40 (0.86) |
2.41 (0.82) |
2.52 (0.84) |
2.70 (0.93) |
|
2.15 (0.57) |
2.64 (0.71) |
2.28 (0.64) |
2.55 (0.45) |
|
Exercise self-efficacy (10–40) |
35.56 (3.73) |
34.94 (4.99) |
34.50 (5.00) |
34.27 (5.57) |
|
35.87 (3.66) |
36.17 (3.56) |
32.69 (5.42) |
34.30 (4.03) |
|
Proactive coping (1–4) |
3.18 (0.36) |
3.21 (0.49) |
3.04 (0.78) |
3.12 (0.58) |
|
2.96 (0.35) |
3.04 (0.32) |
2.97 (0.53) |
2.92 (0.44) |
|
Social support |
|
|
|
|
|
|
|
|
|
|
Family (0–40) |
20.67 (9.57) |
19.83 (7.38) |
21.21 (10.66) |
19.64 (12.09) |
|
20.85 (9.77) |
22.67 (9.37) |
22.85 (7.98) |
22.00 (10.93) |
|
Friends (0–20) |
11.50 (6.85) |
9.78 (5.88) |
10.79 (5.31) |
9.36 (5.30) |
|
8.69 (5.81) |
8.42 (3.48) |
9.38 (4.50) |
9.50 (5.56) |
|
T1: 2 months before discharge; T2: at discharge; T3: 6 months after discharge; T4: 1 year after discharge. SD: standard deviation. |
|||||||||
|
Table III. Mediating effects on the intervention effect on physical activity |
|||
|
|
n |
B |
Mediating effect (%) |
|
Overall model physical activity |
75 |
0.352 |
|
|
Fatigue |
69 |
0.322 |
8.5 |
|
Pain |
|
|
|
|
Pain intensity |
69 |
0.332 |
5.7 |
|
Pain disability |
441 |
* |
15.3 |
|
Depression |
69 |
0.335 |
4.8 |
|
Illness cognition |
|
|
|
|
Helplessness |
69 |
0.308 |
12.5 |
|
Acceptance |
69 |
0.345 |
2.0 |
|
Disease benefits |
69 |
0.328 |
6.8 |
|
Exercise self-efficacy |
68 |
0.296 |
15.9 |
|
Proactive coping |
69 |
0.290 |
17.6 |
|
Social support |
|
|
|
|
Family |
69 |
0.326 |
7.4 |
|
Friends |
69 |
0.370 |
– |
|
1No T2 measurement available. *Physical activity model without T2 measurement changed from B = 0.470 to B = 0.398. n: number of measurements in analysis. |
|||
DISCUSSION
To our knowledge this is the first longitudinal study assessing the working mechanisms of a behavioural intervention intended to promote an active lifestyle in adults with subacute SCI. No single factor strongly mediated the observed positive effect of the behavioural intervention on physical activity, but multiple factors can partly explain the effect. The strongest mediating effects were found for proactive coping, exercise self-efficacy, pain disability, and helplessness.
This study unravelled different concepts that support the benefits of the behavioural intervention. Firstly, proactive coping, which assumes that persons not only react to presently threatening situations, but can also anticipate and respond to situations that may threaten or influence their goals in the future (23, 27). Secondly, helplessness, which refers to emphasizing the aversive meaning of the disease, and is strongly related to the concept of control. Helplessness has been proposed to be an important mediator between condition and well-being (28). Thirdly, self-efficacy, suggesting that confidence in one’s ability to perform a certain behaviour is strongly related to one’s ability to perform that behaviour. Higher exercise self-efficacy has previously been linked to more physical activity (29). Although exercise self-efficacy levels were found to be rather high in persons with subacute SCI (21), the current study confirms that it is an important concept within physical activity promotion. Furthermore, pain disability, wherein pain interferes with daily activities, seems an important factor when promoting an active lifestyle in persons with subacute SCI. However, we did not assess the locations of pain in the current study. In the behavioural intervention, there was no explicit focus on pain. It is possible that the behavioural intervention can be optimized when coaches are more aware of pain disability with regard to physical activity and incorporate this in the intervention. Pain is one of the most common secondary conditions in persons with SCI (30). While there is increased understanding of the underlying mechanisms of pain in persons with SCI, treatment is still unsatisfactory and there is an unmet need to improve pain relief (31).
Fatigue was not a strong mediator on the intervention effect on physical activity, and no direct intervention effect on fatigue was found. This is in line with our previously published results, that fatigue is not related to physical activity in persons with subacute SCI (32). However, fatigue is an important issue in persons with SCI because it is prevalent in both persons with subacute SCI and persons with SCI in the chronic phase (33) and is known to interfere with daily functioning (34). Since fatigue is a multifactorial problem, a specific fatigue management programme might be necessary to reduce fatigue (35). Further study on fatigue, and the physical component of fatigue, in persons with SCI is necessary.
Depression and family support for exercise behaviour explained only a small part of the intervention effect on physical activity. In previous studies, mental health problems have been identified as an important barrier to, and social support as an important facilitator of, physical activity in persons with SCI (36, 37). Further research is necessary to clarify the roles of depression and social support within the promotion of physical activity in persons with subacute SCI.
Study limitations
Although the sample size of 45 is relatively large considering the incidence of SCI and the vulnerability of this group, the absolute number is still small. Furthermore, power was limited because of missing values and drop-outs. In addition, we studied the different factors as separate working mechanisms; however, they might overlap or be the expression of an underlying general concept. Further study is necessary.
Conclusion
No single factor strongly mediated the effect of the behavioural intervention on physical activity, but multiple physical and psychosocial factors could partly explain the effect.
Proactive coping (the ability to anticipate and deal with potential threats before they occur), exercise self-efficacy (self-confidence with respect to performing exercise and daily physical activities), pain disability (interference with daily activities by pain) and helplessness (emphasizing the aversive meaning of the disease) are important concepts in an intervention intended to promote physical activity in persons with subacute SCI.
Acknowledgement
This study was supported by Children’s Fund Adriaanstichting (KFA) and Johanna Children’s Fund (JKF) [number 2007/0181-063].
The authors declare no conflicts of interest.
REFERENCES
