Bernhard Elsner, PhD1,2, Joachim Kugler, MD1, Marcus Pohl, MD3 and Jan Mehrholz, PhD1,4
From the 1Department of Public Health, Dresden Medical School, Technical University Dresden, Dresden, 2Professorship of Physiotherapy, SRH University of Applied Health Sciences Gera, Gera, 3Neurological Rehabilitation, Helios Klinik Schloss Pulsnitz, Pulsnitz and 4Wissenschaftliches Institut, Private Europäische Medizinische Akademie der Klinik Bavaria in Kreischa GmbH, Kreischa, Germany
OBJECTIVE: To evaluate the evidence regarding transcranial direct current stimulation (tDCS) and to assess its impact on spasticity after stroke.
DATA SOURCES: The following databases were searched up to 6 January 2016: Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library, latest issue), MEDLINE (from 1948), EMBASE (from 1980), CINAHL (from 1982), AMED (from 1985), Science Citation Index (from 1900).
STUDY SELECTION: One author screened titles and abstracts and eliminated obviously irrelevant studies. Two authors retrieved the full text of the remaining studies and checked them for inclusion.
DATA EXTRACTION: Two authors independently extracted data from the studies using predefined data extraction sheets. In case an author of being involved in an included trial, another author extracted data.
DATA SYNTHESIS: Five trials were included, with a total of 315 participants. There was moderate-to-low quality of evidence for no effect of tDCS on improving spasticity at the end of the intervention period. There were no studies examining the effect of tDCS on improving spasticity at long-term follow-up.
CONCLUSION: There is moderate-to-low quality evidence for no effect of tDCS on improving spasticity in people with stroke.
Key words: stroke; rehabilitation; transcranial direct current stimulation; muscle spasticity.
J Rehabil Med 2016; 48: 00–00
Correspondence address: Jan Mehrholz, Wissenschaftliches Institut, Private Europäische Medizinische Akademie der Klinik Bavaria in Kreischa GmbH, Kreischa, Germany. E-mail: jan.mehrholz@klinik-bavaria.de
Accepted Mar 18, 2016; Epub ahead of print May 12, 2016
INTRODUCTION
Stroke affects activities of daily living and quality of life (1). Three out of 4 patients experience impaired function at hospital admission (2) and 6 months after stroke 2 out of 3 patients have not regained normal neurological functioning of the affected arm (3). A common result of stroke is spasticity, often defined as a “velocity-dependent increase in muscle tone with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex”. Nearly every second person with stroke with initially impaired arm function experiences upper limb spasticity at 12 months after stroke (4), and 1 out of 3 stroke survivors has lower limb spasticity (5). Upper limb spasticity contributes to chronic post-stroke disability (6). Severe spasticity at 12 months post-stroke can be predicted with substantial accuracy at 4 weeks (7). Effective rehabilitation strategies for reducing upper and lower extremity spasticity, and thus increasing function, are needed to reduce the burden of stroke (5, 8, 9), particularly for the high-risk population likely to develop severe spasticity.
Regardless of today’s probably most commonly used intervention for focal spasticity after stroke, intramuscular botulinum toxin (10, 11), new rehabilitation strategies have emerged in recent years. These include non-invasive brain stimulation techniques, such as repetitive transcranial magnetic stimulation (rTMS) or transcranial direct current stimulation (tDCS), which can alter cortical excitability and hence improve rehabilitation outcome (12–15). For example, tDCS is relatively safe, easy to administer and inexpensive (16). tDCS works by applying a direct current to the brain, usually transmitted by surface electrodes (16–18). The current, usually with a value of 0.5–2 mA, is generated by a direct current stimulator. Three different types of application can be distinguished: (i) the anodal electrode (+) is placed over the presumed brain area of interest and the cathodal electrode (–) is placed as a reference electrode, either on the contralateral orbit or on the contralateral arm (anodal stimulation, A-tDCS); (ii) the electrodes are placed the other way round (cathodal stimulation, C-tDCS); or (iii) they are placed as a combination of (i) and (ii) simultaneously (bihemispheric tDCS/dual-tDCS).
tDCS might be a promising tool for reducing spasticity and increasing function and activities after stroke. A recent randomized control trial (RCT) suggests that tDCS may improve spasticity after stroke and may also improve arm function and activities (19). However, other RCTs have not shown any beneficial effects of tDCS on improving spasticity (20–22).
There is a need for a comprehensive systematic review of RCTs with meta-analysis, taking into account different application techniques and describing the quality level of the evidence. The aim of the present study was therefore to provide an up-to-date systematic overview of the current evidence of randomized trials of tDCS for improving spasticity after stroke.
METHODS
This review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (23) as well as Methodological Expectations of Cochrane Intervention Reviews (MECIR) (24).
Inclusion criteria
Only genuine randomized controlled trials (RCTs) or genuine randomized crossover trials, which evaluated tDCS vs sham and/or any other therapy for improving upper limb and lower limb spasticity (regardless of the outcome measure used) after stroke, were included. Stroke was defined according to the World Health Organization’s (WHO) criteria. Subjects in the trials had to be 18 years of age or older. Quasi-randomized trials were excluded from the review. tDCS was defined as the long-lasting application of a direct current to the scalp (> 1 min). Sham-tDCS was defined as the application of electrodes to the scalp, either only short-term (< 1 min) or with no direct current applied (this is approximately the time it takes to fade in and fade out the current to produce perceivable sensations similar to the active condition) (25). In case of more than one active or sham group in an included trial, experimental and control groups were combined in order to minimize the number of comparisons. No restrictions were applied due to the language of studies.
Data sources
The following databases were searched up to 6 January 2016: Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library, latest issue), MEDLINE (from 1948), EMBASE (from 1980), CINAHL (from 1982), AMED (from1985), Science Citation Index (from 1900). Our MEDLINE search strategy (shown in Appendix I) was modified for the search in all other databases. In addition, we hand-searched relevant conference proceedings and screened reference lists of relevant studies in order to identify further publications.
Study selection
One author screened titles and abstracts and eliminated obviously irrelevant studies. Two authors retrieved the full text of the remaining studies and ranked them as “relevant”, “possibly relevant” or “irrelevant”, according to our inclusion criteria. Two authors then examined whether the “possibly relevant” and “relevant” publications fitted the PICOS (population, intervention, comparison, outcome, study type) strategy of our study design. Disagreements were resolved by discussion with all authors.
Data extraction
Two authors independently extracted data from the studies using pre-defined data extraction sheets. In cases where an author was involved in an included trial, another author extracted data.
As primary outcome measures spasticity was defined at the end of the intervention. As secondary outcome we defined spasticity at long-term follow-up, which we defined as more than 6 months after the end of intervention. We used the Cochrane Risk of Bias Tool for rating the methodological quality of included studies (26). For statistical pooling the appropriate outcome measures for spasticity were used, as reported in the studies. The quality of evidence among studies was rated by the GRADE approach (27), using the software GRADEpro, Version 3.2 (28). Data were extracted regarding the effect of tDCS on improving spasticity at the end of the study and at long-term follow-up, which is more than 6 months after study end.
Statistical analysis
For all continuous data, means and standard deviations were entered. A pooled estimate of the mean difference (MD) with 95% confidence intervals (95% CI) was then calculated. If studies did not use the same measure for an outcome standardized mean differences (SMD) were calculated instead of MD. Heterogeneity was assessed across the included studies by using I2 statistics. A random-effects model was used regardless of the level of heterogeneity. RevMan 5.3 was used for all statistical comparisons (29).
RESULTS
The abstracts and titles of 5,283 unique records were screened for inclusion, 154 full-text articles were screened for eligibility, and 5 trials with 315 participants were included in the quantitative analysis. A flow chart for the study search process is shown in Fig. 1.
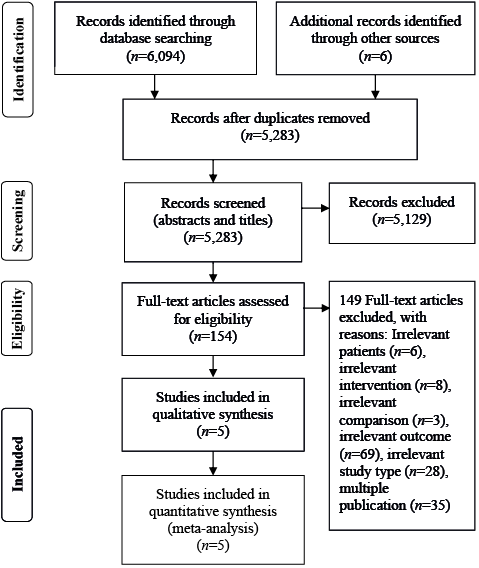
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
Five studies, with a total of 315 participants, evaluated the effect of tDCS on improving spasticity. All trials were RCTs. There was no trial examining the effect of tDCS on improving spasticity at long-term follow-up.
Included studies varied regarding study design, sample size, age of participants, time post-stroke, and initial severity of impairment, as well as treatment modalities and location of stimulation. Table I provides an overview of the demographics of included studies. Table II provides an overview of the study characteristics.
Table I. Demographics of included studies | ||
| Studies examining the effects of tDCS on improving spasticity after stroke | |
Experimental group | Control group | |
Total, n | 183 | 132 |
Age, years, mean (SD) | 56 (14) | 55 (15) |
Time post-stroke, months, mean (SD) | 4 (8) | 5 (10) |
Males, % | 68 | 71 |
Right-sided paresis, % | 50 | 68 |
Severity (MAS), mean (SD) | 2.1 (2.3) | 2.3 (1.8) |
SD: standard deviation; MAS: Modified Ashworth Scale; tDCS: transcranial direct current stimulation. Table shows pooled values (SD) from included studies, n describes the (pooled) number of subjects in the different groups. | ||
|
Table II. Characteristics of included studies |
|||||||||
|
Study |
Method of randomization |
Number of subjects |
Mean age, years |
Time since stroke |
Severity |
Outcome measures for spasticity |
Type and location of stimulation |
Additional therapy |
Treatment regime |
|
Hesse et al., 2011 (22) |
An independent person drew lots out of an envelope, indicating A, B or C |
96 |
65 |
4 weeks |
MAS sum score for wrist, elbow, shoulder 3 |
MAS sum score for wrist, elbow, shoulder |
A-tDCS (2 mA for 20 min) over the lesioned M1, C-tDCS (2 mA for 20 min) over non-lesioned M1, S-tDCS (0 mA for 20 min). Electrode size: 35 cm2 |
Robot-assisted arm training (20 min) 5 days a week for 6 consecutive weeks |
Robot-assisted arm training plus A-tDCS, C-tDCS or S-tDCS |
|
Lee & Chun, 2014 (21) |
Random number table |
59 |
61 |
17 days |
MAS 0.5 |
MAS |
C-tDCS (2 mA; 20 min) over hand area of M1 of the non-lesioned hemisphere. Electrode size: 25 cm² |
VR or OT for 30 min per day, 5 times per week for 3 weeks
|
tDCS plus VR, tDCS plus OT or VR alone |
|
Qu et al., 2009 (30) |
Unclear |
50 |
45 |
5 months |
MAS 2 |
MAS |
C-tDCS (0.5 mA; 20 min) over non-lesioned M1 once a day for 5 consecutive days for 4 weeks. Electrode size: 18 cm2 |
PT according to the Bobath, Brunnstrom and Rood approaches 40 min twice a day) for 5 consecutive days for 4 weeks |
tDCS + PT or PT only |
|
Viana et al., 2014 (20) |
Sealed opaque envelopes with lots |
20 |
56 |
33 months |
MAS 2 |
MAS |
A-tDCS (2 mA; 13 min) placed over M1 of the lesioned hemisphere or S-tDCS (2 mA; 30 s) 3 times a week for 5 weeks. Electrode size: 35 cm2 |
VR (60 min per session), passive stretching before and after each VR session, 3 days a week for 5 weeks |
A-tDCS plus VR or S-tDCS plus VR |
|
Wu et al., 2013 (19) |
Computer-generated randomization scheme |
90 |
48 |
5 months |
MAS 2 |
MAS |
C-tDCS (1.2 mA; 20 min) over non-lesioned M1 and S-tDCS (1.2 mA; 30 s) 5 days a week for 4 weeks |
PT twice daily for 30 min, 5 days a week for 4 weeks |
C-tDCS plus PT or S-tDCS plus PT |
|
A-tDCS: anodal transcranial direct current stimulation; C-tDCS: cathodal transcranial direct current stimulation; MAS: Modified Ashworth Scale; M1: primary motor cortex; OT: occupational therapy; PT: physical therapy; S-tDCS: sham transcranial direct current stimulation; VR: virtual reality. |
|||||||||
Comparison 1: active vs sham tDCS for improving spasticity at the end of the intervention phase
Three studies, with a total of 206 participants, examining the effect of active vs sham tDCS for improving spasticity (19, 20, 22, 30) were found, thus allowing statistical pooling. The difference in absolute values between active and sham groups at the end of the intervention phase was not statistically significant when using an inverse variance method with a random-effects model (SMD –0.61; 95% CI –1.51 to 0.30, p = 0.19) (Fig. 2). The risk of bias of studies was low (Fig. 2). The quality level of evidence for this comparison is moderate according to the GRADE approach.
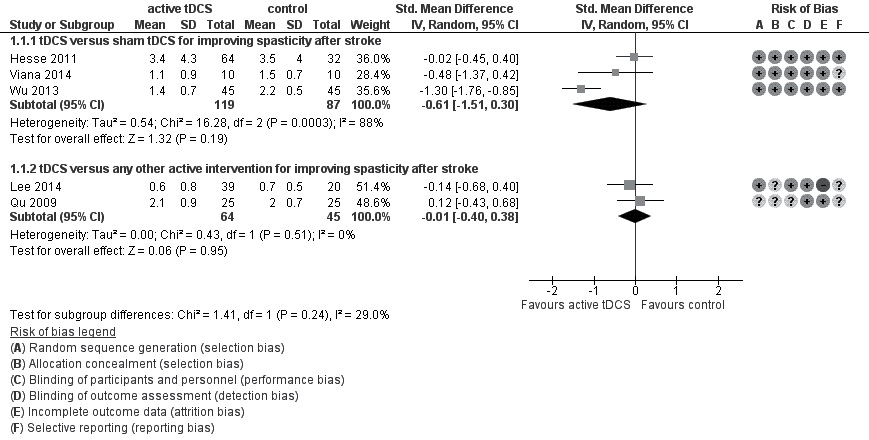
Fig. 2. Forest plot of comparison 1 and 2; active transcranial direct current stimulation (tDCS) vs sham tDCS and active tDCS vs any other active control intervention.
Comparison 2: active tDCS vs active control for improving spasticity at the end of the intervention phase
Two trials, with a total of 109 participants, evaluated the effect of active tDCS vs active control (physical therapy or virtual reality) for improving spasticity at the end of the intervention phase (21, 30). There was no statistically significant difference in spasticity (SMD –0.01; 95% CI –0.40 to 0.38, p = 0.51; inverse variance method with random-effects model) (Fig. 2). The risk of bias of studies was low to high (Fig. 2). The quality level of evidence for this comparison is low according to the GRADE approach.
DISCUSSION
This systematic review describes all randomized studies published to date of the effects of tDCS on improving spasticity. Five trials, with a total of 315 participants, were included and outcome data analysed with combined intervention and control groups, respectively. No evidence was found for an effect that tDCS improves spasticity.
There are several published systematic reviews of the effects of tDCS on function and motor learning (31–37). However, none have focused on spasticity as an outcome and, to our knowledge, there is no similar systematic review of the effects of tDCS on spasticity.
The results of the present review appear to be quite generalizable to the population of stroke patients. It could be argued, however, that the applicability of our results is limited because only spasticity of the upper limbs was investigated, whereas spasticity in the lower limbs was not.
In general, the reasons for downgrading the quality level of evidence for our analysis according to the GRADE approach are concerns regarding risk of bias in included studies and imprecision of effect estimation. In detail, we found heterogeneity between trials regarding design (2 vs 3 study arms, single centre vs multicentre), study sample size and patient characteristics (e.g. age, severity of impairment and time post-stroke) as well as stimulation parameters (location of and type of stimulation, number and duration of treatment sessions, dosage of electrical charge) and base treatment. Despite the absence of unexplained or statistically significant heterogeneity in our analysis, this might have influenced our results.
We used a clinical definition of spasticity as our primary outcome measure. The clinical measurement of spasticity is, however, discussed very controversially and questions the Modified Ashworth Scale (MAS) as the gold standard for assessing spasticity in clinical practice and research. There are concerns regarding the MAS as a reliable, valid and sensitive outcome measure for spasticity (38) and other scales, such as the modified Tardieu Scale, have been suggested by some (39). The MAS is, however, widely used in many clinical studies (40). For instance, all our eligible and included studies used the modified Ashworth Scale to measure spasticity clinically. Eventually our results might therefore be limited by the described limitation of the assessment of spasticity.
All of the 5 included studies aimed at rebalancing interhemispheric inhibition and thus improving rehabilitation outcome. However, recently doubts have emerged that the rationale of these comparisons, the interhemispheric competition model, may be oversimplified or even incorrect (41). Moreover, the optimal tDCS paradigm regarding polarization, electrode location, amount of direct current applied and time administered still has to be established (16). Despite the individualized approach of mapping M1 with transcranial magnetic stimulation prior to stimulation, further improvements may be achieved by involving functional imaging techniques during tDCS to identify other target areas of potential interest (42). Further research into individualized stimulation protocols is therefore warranted.
One could argue that further potentially relevant studies may have been missed by our literature search. However, by searching common databases using a comprehensive and sensitive search strategy and by making an additional hand-search, this possibility should have been avoided. Furthermore, one could argue that the results might be prone to publication bias. However, by visual inspection of funnel plots (not figured here) we were not able to identify funnel plot asymmetry as a possible marker of publication bias.
In conclusion, when comparing active with sham tDCS, there is moderate-quality evidence for no effect of tDCS on improving spasticity. There is low-quality evidence for no effect of tDCS on improving spasticity compared with any other active control intervention.
Future research should aim at conducting further multicentre RCTs in a parallel group design in order to examine the impact of tDCS on spasticity together with function and activities. It is likely that more accurate measures of spasticity, perhaps including neurophysiological measures, would strengthen further studies in this area.
At present, there is insufficient evidence to support or refute the use of tDCS as an intervention for improving spasticity after stroke in daily routine practice.
REFERENCES
Appendix I. Search strategy for MEDLINE (via OvidSP) | |
Search strategy for searching MEDLINE via Ovid | |
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp “intracranial embolism and thrombosis”/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vertebral artery dissection/ 2. (stroke or poststroke or post-stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic or hemineglect or hemi-neglect or ((unilateral or spatial or hemi?spatial or visual) adj5 neglect)).tw. 7. or/1-6 8. Electric Stimulation Therapy/ 9. Electric Stimulation/ 10. Electrodes/ 11. (transcranial adj5 direct current adj5 stimulation).tw. 12. (transcranial adj5 DC adj5 stimulation).tw. 13. (transcranial adj5 electric$ adj5 stimulation).tw. 14. (tDCS or A-tDCS or C-tDCS or S-tDCS or electrode$ or anode or anodes or anodal or cathode or cathodes or cathodal).tw. 15. or/8-14 16. Randomized Controlled Trials as Topic/ 17. random allocation/ 18. Controlled Clinical Trials as Topic/ 19. control groups/ | 20. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ 21. double-blind method/ 22. single-blind method/ 23. Placebos/ 24. placebo effect/ 25. cross-over studies/ 26. randomized controlled trial.pt. 27. controlled clinical trial.pt. 28. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt. 29. (random$ or RCT or RCTs).tw. 30. (controlled adj5 (trial$ or stud$)).tw. 31. (clinical$ adj5 trial$).tw. 32. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 33. (quasi-random$ or quasi random$ or pseudo-random$ or pseudo random$).tw. 34. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 35. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 36. (cross-over or cross over or crossover).tw. 37. (placebo$ or sham).tw. 38. trial.ti. 39. (assign$ or allocat$).tw. 40. controls.tw. 41. or/16-40 42. 7 and 15 and 41 43. exp animals/ not humans.sh. 44. 42 not 43 |
