Jordan W. Squair, MSc1–4, Anna Bjerkefors, PhD1–4, J. Timothy Inglis, PhD1,2, Tania Lam, PhD1,2 and Mark G. Carpenter, PhD1,2
From the 1School of Kinesiology, 2International Collaboration on Repair Discoveries, University of British Columbia, Vancouver, BC, Canada, 3Department of Neuroscience, Karolinska Institutet, and 4The Swedish School of Sport and Health Sciences (GIH), Stockholm, Sweden
OBJECTIVE: To use a combination of electrophysiological techniques to determine the extent of preserved muscle activity below the clinically-defined level of motor-complete spinal cord injury.
METHODS: Transcranial magnetic stimulation and vestibular-evoked myogenic potentials were used to investigate whether there was any preserved muscle activity in trunk, hip and leg muscles of 16 individuals with motor-complete spinal cord injury (C4–T12) and 16 able-bodied matched controls.
RESULTS: Most individuals (14/16) with motor-complete spinal cord injury were found to have transcranial magnetic stimulation evoked, and/or voluntary evoked muscle activity in muscles innervated below the clinically classified lesion level. In most cases voluntary muscle activation was accompanied by a present transcranial magnetic stimulation response. Furthermore, motor-evoked potentials to transcranial magnetic stimulation could be observed in muscles that could not be voluntarily activated. Vestibular-evoked myogenic potentials responses were also observed in a small number of subjects, indicating the potential preservation of other descending pathways.
CONCLUSION: These results highlight the importance of using multiple electrophysiological techniques to assist in determining the potential preservation of muscle activity below the clinically-defined level of injury in individuals with a motor-complete spinal cord injury. These techniques may provide clinicians with more accurate information about the state of various motor pathways, and could offer a method to more accurately target rehabilitation.
Key words: spinal cord injury; transcranial magnetic stimulation; vestibulospinal; vestibular-evoked myogenic potentials; corticospinal; electromyography.
J Rehabil Med 2016; 48: 00–00
Correspondence address: Mark. G. Carpenter, School of Kinesiology, University of British Columbia, Osborne Centre Unit I, 6108 Thunderbird Blvd, Vancouver, BC, V6T 1Z3, Canada. E-mail: mark.carpenter@ubc.ca
Accepted Mar 31, 2016; Epub ahead of print Jun 1, 2016
INTRODUCTION
Accurately determining the level of motor function following spinal cord injury (SCI) is crucial to properly target rehabilitation programs aimed at retaining or regaining motor function. Even in clinically defined motor-complete injuries, an SCI does not typically result in complete transection of all motor fibres (1). Recent evidence suggests that surface electromyography (EMG) can be used to detect preserved abdominal muscle activity in individuals classified with high-thoracic motor-complete SCI (mc-SCI) (2). Furthermore, techniques such as transcranial magnetic stimulation (TMS) have been demonstrated to elicit muscle activity even in the absence of voluntary activation recorded using surface EMG (3). In general, studies examining the use of TMS in individuals with mc-SCI show sparse preservation of motor responses that are typically delayed; however, significant methodological differences across studies make it difficult to establish a definitive result (3–10). For example, many previous studies restricted their investigation to motor-evoked potentials (MEPs) in only one (8) or two (5, 9, 10) lower limb muscles, and have generally ignored potential activity that may exist in abdominal or hip muscles that are innervated below the level of injury. Secondly, only 1 study (4) has used a double-cone coil, which has been shown to be a most effective method for stimulating the leg region of the primary motor cortex (11, 12). Thirdly, only one study has utilized TMS in conjunction with remote and focal contractions to elicit motor activity in individuals with mc-SCI (13). However, a double-cone coil was not used in this study, which may have precluded a better response rate.
While most studies focus on the preservation of cortico-spinal pathways due to its role in volitional movement, other descending motor pathways, such as vestibulo-spinal and reticulo-spinal pathways, may also contribute to preserved motor function below the level of injury. Galvanic vestibular stimulation (GVS) has been studied previously as a means to test the integrity of vestibulospinal pathways in motor-incomplete SCI (14, 15). However, GVS requires subjects to be standing and engaged in a balance task (14), and thus is not suitable for application in individuals with mc-SCI who are unable to stand independently. In contrast, vestibular-evoked myogenic potentials (VEMPs) can be applied while the patient is lying supine and do not require muscles to be posturally engaged (16). VEMPs are elicited through high-intensity short-duration tone bursts (4–20 ms) or clicks (0.1–0.5 ms) (17, 18), which stimulate hair cells in the vestibular organs and elicit short-latency reflexes in the muscles of the neck and distal limbs, through vestibulospinal and/or vestibulo-reticulospinal pathways (19, 20). Therefore, VEMPs may provide an alternative means to assess preserved motor activity in muscles below the level of injury in individuals with a mc-SCI.
The aim of this study was to combine surface EMG, TMS and VEMPs into a comprehensive electrophysiological protocol to determine the extent of preserved muscle activity below the clinically-defined level of mc-SCI. We hypothesized that direct stimulation of cortical and vestibular neurons would evoke motor activity via descending pathways in abdominal and leg muscles below the level of injury in individuals with a mc-SCI, but at longer latencies compared with able-bodied (AB) controls.
METHODS
Participants
Sixteen individuals (4 women, mean age, height and weight (and standard deviation (SD)) 44 years (SD 13 years); 1.73 m (SD 0.07 m), 67.3 kg (SD 14.9 kg)) with a stable (chronic) mc-SCI (American Spinal Injury Association Impairment Scale (AIS) A/B) and 16 AB matched controls (4 women, 42 years (SD 14 years); 1.76 m (SD 0.06 m), 77.7 kg (SD 11.0 kg)) volunteered for the study (Table I). Inclusion criteria for participants with an SCI were as follows: sustained a cervical or thoracic SCI resulting in a motor-complete (AIS A/B) clinical classification at or above the T12-level at least one year earlier, with stable neurological and medical status and no cognitive impairments. Individuals with an SCI were excluded if they had: frequent experience with autonomic dysreflexia, severe spasticity, or history of epilepsy/seizure, and/or disturbances of the nervous system other than the SCI, such as cauda equina syndrome (3). Exclusion criteria for all participants included any of the following contra-indications for TMS: recurring or severe headaches, skull fracture or head injury including concussion, head or brain surgery, hearing problems, psychiatric impairment and/or sleep deprivation, pregnancy, heart disease, diabetes and electrodes implanted in the central or peripheral nervous system (21). The Stockholm Regional Ethical Committee and University of British Columbia Clinical Research Ethics Board reviewed and approved this study. All participants received oral and written information describing the study and provided written consent prior to participating.
|
Table I. Detailed description of the participants with spinal cord injury (SCI) |
||||||||||||||
|
Participant |
Age (years) |
Height (m) |
Sex |
Years post-injury |
NLI |
Sensory score |
LEMS |
AIS |
ZPP R & L |
Traumatic injury |
Spasticity |
Negative impact on ADL |
Medication |
|
|
Pin-prick |
Light touch |
|||||||||||||
|
SCI1 |
37 |
1.70 |
M |
3.5 |
C4 |
12 |
13 |
0 |
A |
C5/C4 |
Yes |
Yes |
No |
Yes |
|
SCI2 |
27 |
1.61 |
F |
5.5 |
C5 |
67 |
66 |
0 |
B |
– |
Yes |
Yes |
No |
Yes |
|
SCI3 |
55 |
1.78 |
M |
13 |
C5 |
10 |
24 |
0 |
B |
– |
Yes |
Yes |
No |
No |
|
SCI4 |
36 |
1.73 |
M |
10 |
C6 |
20 |
22 |
0 |
A |
C6/C7 |
Yes |
No |
No |
No |
|
SCI5 |
43 |
1.65 |
F |
19 |
C7 |
28 |
40 |
0 |
B |
– |
Yes |
Yes |
Yes |
No |
|
SCI6 |
27 |
1.65 |
M |
3 |
C8 |
28 |
33 |
0 |
B |
– |
Yes |
Yes |
No |
Yes |
|
SCI7 |
51 |
1.70 |
F |
30 |
T3 |
50 |
68 |
0 |
B |
– |
Yes |
Yes |
No |
Yes |
|
SCI8 |
38 |
1.72 |
M |
1.5 |
T3 |
42 |
43 |
0 |
A |
T4/T5 |
Yes |
No |
No |
No |
|
SCI9 |
70 |
1.73 |
M |
16 |
T5 |
48 |
49 |
0 |
A |
T6/T5 |
Yes |
Yes |
No |
No |
|
SCI10 |
66 |
1.84 |
M |
27 |
T5 |
48 |
54 |
0 |
A |
T7/T6 |
Yes |
Yes |
No |
No |
|
SCI11 |
44 |
1.83 |
M |
25 |
T8 |
61 |
61 |
0 |
A |
T9/T9 |
Yes |
Yes |
No |
No |
|
SCI12 |
31 |
1.63 |
F |
1 |
T10 |
66 |
67 |
0 |
A |
T11/T10 |
Yes |
Yes |
Yes |
Yes |
|
SCI13 |
53 |
1.83 |
M |
29 |
T10 |
90 |
90 |
0 |
B |
– |
Yes |
Yes |
No |
No |
|
SCI14 |
46 |
1.65 |
M |
26 |
T10 |
74 |
78 |
2 |
A |
L3/L2 |
Yes |
Yes |
No |
Yes |
|
SCI15 |
48 |
1.79 |
M |
28 |
T11 |
74 |
77 |
0 |
A |
L2/L1 |
Yes |
No |
No |
No |
|
SCI16 |
24 |
1.77 |
M |
6 |
T12 |
77 |
79 |
1 |
A |
L1/L1 |
Yes |
Yes |
No |
No |
|
F: female; M: male; NLI: neurological level of injury; LEMS: lower-extremity motor score; AIS: ASIA impairment scale; ZPP: zone of partial preservation; R: right; L: left; ADL: activities of daily living. |
||||||||||||||
Experimental protocol
Experiment 1. Participants laid in a supine position on a plinth with their arms folded and hips and knees supported in a flexed position using a foam wedge. Participants wore a tight-fitting swimming cap over the head, and the location of the vertex (CZ) was identified using the international 10/20 system. Magnetic stimulation was applied over the scalp site using a MagStim 2002 stimulator, Mono Pulse (The MagStim Company Ltd, Dyfed, UK) connected to a stimulating coil (Double cone coil, diameter 110 mm). The coil was placed approximately 1 cm anterior to the vertex, with the intersection of the coil placed over the stimulation site and the point of optimal excitability (POE) over the primary motor cortex was determined. The stimulus intensity was initially set to 50% of maximal stimulator output (MSO), and then increased to 70–100% of MSO, while the orientation of the coil and the location was adjusted slightly until the POE was localized, and identifiable MEPs were recorded in both legs. Participants were asked to perform various sub-maximal contractions, as previous studies have shown that the POE is easier to identify if the participant maintains a sub-maximal (or attempted) contraction (7, 13). The number of stimuli, the POE, and the percentage of MSO were documented for all participants.
Participants were then instructed to perform sub-maximal voluntary (or attempted) contractions during trunk rotation to the right, hip flexion, knee extension, plantarflexion, dorsiflexion, and great toe extension, while also performing a maximal hand-clench, as remote facilitation coupled with focal contraction has been shown to increase MEP amplitude to a greater extent than focal contractions alone (13). Ten stimuli were delivered while participants performed each task using the same stimulation intensity as when the POE was defined. A 30 s rest between trials and a 2 min break between tasks were given.
Following the TMS protocol, participants performed the 6 different maximal muscle contractions while lying supine on a plinth. The legs and trunk were secured to a plinth with straps placed over the shoulders, knees and feet to minimize movements of the upper and lower body. Each task was preceded by a verbal explanation by the examiner. Participants performed 2 trials for each task, for each leg with a 30 s rest between trials and a 2 min break between tasks.
Experiment 2. A sequence of short-tone burst (duration 4 ms, 500 Hz, 125 dB) acoustic stimuli was presented to each participant monaurally, through Telephonics earphones (Farmingdale, USA) via a stereo amplifier. Tones of this duration have been shown to preferentially activate the vestibulospinal system (22). Stimuli were generated using a custom-made sequencer (Spike 2, CED, Cambridge, UK) and calibrated to the correct amplitude, which was confirmed prior to each participant. Participants’ heads were turned contralateral to the side of stimulation. For this experiment, each participant completed 2 trials. Each trial consisted of 2 blocks of 128 acoustic stimuli with an interstimulus interval of 0.2–1.8 s. For participants with SCI, the trials were completed during maximal (or attempted) contraction of the soleus (SOL) on each side in order to facilitate the VEMP response (23). Stimulations were delivered to the left ear, and then repeated in the right ear. AB participants completed 1 trial during ~10% maximal voluntary contraction (HARD) of SOL and 1 trial during palpable contraction of SOL (SOFT) in order to replicate the muscle activity found in the participants with SCI. HARD and SOFT trials were always completed on opposite ears and the order of trials was randomized in both SCI and AB participants. Responses were recorded in the sternocleidomastoid (SCM) and SOL EMG.
Measurements
Experiment 1. Muscle activity was recorded with surface EMG (Myosystem 1400A, Noraxon, USA) from left external oblique (OE), right internal oblique (OI)/transverse abdominus (TrA) and bilaterally from: sartorius (SAR), rectus femoris (RF), tibialis anterior (TA), SOL, and extensor hallucis (EH) using belly-belly preparations. Electrodes were moved from the abdominal muscles (OE, OI/TrA) to the left and right SCM during the VEMP protocol. Signals were band-pass filtered between 10 and 1000 Hz online, amplified 500 ×, A/D converted (Micro1401, CED, Cambridge, UK), and digitally collected at 5000 Hz (Spike2, CED, Cambridge, UK). Prior to placing the electrodes, the skin was shaved and cleaned with alcohol. Pairs of self-adhesive electrodes (10 mm diameter, BlueSensor, Ambu, Ballerup, Denmark) were attached with approximately 2 cm inter-electrode separation.
MEP onset was calculated as the time at which the MEP exceeded 2 SD above the mean baseline EMG activity (100 ms prior to the stimulation onset) and remained beyond this threshold for at least 2 ms. All MEP onsets were visually confirmed by the experimenter. If an onset latency exceeded 2 times the mean AB latency, that response was excluded from further analysis (4). Muscles with detectable MEP onsets in at least 3 out of 10 trials were defined as “present” and were used to calculate individual participant and group mean latency. Muscles with detectable MEP onsets in fewer than 3 out of 10 trials were defined as “inconsistent” and were not included in individual and group latency averages. Analysis was also performed to determine the number of participants with present and inconsistent responses for the target muscle, during each task.
EMG recorded during voluntary tasks was used to calculate the root mean square (RMS) amplitude over a 500 ms time-period for each muscle and task during rest and voluntary contraction. If the mean RMS of the 2 contraction trials for a given muscle and task exceeded 2 SD above the mean resting value, the value was defined as “present” and included in subsequent analysis. Analysis was then performed to determine the number of subjects with responses for the target muscle during each task.
Experiment 2. VEMP responses were analysed in a staged approach on the ipsilateral side to stimulation. Response latencies for the VEMP in SCM were determined from the mean ensemble of 256 trials for each participant. All data were baseline corrected prior to analysis. SCM VEMP latencies were determined as the point of peak amplitude of the first peak to exceed 2 SD of background activity 250 ms before the onset of stimulus and the following peak in the unrectified signal (24). Only muscles with a present SCM response on that side were included in the subsequent SOL analysis.
As responses are more difficult to find in SOL unrectified signals, the data were rectified prior to the ensemble averaging (18). SOL latencies were then determined as the first peak that exceeded 2 SD of background activity (250 ms prior to onset of stimulus) within a 40–120 ms latency window post-stimulus onset. Responses that passed the criteria were then included in a descriptive analysis to determine the number of participants with a detectable SOL VEMP.
Statistical analysis
To compare MEP latencies with AB data, SCI MEPs were paired to their respective AB control (matched to the correct muscle and task) and included in a paired sample Student’s t-test. The number of present MEPs and responses to voluntary (or attempted) contraction in a given muscle, for a given task were descriptively compared across participants and groups. Latencies for SCM VEMPs were descriptively compared. Significance was set at p < 0.05.
RESULTS
Participants
All participants completed the TMS protocol. One participant reported a headache following the protocol, but all other participants were free from headaches or any other side-effects from the TMS. Consistent with previous studies, the %MSO ranged from 45% to 80% in the AB group and 80% to 100% in the SCI group when stimulating over the leg region of primary motor cortex (4). The number of stimulations ranged from 93 to 105 in the AB group and 93 to 102 in the SCI group and this number was well tolerated by all participants. All participants also completed the maximal voluntary contraction (MVC) protocol. For the VEMP protocol, 2 of the AB participants could only complete 2 stimulation trials, due to muscle cramps and ear irritation. All participants with SCI completed the VEMP protocol and tolerated it well. All participants who completed the VEMP protocol received all 4 trials of 256 stimulations.
Experiment 1
Motor-evoked potentials. Biphasic MEPs were elicited by TMS in 100% of the target musculature for all AB participants (Table II). In addition, TMS elicited MEPs in some abdominal and leg muscles of individuals with motor-complete SCI (AIS A/B) at or above T12 (Fig. 1; Tables II and III).
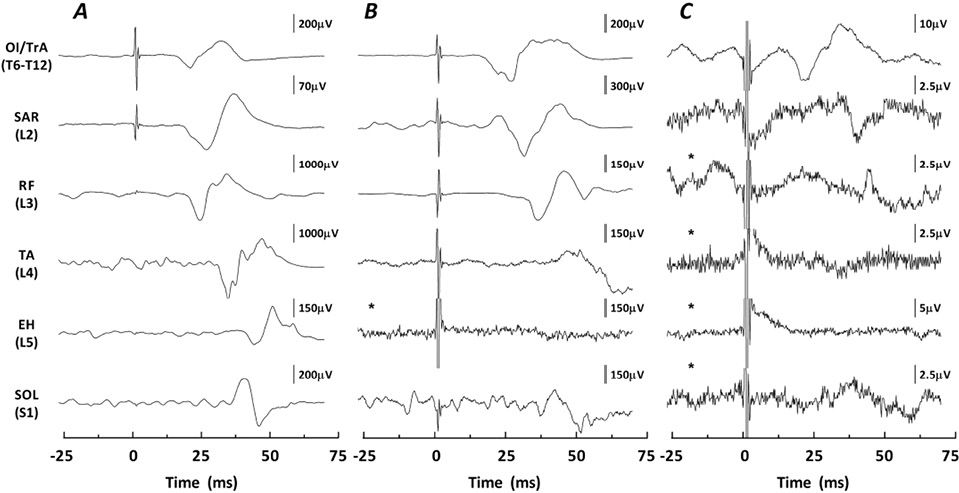
In individuals with an SCI, biphasic “present” MEPs were elicited by TMS below the level of injury in 14 of 16 participants (9 AIS A, 5 AIS B) with similar probability in participants with AIS A (90%) and AIS B (83%) injuries. Nine of the participants with SCI with MEPs had an injury above T6 (innervation to the abdominal muscles) and therefore abdominal muscle responses were considered as below the level of injury. In these participants MEPs were most commonly elicited in the abdominal muscles and, to a lesser extent, in the leg muscles. In particular, 4 participants with injuries above T6 had a present MEP in OE only, 1 in OI/TrA only, and 4 in both OE and OI/TrA, whereas 3 had a present MEP in SAR and 1 had a present MEP in SOL. Of the 5 participants with injuries at or below T6, 2 participants had present MEPs in SAR only; 2 had MEPs in both SAR and RF; and 1 had MEPs in SAR, RF, TA and SOL (Table II).
In addition to “present” MEPs detected below the level of injury, 9 participants with SCI had “inconsistent” MEPs, having only 1–2 detectable MEPs out of 10 (Table II). These MEPs were found in both the abdominal muscles (n = 3) and in the leg muscles (n = 9).
|
Table II. Frequency of motor-evoked potentials (MEPs) and responses to maximal voluntary contraction (MVC) in persons with spinal cord injury (SCI) |
|||||||||||||||||||
|
Participant |
AIS |
Injury level |
Trunk |
|
SAR |
|
RF |
|
TA |
|
EH |
|
SOL |
||||||
|
OE |
OI/TrA |
|
Left |
Right |
|
Left |
Right |
|
Left |
Right |
|
Left |
Right |
|
Left |
Right |
|||
|
SCI1 |
A |
C4 |
+++ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SCI2 |
B |
C5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SCI3 |
B |
C5 |
+++ |
+ |
|
+++ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SCI4 |
A |
C6 |
+++ |
+++ |
|
+ |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
SCI5 |
B |
C7 |
+++ |
+++ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SCI6 |
B |
C8 |
+++ |
+++ |
|
+++ |
+++ |
|
|
|
|
|
|
|
|
|
|
|
|
|
SCI7 |
B |
T3 |
+ |
+++ |
|
|
+++ |
|
|
|
|
|
|
|
|
|
|
|
+++ |
|
SCI8 |
A |
T3 |
+++ |
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
SCI9 |
A |
T5 |
+++ |
+++ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SCI10 |
A |
T5 |
+++ |
+ |
|
+ |
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
SCI11 |
A |
T8 |
+++ |
+++ |
|
+++ |
+++ |
|
|
|
|
|
|
|
|
|
|
+ |
|
|
SCI12 |
A |
T10 |
+++ |
+++ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SCI13 |
B |
T10 |
+++ |
+++ |
|
+++ |
+++ |
|
+++ |
+++ |
|
|
|
|
|
+ |
|
|
+ |
|
SCI14 |
A |
T10 |
+++ |
+++ |
|
+++ |
+++ |
|
+++ |
+++ |
|
|
+++ |
|
|
|
|
|
+++ |
|
SCI15 |
A |
T11 |
+++ |
+++ |
|
+++ |
+++ |
|
|
|
|
|
|
|
|
|
|
|
|
|
SCI16 |
A |
T12 |
+++ |
+++ |
|
+++ |
+++ |
|
+++ |
|
|
+ |
|
|
|
|
|
|
|
|
Participants were given a “+++” if the participants’ MEP response was defined as present, a “+” if the MEP response was defined as inconsistent. Grey cells represent muscles in which voluntary muscle activation was also defined as present. Note: Trunk muscle responses in individuals with injuries below T5 are separated by a dotted line, as they were not included in the mean latency analysis due to their injury being below the level of abdominal muscle innervation. AIS: ASIA impairment scale; OE: left external oblique; OI/TrA: right internal oblique, transverse abdominus; SAR: sartorius; RF: rectus femoris; TA: tibialis anterior; EH: extensor hallucis; SOL: soleus. |
|||||||||||||||||||
To examine latencies within participants with SCI, MEPs were collapsed across sides and across injury levels; abdominal muscle activity was examined only in individuals with an injury level above T6. MEPs were observed in individuals with SCI with mean latencies of 23.6 ms (SD 3.2) in OE/OI/TrA, 31.3 ms (SD 6.9) in SAR, 27.8 ms (SD 7.1) in RF, 48.3 ms in TA, and 52.7 ms (SD 9.1) in SOL (see Table III for summary). The latencies in individuals with SCI were significantly longer than AB latencies for OE/OI/TrA, with similar trends for longer latencies in SCI for SAR (t(13) = 2.124, p = 0.053) and RF (t(4) = 2.532, p = 0.065).
|
Table III. Absolute onset latencies of motor-evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) MEP responses |
||||||||||||||||||
|
|
Left side |
|
Right side |
|
Collapsed |
|||||||||||||
|
SCI |
|
AB |
|
SCI |
|
AB |
|
SCI |
|
AB |
|
|
||||||
|
n |
Latency (ms) Mean (SD) |
|
n |
Latency (ms) Mean (SD) |
|
n |
Latency (ms) Mean (SD) |
|
n |
Latency (ms) Mean (SD) |
|
n |
Latency (ms) Mean (SD) |
|
Latency (ms) Mean (SD) |
MD ± SE |
p-value |
|
|
OE/OI/TrA† |
8 |
23.0 (3.6) |
|
16 |
20.9 (2.1) |
|
5 |
24.5 (2.6) |
|
16 |
21.0 (2.6) |
|
13 |
23.6 (3.2) |
|
21.8 (2.9) |
1.8 ± 0.8 |
0.040* |
|
SAR |
8 |
34.5 (19.7) |
|
16 |
23.1 (2.0) |
|
6 |
39.3 (24.5) |
|
16 |
22.7 (2.6) |
|
14 |
27.8 (7.1) |
|
23.4 (2.5) |
4.3 ± 2.0 |
0.053 |
|
RF |
3 |
32.1 ( 9.6) |
|
16 |
23.0 (1.6) |
|
2 |
30.2 (1.8) |
|
16 |
23.1 (1.9) |
|
5 |
31.3 (6.9) |
|
22.8 (1.7) |
7.5 ± 3.4 |
0.065 |
|
TA |
0 |
̶ |
|
16 |
37.9 (3.3) |
|
1 |
48.3 |
|
16 |
38.6 (4.3) |
|
1 |
48.3 |
|
33.3 |
̶ |
̶ |
|
EH |
0 |
̶ |
|
16 |
45.6 (3.9) |
|
0 |
̶ |
|
16 |
45.0 (3.3) |
|
0 |
̶ |
|
̶ |
̶ |
̶ |
|
SOL |
0 |
̶ |
|
16 |
34.1 (2.6) |
|
2 |
52.7 (9.1) |
|
16 |
33.9 (2.1) |
|
2 |
52.7 (9.1) |
|
34.5 (3.3) |
̶ |
̶ |
|
“n” indicates the number of muscles with MEP responses defined as “present” in at least 3 out of 10 trials. Note: dashed lines indicate no responses to TMS recorded for the muscle. *Significance with α=0.05 and Bonferroni correction. †Left = OE, Right = OI/TrA as only trunk rotation to the right was performed. SCI: spinal cord injury; AB: able-bodied; OE: left external oblique; OI/TrA: right internal oblique/transverse abdominus; SAR: sartorius; RF: rectus femoris; TA: tibialis anterior; EH: extensor hallucis; SOL: soleus; MD: mean difference; SE: standard error of mean. |
||||||||||||||||||
Muscle responses above the level of injury. As noted in Table II, 6 participants with an SCI had lesions between T6 and T12. All participants had present MEPs in the abdominal muscles, and their latencies (20.6 ms (SD 4.1)) were similar to their matched AB controls (20.6 ms (SD 1.9)).
Maximal voluntary contractions. All AB participants were able to elicit voluntary EMG activity above resting levels in all tasks. Of the 10 individuals with an SCI above T6, only one had voluntary muscle activity that exceeded 2 SD above resting levels in OE, OI/TrA and SAR. In this participant there was a present TMS MEP in OI/TrA and SAR, but not OE (Table II).
Of the 6 participants with SCI with injuries at or below T6, 3 had voluntary EMG activity observed in SAR and 1 in SAR, RF, TA and SOL. In 13 of 14 cases of voluntary muscle activation (Table II), a present response to MVC was accompanied by a present response to TMS. Conversely, there were 22 cases in which a response to MVC was absent in spite of a present response to TMS. Despite having an injury below the innervation of the abdominal muscles, 2 participants with injuries at or below T6 were not able to raise their muscle activity above baseline in their OE.
Experiment 2
Vestibular-evoked myogenic potentials. Present VEMP responses were found in 81% of the SCM muscles in individuals with SCI and 75% of the SCM muscles in AB participants. Response latencies were found to be consistent, with mean first peak latencies in SCM of 16.3 ms (SD 1.4) and 16.2 ms (SD 2.2) in the SCI and AB groups, respectively (Fig. 2). Mean second peak latencies were 25.0 ms (SD 2.4) in the SCI group and 24.5 ms (SD 2.3) in the AB group. No between-group differences were observed in SCM VEMP first peak (p = 0.85) or second peak (p = 0.54) latencies.
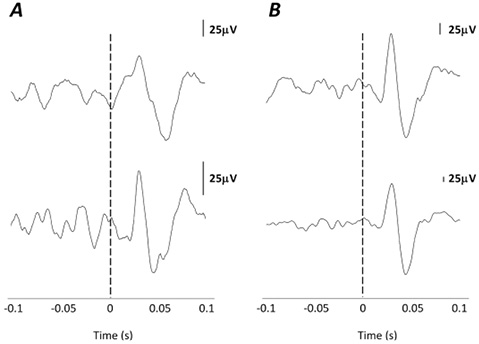
Fig. 2. Vestibular-evoked myogenic potentials (VEMPs) in the sternocleidomastoid (SCM) ipsilateral to the side of stimulation. (A) Two participants with spinal cord injury (SCI) showing a detectable VEMP in SCM. (B) Two representative able-bodied participants for comparison purposes.
SOL responses were more difficult to elicit than SCM responses in response to short-tone bursts. In AB subjects present responses were found ipsilateral to the side of stimulation in 58% of the AB SOL muscles, with a mean latency of 84.6 ms (SD 7.9). Responses were detected in only 2 SOL muscles in the SCI group, with a mean latency of 79.6 ms (SD 22.5). Of the 2 SCI participants, SCI14 had a present MEP in response to TMS and present voluntary activation in SOL, and SCI01 had no observable activation of SOL. Two representative AB responses and the 2 SCI responses are shown in Fig. 3.
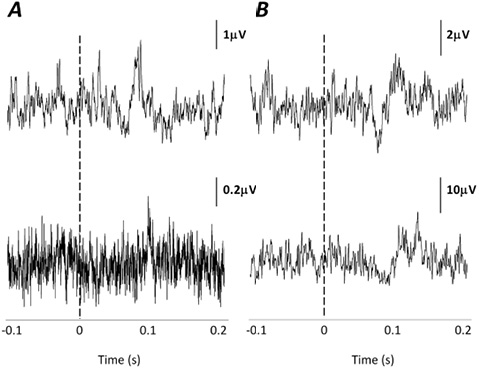
Fig. 3. Vestibular-evoked myogenic potentials (VEMPs) in the soleus (SOL) ipsilateral to the side of stimulation. (A) Two participants with spinal cord injury (SCI) showing a detectable VEMP in SOL. (B) Two representative able-bodied participants for comparison purposes.
DISCUSSION
Frequency of motor-evoked potentials
Previous studies have shown that it is possible to record MEPs in response to TMS below the level of injury in individuals with a mc-SCI; however, their detection rates have been, in general, lower than the detection rate in this study (14 of 16 individuals with SCI) (3–12). By utilizing attempted contractions it is likely the cortical motor thresholds were reduced, as has been seen during imagined movements (25), leading to the increased rate of detection. While we did observe an increased rate of detection, the current data represents only the most conservative observations, as the threshold for activation may not have been reduced to the same degree in all participants.
Latencies of motor-evoked potentials
MEPs evoked by TMS in the AB group are within the range of published values for the OI/TrA/OE (26–28), RF (29), TA (11) and SOL (30). Furthermore, there was a clear rostro-caudal pattern of activation, as would be expected due to conduction distance for each muscle. Although a statistical difference in MEP latencies between SCI and AB was observed only in the abdominal muscles, the trend of delayed responses observed in SCI MEPs below the level of injury is consistent with latencies reported in individuals with motor-complete SCI (7) and motor-incomplete SCI (4). Many mechanisms have been discussed to account for MEP latency delays in individuals with SCI. These include, but are not limited to, peripheral nerve damage (31, 32), summation delays (31), focal myelin damage (4) and indirect activation of bulbospinal pathways (33).
Maximal voluntary contractions
EMG activity in response to MVC confirmed the TMS findings in 13 cases (Table II), and in 1 case was present without a motor response to TMS. These results support those of previous work showing that individuals with a clinically classified mc-SCI may still be able to voluntarily activate the muscles below the level of their injury, termed a “discomplete” SCI (34, 35).
Voluntary activation in the abdominal muscles was only observed below the level of injury in one participant with a mc-SCI. This finding is in contrast to previous work, which showed a much higher frequency of detection (2, 3). However, the current study only used 1 trunk task, as opposed to 6 different trunk tasks used by Bjerkefors et al. (2, 3), which could explain the lower detection rate. In addition, it is worth noting that, in 2 cases, the individuals with an injury at or below T6 were not able to voluntarily activate their abdominal muscles to a measureable level. The lack of relationship between the level of injury and the ability to voluntarily activate the abdominal muscles further demonstrates that determining the motor level solely from the sensory level in the thoracic segments may lead to an inaccurate determination of abdominal muscle activity (2, 3, 36, 37).
In addition to voluntary abdominal muscle responses, 5 participants showed present volitional EMG activation in leg muscles, below their level of injury, with the majority of activity observed in SAR. No studies to date have examined hip flexor activity in individuals with mc-SCI and, as such, this finding provides novel insight into a possible therapeutic target.
The results of this study also confirm that TMS may be more sensitive compared with MVC and surface EMG in its ability to detect preserved motor activity in individuals with mc-SCI. This is demonstrated by a present TMS MEP in the absence of volitional activity (Table III; 2, 3, 6). In a previous study, 5 MEPs in 4 individuals classified as mc-SCI were disregarded because no volitional movement from these subjects was observed (4). However, more recent studies have highlighted the importance of detecting minimal preservation in the absence of volitional movement (2, 3, 6), as this may have rehabilitation significance.
Vestibular-evoked myogenic potentials
To our knowledge, this is the first study to attempt to utilize vestibular stimulation to elicit motor activity in the lower legs of 2 individuals with a mc-SCI at or above T12. The limitations of the current VEMP protocol are highlighted by the relatively low detection rates for VEMP responses in the AB group for SCM (75%) and SOL (58%) compared with earlier reports (17, 18). The most distinct difference between the current and earlier studies is the supine posture of the subjects, which removes the balance element and reduces background activity associated with upright standing. The paucity of responses in both the AB and SCI group (2 of 26 muscles) highlight the need to further refine this technique in an AB population before extrapolating to a clinical population. Improvements may include more optimal leg positions for activation of SOL and an increased delivery rate to reduce fatigue. In addition, there is evidence that short-tone bursts may be delivered at rates of 5 Hz or above (23). This would reduce both central and muscle fatigue in AB individuals and in individuals with SCI. Lastly, the dramatically reduced background muscle activity in the SCI population may obviate the detection of preserved pathways, as this is a critical factor in eliciting VEMPs. However, as some SOL VEMPs were seen in the SCI group, the results from this study provide encouraging evidence to support vestibular stimulation as a potential method to investigate descending motor pathways in individuals with SCI, which has been shown to elicit muscle activity not otherwise detected using TMS or voluntary EMG.
Implications for classification and rehabilitation
The observed abdominal muscle preservation in this study confirms previous findings (2, 3, 36, 37) highlighting the inaccuracy of determining motor levels from sensory levels in the thoracic segments and emphasizing the need to include a trunk assessment in the current International Standards for Neurological Classification of Spinal Cord Injury (38). In some cases (5/10 individuals with AIS A), we observed similarities between the degree of electrophysiologically-detected motor preservation and the clinically defined zone of partial preservation (ZPP). However, in the remaining individuals, the extent of electrophysiologically-determined motor preservation extended beyond the clinically defined ZPP. While some of this discrepancy can be explained by the ability of an electrophysiological measure to detect subtle levels of motor activity, lack of motor testing in the thoracic segments also precludes the finding of preserved motor activity in the abdominal muscles. Therefore, the inclusion of a controlled trunk assessment would assist in providing more accurate clinical information about the state of the abdominal musculature.
Correctly determining the amount and nature of neural preservation to muscles below the injury may also have important implications from a rehabilitation perspective. Previous work has shown that, following rigorous rehabilitation on a kayak ergometer, individuals with high-thoracic mc-SCI show improvements in sitting trunk stability, upper body coordination and functional performance (37). Therefore, when considering rehabilitation for sitting posture, it is beneficial to know the extent of preservation of various motor pathways in order to better determine the potential for improvement.
Conclusion
In conclusion, by combining various electrophysiological methodologies (i.e. surface EMG, TMS, VEMPs), a large number of preserved motor pathways can be detected in individuals with mc-SCI. Furthermore, we provide the first evidence that VEMPs may be used as a method to stimulate vestibular neurones and evoke motor activity in the lower limbs of individuals with mc-SCI.
ACKNOWLEDGEMENTS
The authors would like to thank the Swedish Centre for Sports Research (CIF) and the Canadian Institute for Health Research (CIHR) for financial support. Special thanks are due to the participants.
The authors declare no conflicts of interest.
REFERENCES
