Peter Michaelson, PhD, RPT1, David Holmberg, MSc, RPT2, Björn Aasa, MSc, RPT3 and Ulrika Aasa, PhD, RPT4
From the 1Department of Health Science, Division of Health and Rehabilitation, Luleå University of Technology, Luleå, 2Cederkliniken Primary Health Care Centre, Piteå, Department of Research, The Norrbotten County Council, Luleå, 3Department of Surgical and Perioperative Sciences, Umeå University, Norrlandskliniken Primary Health Care Centre and 4Department of Community Medicine and Rehabilitation, Division of Physiotherapy, Umeå University, Umeå, Sweden
OBJECTIVE: The aim of this study was to compare the effects of a high load lifting exercise with low load motor control exercises on pain intensity, disability and health-related quality of life for patients with mechanical low back pain.
DESIGN: A randomized controlled trial.
SUBJECTS: Patients with mechanical low back pain as their dominating pain mechanism.
METHODS: The intervention programme consisted of a high load lifting exercise, while the control group received low load motor control exercises over 8 weeks (12 sessions) with pain education included in both intervention arms. The primary outcome was pain intensity and disability, and the secondary outcome was health-related quality of life.
RESULTS: Each intervention arm included 35 participants, analysed following 2-, 12- and 24-month follow-up. There was no significant difference between the high load lifting and low load motor control interventions for the primary or secondary outcome measures. Between 50% and 80% of participants reported a decrease in perceived pain intensity and disability for both short- and long-term follow-up.
CONCLUSION: No difference was observed between the high low load lifting and low load motor control interventions. Both interventions included retraining of movement patterns and pain education, which might explain the positive results over time.
Key words: mechanical low back pain; exercise therapy; pain intensity; disability; follow-up.
J Rehabil Med 2016; 48: 456–463
Correspondence address: Peter Michaelson, Luleå University of Technology, Department of Health Sciences, SE-971 87 Luleå, Sweden. E-mail: peter.michaelson@ltu.se
Accepted Feb 25, 2016; Epub ahead of print Apr 13, 2016
INTRODUCTION
Low back pain (LBP) is a common health problem (1). Patients with LBP can be classified into sub-groups based on assumptions about the neurophysiological mechanisms responsible for generating and maintaining the pain (2). Nociceptive pain (NP) has been proposed as one category (3), where the pain condition is assumed to be predominantly driven by activation of peripheral nociceptive neurones (4) in response to noxious chemical, mechanical or thermal stimuli (5). In this sub-group, the pain is distinct, with a consistent and proportionate mechanical pattern that can be reproduced by movements. It has been suggested that movements that are not performed optimally (6–8) can overload structures in the lumbar spine and/or aggravate an injury (9), thereby increasing pain perception (10). Physical therapists worldwide commonly use low load motor control (LMC) exercises to correct motor control deficiencies, in order to retrain movement patterns and regain control of spinal motions. In a recent review, the authors concluded that LMC exercises reduce pain more efficiently than general exercises (11).
It is unclear whether exercises other than LMC can optimize the spinal load and reduce pain arising from nociceptive neurones due to mechanical tissue loading. A pilot study using high-intensity dead-lift training as treatment for LBP has shown improvements regarding both pain and function (12), which might be explained by the strengthening of stabilizing muscles, such as the multifidus, longissimus, transversus abdominis, obliqus internus and externus and/or retraining of movement patterns. A recent review concluded that deconditioning of lumbar erector spinae commonly occurs among LBP patients (13), which also motivates retraining of the lumbar extensors. High-intensity exercises generate increased synchronization of motor units and stronger impulses from the central nervous system to motor units (14), which can motivate the implementation of such exercises. Performing dead-lift as a high load lifting (HLL) exercise can stress almost the entire muscular system, especially the back extensors and trunk stabilizing muscles (15). The exercise includes motor control components, since it activates the stabilizing muscles while the lumbar spine is held in a neutral position with a concurrent movement in the hip and knee, much like a functional dynamic stabilizing exercise. If performed with sufficient intensity, it can activate the stabilizing muscles to a greater extent than LMC (16, 17). Altogether, this indicates that HLL might be effective as treatment for LBP. In a previous article from our research group, the effects of HLL were compared with LMC exercises for participants with mechanical LBP, evaluating pain intensity, activity and physical capacity (18). Aasa et al. (18) showed that LMC was superior regarding activity and movement control, but not regarding pain intensity, strength and muscle endurance. However, this study did not evaluate the domains of disability, health-related quality of life and pain intensity with long-term follow-up. The aim of this study was therefore to compare HLL with LMC exercises regarding effects on pain intensity, disability, and health-related quality of life up to 24 months.
METHODS
Design
The design was a randomized controlled trial (Fig. 1). The protocol is registered in the Clinical Trial Registry of the US National Institute of Health (NCT01061632), approved by the Regional Ethical Review Board at the University of Umeå (nr 09-200M), and conducted in accordance with the Declaration of Helsinki. This article is part of a larger data collection.
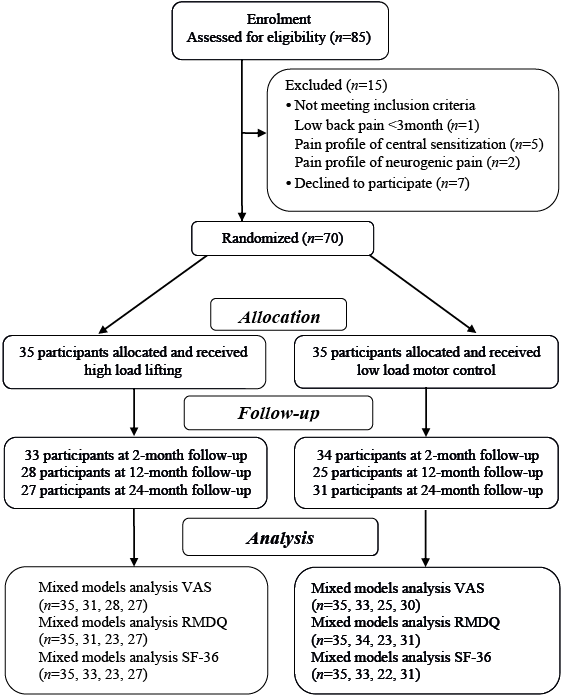
Fig. 1. Flow chart of the randomised controlled trial. VAS: visual analogue scale; RMDQ: Roland Morris Disability Questionnaire; SF-36: The 36-item Short Form Health Survey.
Participants
Consecutive patients aged 25–60 years seeking care from physiotherapists (PT) at 2 occupational healthcare services in late 2009 and classified with nociceptive mechanical LBP of more than 3-month duration (i.e. inclusion criteria) (3) were screened for inclusion and, if they fulfilled the inclusion criteria, were invited to participate in the trial. Thereafter, potential participants (n = 85) were contacted by a study administrator (physiotherapist; PT), who continued the inclusion process by controlling the presence of any exclusion criteria by asking specific questions about suspected or X-ray-confirmed spinal pathology (e.g. tumour, infection, spinal deformity, fracture and inflammatory disease), pregnancy, nerve root compression, acute disc herniation, systemic illness, rheumatic, neurological and psychiatric diseases or contraindications to exercises.
Thereafter, a referent PT specialized in orthopaedic manual therapy established the participants’ eligibility (Fig. 1), confirming that the LBP was mainly of a nociceptive mechanical character (3). Two potential participants younger than 25 years were included, since all other inclusion and exclusion criteria were met.
Procedure
The participants were assigned consecutive numbers, i.e. the participant first included was labelled 1, the next participant 2, and so on. A study administrator collected baseline data after inclusion was complete (UA). Thereafter, an investigator (PM), who had not met any of the participants, and who was blinded to all patient characteristics, performed a blinded randomization procedure to provide a concealed allocation. The randomization was stratified according to sex (male/female) and age (“young” ≤ 42 years and “old” 43–60 years), forming 4 groups. For each group, randomization was performed by applying a computer-generated procedure of n out of N. This procedure randomly draws n cases out of a population of size N, forming the HLL group. This list of numbers was then matched with the list of participants, by another investigator (UA), who allocated the participants to each intervention arm.
The intervention consisted of 12 treatments over an 8-week period, with 2 sessions per week in the first month and 1 session per week thereafter. Both PTs involved in the study were instructed to teach the participants about the mechanisms of the disorders and support self-management (19). Their fear and anxiety of movement were confronted and discussed (20), and correct movement techniques were taught.
At the 2-month follow-up session, questionnaires were delivered and collected by a study administrator blinded to group allocation. At the 12- and 24-month follow-up sessions, a study investigator (PM) posted questionnaires to participants.
Interventions
High load lifting exercise. The HLL intervention consisted of the dead-lift exercise that efficiently activates the stabilizing muscles of the lower back through optimal alignment of the spine (15–17). The exercise was performed as described in the pilot study (12), but with a reduced load and increased number of repetitions (18). Before each lift, the participant was instructed to take a deep breath (i.e. Valsalva manoeuvre) and contract the stabilizing muscles of the trunk (i.e. abdominal bracing). The training started at a low load (10 kg including the barbell), while the PT (DH) ensured that the spine was held in a neutral position through the lift. When the technique was correct through the ascent/descent phases of the lift, the load was gradually increased during the intervention period by increasing the number of lifts and/or the weight on the bar. The participants reported their pain intensity before, during and after the dead-lift session. If the pain had not increased, the training was progressed. No 1 repetition maximum (RM) test was performed, but based on the PT’s extensive experience, most participants reached approximately 70–85% of 1 RM. To minimize the risk of discomfort or injury the progression was individually adjusted throughout the intervention, at the discretion of the PT together with the participant. In this case, individual adjustment was to decide whether or not to increase the load, or increase the number of repetitions or number of sets on the same load. The overall goal was trying to lift a greater total weight (kg) compared with the previous session. Before each session, participants were asked if their symptoms had increased since the previous session. If so, this was taken into consideration when adjusting the training. The training was carried out in groups of 2–6 participants. A PT (DH) with extensive experience of the dead-lift exercise performed the HLL intervention. The intervention has been described in detail elsewhere (18).
Low load motor control exercises. The LMC intervention used low load motor control exercises to retrain identified faulty movement patterns. The choice of the exercises was based on the anamnesis and physical examination performed to identify provocative and relieving postures and movements (6, 7, 21–23). The LMC intervention was divided into 3 stages. In the first stage, the participants’ ability to activate the stabilizing muscles in order to control the lumbar spine in neutral positions was retrained in supine, sitting, four-point-kneeling, and standing positions. The exercises progressed through movements of the arms and legs, while participants maintained control over lumbar spine movements. In the second stage, the participant was assessed and evaluated on provocative and relieving movements using postural correction exercises including static control. This part also included specific training to dissociate movements between the upper and lower back and aimed at reducing over-activity and stiffness of superficial mobilizing muscles. In the third stage, an implementation of the desired movement pattern into various dynamic tasks and functional positions used in everyday life was performed, based on activities the participants reported to be provocative. After each treatment session, the participant received 1–3 home exercises to perform each day until the next appointment. A PT (BA) that had used motor control exercises in clinical practice for several years provided the intervention. The exercises have been described in detail elsewhere (18).
Outcome measures
The outcome measures chosen were recommended as core outcome for chronic pain clinical trials (24). Participant characteristics, such as age, sex, weight, height, smoking habits, and physical activity, were collected at baseline. At the 24-month follow-up sessions, a Credibility/Expectancy Questionnaire (25) was applied together with questions on additional treatment and exercises performed since the intervention ended.
Primary outcomes. Pain: Pain intensity was evaluated with the visual analogue scale (VAS) using the descriptor average pain intensity during the last 7 days (VAS 7 days). The scale had the anchors 0 = no pain and 100 = worst possible pain. The validity, reliability, and responsiveness of the VAS scale are well documented (24). Disability: The 24-item Roland-Morrison Disability Questionnaire (RMDQ) was used to assess self-rated physical disability through a series of Yes/No questions regarding aspects of disability, with 24 points representing maximum disability. The RMDQ has been recommended as a valid questionnaire to measure disability for interventions regarding LBP (24).
Secondary outcomes. Health-related quality of life: The 36-item Short Form Health Survey (SF-36) was used to measure how the participants experienced physical and mental health as well as quality of life (24). SF-36 is divided into the categories physical function (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). Within each category, a value between 0 and 100 is possible, with a value of 100 corresponding to the best possible health and quality of life. The Swedish version of SF-36 has good validity and reliability (26).
Minimal important change. An individual reduction of 30% or more of each outcome measure (pain intensity, disability, and health-related quality of life) was considered to represent a clinically meaningful improvement presented as minimal important change (MIC) (27). The numbers of participants who achieved MIC improvements are presented in percentages.
Data processing and statistical analysis
The sample size was calculated with 80% power (alpha level 0.05) to detect a between-group difference on VAS 7 days of 15 units (SD 21), giving an estimated group size of 31, enrolling 35 participants in each intervention to ensure power even with potential drop-outs.
Statistical analyses were performed using SPSS version 22 and the level of significance was set at p < 0.05. If a single answer in the RMDQ questionnaire was missing (5 cases), the missing answers were replaced by imputation of the mean value of the participant. Data were not imputed if missing on VAS 7 days, the entire RMDQ, or SF-36 questionnaires (Fig. 1), but handled within the generalized linear mixed model. As the outcome variables were not normally distributed according to Shapiro–Wilk and q-q plots, non-parametric statistics were applied. For evaluating the aim of the study, a generalized linear mixed model with the scores of the VAS 7 days, RMDQ and SF-36 categories entered as dependent variables using an ordinal response distribution. The independent variables included Group (HLL, LMC), Time (Baseline, 2-, 12- and 24-month) and the interaction Group*Time entered as fixed effects, while age (years) and sex (male, female) were covariates in the overall model. The between-group and within-group comparison of Baseline–2 months, Baseline–12 months, and Baseline–24 months were evaluated using χ2 statistics of likelihood ratio calculated within the overall model.
RESULTS
In total, 15 of the initial 85 participants did not enter the study for various reasons, while 70 signed informed consent (Fig. 1). The participants’ mean age was 42.1 years, with the youngest aged 22 and the oldest 60 years. None of the participants were on full-time sick leave when entering the study and most were blue-collar workers. Descriptive statistics of included participants are presented in Table I. There were no differences in baseline value between those attending the 24-month follow-up sessions and the dropouts for any outcome measure. Two participants from the HLL group reported adverse effects, and 1 of these dropped out during intervention. Another participant also dropped out of the HLL group without giving a reason. One participant withdrew from the LMC group due to abdominal surgery unrelated to the intervention. No adverse effects were reported from the LMC group. The HLL participants attended almost all intervention sessions (mean 11.0, SD 2.7), while the LMC group attended 6.1 (SD 2.0) intervention sessions on average. For the HLL group, the highest load lifted by the men was mean 100 kg (SD 31, range 55–200 kg), while the women lifted mean 53 kg (SD 21, range 15–102.5 kg). As shown in Table II, there were no significant differences between the groups regarding the 24-month follow-up scores of expectancies of the intervention. Following the intervention, 33–42% of the participants in the intervention groups received treatment approximately 3–4 times over the 24-month period (Table II).
|
Table I. Baseline characteristics of the participants in the high load lifting (HLL) group and the low load motor control (LMC) group, displayed with mean and standard deviations unless other indicated |
||
|
|
HLL (n = 35) |
LMC (n = 35) |
|
Sex Male Female |
15 20 |
16 19 |
|
Age, years, mean (SD) |
41.9 (9.9) |
42.2 (10.4) |
|
Height, m, mean (SD) |
173.8 (8.3) |
172.2 (10.4) |
|
Weight, kg, mean (SD) |
74.0 (12.9) |
78.2 (14.8) |
|
BMI, mean (SD) |
24.4 (2.7) |
26.3 (3.7) |
|
Pain duration, weeks, mean (SD) |
312 (310) |
340 (290) |
|
Smoker, n (%) |
3 (9) |
3 (9) |
|
Taking analgesic, n (%) |
18 (51) |
18 (51) |
|
Physical activitya, min/week, mean (SD) |
179 (148) |
165 (160) |
|
aPhysical activity with moderate intensity. BMI: body mass index; SD: standard deviation. |
||
|
Table II. Treatment expectancies and credibility at 24-month follow-up for the high load lifting (HLL) group and low load motor control (LMC) group, and treatment and exercise performed following interventions |
|||
|
|
HLL |
LMC |
p-value |
|
How logical does the therapy offered to you seem? Mean (SD) |
74.2 (26.8) |
79.4 (24.9) |
0.45* |
|
How successful do you think this treatment was in reducing your pain? Mean (SD) |
67.5 (30.5) |
72.9 (25.5) |
0.48* |
|
How confident would you be in recommending this treatment to a friend? Mean (SD) |
73.1 (28.9) |
81.7 (24.4) |
0.23* |
|
How easy was it to complete this intervention? Mean (SD) |
65.8 (26.4) |
75.6 (24.8) |
0.54* |
|
Treatment for LBP after interventiona, % |
42 (n = 26) |
33 (n = 30) |
0.34** |
|
Number of treatmentsb, median (range) |
4 (1–10) (n = 10) |
3 (1–10) (n = 11) |
|
|
Exercise according to intervention, % |
100 (n = 7) |
100 (n = 2) |
|
|
Performing physical exercise, % |
82 (n = 26) |
73 (n = 31) |
0.42** |
|
*Independent samples t-test; **χ2 test. aTreated by: physician (n = 1), physiotherapist (n = 6), chiropractor/naprapath (n = 7). bType of treatment: massage (n = 2), mobilization (n = 2), manipulation (n = 3). n = : numbers of participants answering the question. |
|||
Pain intensity and disability
Scores of the VAS 7 days and RMDQ are presented in Table III. There was no difference between intervention arms over time regarding VAS 7 days (Group*Time, p = 0.98), with no significant difference between Group (p = 0.17). The within-group analysis showed a significant decrease in the pain intensity over Time (p < 0.000), with a decrease in pain intensity on 20.2 mm VAS 7 days at 2 months, 19.5 mm at 12 months, and 13.5 mm at 24 months. Age was significant (p = 0.012), while sex was not significant (p = 0.24) in the model. A MIC improvement was achieved for 53–70% of the participants in the intervention groups (Table III). Regarding RMDQ, no significant difference was found between the intervention arms (Group*Time, p = 0.98), with no significant difference between Group (p = 0.54). Within-group analyses showed a significant difference over Time (p < 0.000) with a treatment effect of 3.2 points at 2 months and 2.8 points at 24 months. Age was significant (p = 0.000), while sex was not significant (p = 0.12) in the model. Among the LMC participants 74–78% achieved a MIC improvement, compared with 63–74% for the HLL participants.
|
Table III. Scores for visual analogue scale (VAS) 7 days and Roland Morris Disability Questionnaire at baseline, 2 months, 12 months, and 24 months for the high load lifting (HLL) group and low load motor control (LMC) group. Values are presented with mean values and standard deviations. Also presented are the between-group adjusted B-value of change with a 95% Wald confidence interval along with the within-group adjusted B-value of change over time with a 95% Wald confidence interval for both intervention groups. The percentage (n %) of participants that improved by at least 30% (minimal important change (MIC)) on that outcome measure is also shown |
|||||||||||
|
|
Unadjusted outcome values |
|
Adjusted treatment effect |
||||||||
|
HLL |
|
LMC |
|
Between-group changea B (95% Wald CI) |
p-value |
|
Within-group changeb B (95% Wald CI) |
p-value |
|||
|
Mean (SD) |
MIC |
|
Mean (SD) |
MIC |
|
|
|||||
|
VAS 7 days |
|
|
|
|
|
|
|
|
|
|
|
|
Baseline |
43 (24) |
|
|
47 (28) |
|
|
|
|
|
|
|
|
2 months |
22 (21) |
70 |
|
30 (26) |
53 |
|
0.2 (–1.0 to 1.4) |
0.74 |
|
–1.4 (–2.2 to –0.5) |
0.001 |
|
12 months |
24 (27) |
67 |
|
25 (22) |
61 |
|
0.05 (–1.2 to 1.3) |
0.94 |
|
–1.6 (–2.5 to –0.7) |
0.001 |
|
24 months |
27 (27) |
52 |
|
30 (29) |
60 |
|
–0.1 (–1.3 to 1.2) |
0.89 |
|
–1.3 (–2.2 to –0.4) |
0.006 |
|
RMDQ |
|
|
|
|
|
|
|
|
|
|
|
|
Baseline |
7.2 (4.3) |
|
|
7.1 (3.9) |
|
|
|
|
|
|
|
|
2 months |
3.8 (4.0) |
71 |
|
3.6 (4.2) |
74 |
|
–0.2 (–1.3 to 1.0) |
0.77 |
|
–1.6 (–2.5 to –0.8) |
0.000 |
|
12 months |
3.6 (4.2) |
74 |
|
3.3 (3.6) |
78 |
|
–0.2 (–1.5 to 1.1) |
0.74 |
|
–1.7 (–2.7 to –0.8) |
0.000 |
|
24 months |
3.8 (3.9) |
63 |
|
3.6 (3.7) |
74 |
|
0.01 (–1.2 to 1.2) |
0.99 |
|
–1.7 (–2.6 to –0.8) |
0.000 |
|
aInteraction. bWithin-group analysis. RMDQ: Roland Morris Disability questionnaire. |
|||||||||||
Health-related quality of life
Descriptive statistics for the categories of the SF-36 along with the percentage of reported MIC are shown in Table IV. There were no significant differences between the intervention arms over time (Group*Time) for the categories PF (p = 0.57), RF (p = 0.73), BP (p = 0.68), GH (p = 0.67), VT (p = 0.82), SF (p = 0.84), MH (p = 0.97), or RE (p = 0.83). In the models, sex was a significant covariate in the PF- (p = 0.000) and MH model (p = 0.007). Age was significant for the PF- (p = 0.000), GH- (0.025), VT- (p = 0.03), SF- (p = 0.006), MH- (p = 0.001) and RE model (p = 0.002). Within-group analysis over Time showed significantly increased scores for the categories PF (p < 0.000), RF (p < 0.000), BP (p < 0.000), GH (p < 0.002), VT (p < 0.000), SF (p < 0.000), and MH (p < 0.000), but not for RE (p = 0.39). Sex was significant as covariate in models for PF (p = 0.000) and MH (p = 0.007), while age was significant for PF (p = 0.000), RP (p = 0.044), GH (p = 0.025), VT (p = 0.03), SF (p = 0.006), MH (p = 0.001) and RE (p = 0.002). For the categories RF, BP, and VT, 48–70% of the participants showed a MIC on all follow-up occasions, with lower scores, ranging from 12% to 34%, for PF, GH, SF, RE and MH.
|
Table IV. Values for categories of the 36-item Short Form Health Survey at baseline, 2 months, 12 months, and 24 months for the high load lifting (HLL) group and low load motor control (LMC) group. Values are presented with mean values and standard deviations. Also presented are between-group adjusted B-value of change with a 95% Wald confidence interval along with the within-group adjusted B-value of change over time with a 95% Wald confidence interval for both intervention groups. The percentage (n %) of participants that improved by at least 30% (minimal important change (MIC)) on that outcome measure is also shown |
||||||||||
|
|
Unadjusted outcome values |
|
Adjusted treatment effect |
|||||||
|
HLL |
|
LMC |
|
Between-group changea B (95% Wald CI) |
p-value |
Within-group changeb B (95% Wald CI) |
p-value |
|||
|
Mean (SD) |
MIC |
|
Mean (SD) |
MIC |
|
|||||
|
Physical function |
|
|
|
|
|
|
|
|
|
|
|
Baseline |
76.4 (13.5) |
|
|
77.0 (12.1) |
|
|
|
|
|
|
|
2 months |
90.2 (10.4) |
19 |
|
87.8 (9.9) |
24 |
|
–0.7 (–1.9 to 0.5) |
0.24 |
2.4 (1.5 to 3.3) |
0.000 |
|
12 months |
89.6 (14.4) |
22 |
|
87.4 (10.3) |
15 |
|
–0.7 (–2.1 to 0.7) |
0.37 |
2.6 (1.5 to 3.6) |
0.000 |
|
24 months |
90.9 (9.3) |
22 |
|
90.8 (9.4) |
19 |
|
–0.1 (–1.3 to 1.2) |
0.83 |
2.5 (1.5 to 3.4) |
0.000 |
|
Role physical |
|
|
|
|
|
|
|
|
|
|
|
Baseline |
50.7 (41.8) |
|
|
50.0 (38.4) |
|
|
|
|
|
|
|
2 months |
87.9 (23.2) |
52 |
|
86.7 (27.0) |
61 |
|
0.1 (–1.3 to 1.5) |
0.92 |
2.0 (1.0 to 3.0) |
0.000 |
|
12 months |
85.9 (35.2) |
44 |
|
77.4 (37.8) |
60 |
|
–0.9 (–2.5 to 0.8) |
0.30 |
2.3 (1.0 to 3.6) |
0.000 |
|
24 months |
84.3 (30.3) |
52 |
|
85.5 (24.0) |
65 |
|
–0.2 (–1.6 to 1.2) |
0.78 |
1.9 (0.8 to 3.0) |
0.000 |
|
Bodily pain |
|
|
|
|
|
|
|
|
|
|
|
Baseline |
42.4 (14.9) |
|
|
45.8 (15.2) |
|
|
|
|
|
|
|
2 months |
67.4 (20.5) |
68 |
|
70.6 (17.5) |
74 |
|
–0.1 (–1.2 to 1.0) |
0.87 |
2.0 (1.2 to 2.9) |
0.000 |
|
12 months |
68.2 (24.6) |
78 |
|
60.6 (27.3) |
43 |
|
–0.8 (–2.2 to 0.5) |
0.24 |
2.2 (1.2 to 3.2) |
0.000 |
|
24 months |
67.5 (24.1) |
59 |
|
65.8 (26.8) |
65 |
|
–0.2 (–1.4 to 1.0) |
0.75 |
2.0 (1.1 to 2.9) |
0.000 |
|
General health |
|
|
|
|
|
|
|
|
|
|
|
Baseline |
66.4 (16.9) |
|
|
64.5 (18.2) |
|
|
|
|
|
|
|
2 months |
78.2 (18.8) |
26 |
|
75.6 (18.9) |
27 |
|
–0.1 (–1.2 to 1.1) |
0.89 |
1.1 (0.3 to 2.0) |
0.007 |
|
12 months |
76.4 (19.3) |
17 |
|
67.5 (23.2) |
19 |
|
–0.7 (–2.1 to 0.6) |
0.29 |
1.0 (0.0 to 1.9) |
0.044 |
|
24 months |
74.2 (18.3) |
15 |
|
72.2 (23.7) |
16 |
|
0.1 (–1.1 to 1.4) |
0.84 |
0.7 (–0.1 to 1.6) |
0.10 |
|
Vitality |
|
|
|
|
|
|
|
|
|
|
|
Baseline |
40.7 (19.1) |
|
|
41.6 (19.0) |
|
|
|
|
|
|
|
2 months |
64.1 (19.7) |
74 |
|
60.4 (24.7) |
62 |
|
–0.2 (–1.4 to 1.0) |
0.74 |
1.9 (1.0 to 2.7) |
0.000 |
|
12 months |
57.4 (20.6) |
44 |
|
62.4 (20.3) |
62 |
|
0.4 (–0.9 to 1.7) |
0.56 |
1.3 (0.4 to 2.2) |
0.006 |
|
24 months |
59.8 (19.0) |
41 |
|
56.5 (24.2) |
55 |
|
–0.2 (–1.4 to 1.0) |
0.75 |
1.4 (0.6 to 2.3) |
0.001 |
|
Social functioning |
|
|
|
|
|
|
|
|
|
|
|
Baseline |
76.4 (22.6) |
|
|
78.6 (20.5) |
|
|
|
|
|
|
|
2 months |
90.7 (17.7) |
36 |
|
88.6 (18.3) |
32 |
|
–0.4 (–1.7 to 0.9) |
0.55 |
1.5 (0.5 to 2.5) |
0.003 |
|
12 months |
87.5 (16.9) |
22 |
|
89.3 (19.1) |
38 |
|
0.2 (–1.3 to 1.7) |
0.77 |
0.9 (–0.0 to 1.9) |
0.060 |
|
24 months |
89.8 (15.1) |
22 |
|
90.7 (17.7) |
39 |
|
0.2 (–1.2 to 1.6) |
0.79 |
1.2 (0.2 to 2.2) |
0.015 |
|
Role emotional |
|
|
|
|
|
|
|
|
|
|
|
Baseline |
84.8 (31.7) |
|
|
88.6 (22.8) |
|
|
|
|
|
|
|
2 months |
84.9 (29.6) |
16 |
|
87.3 (23.2) |
12 |
|
–0.2 (–1.7 to 1.4) |
0.85 |
–0.0 (–1.2 to 1.1) |
0.94 |
|
12 months |
85.5 (33.1) |
5 |
|
93.7 (22.7) |
13 |
|
0.7 (–1.5 to 2.8) |
0.54 |
0.2 (–1.2 to 1.5) |
0.81 |
|
24 months |
91.4 (27.1) |
15 |
|
90.3 (24.6) |
19 |
|
–0.4 (–2.3 to 1.5) |
0.66 |
0.8 (–0.7 to 2.2) |
0.28 |
|
Mental health |
|
|
|
|
|
|
|
|
|
|
|
Baseline |
70.9 (14.6) |
|
|
73.4 (17.0) |
|
|
|
|
|
|
|
2 months |
83.0 (14.9) |
29 |
|
83.3 (15.6) |
21 |
|
–0.2 (–1.4 to 0.9) |
0.70 |
1.5 (0.7 to 2.4) |
0.000 |
|
12 months |
77.0 (15.7) |
17 |
|
81.7 (14.6) |
19 |
|
0.1 (–1.2 to 1.4) |
0.89 |
0.8 (–0.1 to 1.7) |
0.087 |
|
24 months |
79.0 (17.4) |
30 |
|
80.4 (15.8) |
16 |
|
–0.1 (–1.3 to 1.1) |
0.86 |
1.0 (0.1 to 1.9) |
0.032 |
|
aInteraction. bWithin-group analysis. |
||||||||||
DISCUSSION
There was no significant difference between the HLL and LMC groups regarding pain intensity, disability, or health-related quality of life at the 2-, 12- or 24-month follow-ups. Both the HLL and LMC exercises combined with pain education resulted in decreased pain intensity, disability and increased health-related quality of life at the 2-month follow-up. The positive result was still apparent at the 12- and 24-month follow-ups. A majority of the participants reported a MIC (27) for pain intensity and disability (48–86%) at the 2- and 12-month follow-ups, while the numbers at the 24-month follow-up were slightly lower. Similar results were observed for the categories RP, BP, and VT of SF-36, where over 50% of the participants reported a MIC. Due to the study design, it is not possible to elucidate whether the observed effect is a result of the exercises, the pain education, or a combination of both.
To our knowledge, this is the first randomized controlled trial that has used the dead-lift exercise as intervention for participants with LBP with a 24-month follow-up. Compared with LMC exercises, no significant differences were found for self-reported pain intensity, disability, or health-related quality of life. Since our interventions combined exercises and pain education with a behavioural aspect, we cannot exclude the possibility that pain education caused the positive effect over time and explains why no difference between HLL and LMC was observed. The importance of adding a behavioural approach as part of the intervention treatment was shown by Vibe-Fersum et al. (20) when comparing cognitive functional rehabilitation with exercise or manual therapy. Although our pain education was not as comprehensive as in the cognitive functional rehabilitation programme (20), there are similarities, such as including pain education, confronting maladaptive movement patterns of the participants, and implementing specific movement exercises while avoiding provocative movements. There is also a possibility that there is no therapeutic power between the LMC and HLL interventions. Byström et al. (11) recently concluded that motor control exercises were superior to general exercises, regarding disability, for short-, intermediate-, and long-term follow-up. However, this finding was almost immediately refuted by Smith et al. (28), who argued that motor control exercises are not superior in effect compared with general exercises. These conflicting results might be explained by the fact that Smith et al. (28) and Byström et al. (11) used different inclusion criteria, slightly different interventions and different statistical methods. Our LMC intervention would most likely be categorized as motor control exercises in systematic reviews, while HLL probably would be considered a general exercise (29, 30). However, it should be noted that the dead-lift exercise resembles a motor control exercise, since it was most likely performed with activation of stabilizing muscles (16, 17) of the lumbar spine with concurrent disassociation of extremity movements (31), performed at high intensity.
Both the HLL and LMC interventions seem to be effective in decreasing pain intensity and disability at both short- and long-term follow-up. This result is in agreement with recent systematic reviews (11, 28–30). Macedo et al. (29) concluded that motor control exercises were more effective than minimal intervention in reducing pain intensity in the short, intermediate, and long term, and disability in the long term, while Van Middelkoop et al. (30) concluded that exercise therapy was effective for chronic LBP. The observed treatment effect of a 20-mm and 19-mm decrease in VAS 7 days at 2 and 12 months are in accordance with the numbers presented by Smith et al. (28). Regarding MIC, 53–70% of participants perceived reduced pain intensity in their lower back at the 2-month follow-up, with similar figures at the long-term follow-up. For disability, the results were even more impressive; 63% (HLL) to 74% (LMC) of participants reported MIC. However, not all participants reported an improvement in MIC, indicating that the interventions were not successful for all participants. The LMC exercises focused on targeting aberrant and painful movements in flexion, extension, lateral flexion and rotation, with an activation of relevant muscles at sufficient intensity to retrain desired movement patterns (6, 31). For a majority of the participants in this trial, retraining optimal movement patterns seemed to be an important factor in reducing pain and improving function. In the HLL group, the initial focus was technical execution, i.e. maintaining neutral position in the lumbar spine during the dead-lift. After the initial phase, the dead-lift was performed strenuously to challenge the participants’ maximal lifting capacity by activating the lumbar extensors (15) to stimulate neuromuscular adaption (32). In the HLL group, it is possible that hypertrophy of the multifidus muscles occurred due to the intensity of the exercise (13, 33). Previously, Dannels et al. showed an increased cross-sectional area (CSA) of the multifidus muscles only in the intervention arm, where dynamic resistance training was added to stabilizing exercises that served as control group (33). However, later, Hides et al. (34) showed that also low load stabilization exercises could stimulate hypertrophy of the multifidus muscles among male cricket players. The cricket players also reported less pain for the lower back following intervention. These contrasting results imply that future research, designed to explore the pain mechanism of the intervention, is needed. It would be interesting to evaluate the effect on CSA of the multifidus muscles following low and high load motor control interventions. Such studies might provide some insight to the pain mechanisms accountable to the effect of the HLL and LMC interventions shown in this trial.
Methodological considerations
The observed treatment effect over time has to be judged cautiously, since no placebo-controlled intervention arm was included. One weakness of the study is that the physiotherapists (BA, DH) were not blinded to the interventions they managed. It has been shown that positive expectations of intervention (35) influences treatment outcome. However, as both intervention groups graded treatment expectancy and credibility equally, this probably did not affect the results of our intervention. Two participants reported adverse effects from the HLL exercise. Furthermore, a secondary analysis performed by Berglund et al. (36) showed that high pain intensity and low performance on the Biering-Sørensen test at baseline could predict an unsuccessful outcome of the HLL intervention. Altogether, this indicates: (i) that the dead-lift exercise should be used carefully and must be performed correctly, with slow progression under close supervision of an experienced physiotherapist; and (ii) that the dead-lift might not be an optimal exercise for all participants, whereas interventions including pain alleviation or LMC exercises might be more suitable for some participants to start with.
A reason that only 50 participants (71%) completed the 12-month follow-up might be that they were only reminded once or not at all. At the 24-month follow-up, after up to 4 reminders, the compliance rate was 83%, but still lacking 4 participants to ensure full power for the analyses. Since the results for the 12-month follow-up are consistent with the 2- and 24-month follow-ups, we believe the results of the 12-month follow-up are reliable. Furthermore, we used generalized linear mixed models for the statistical analyses, a method that uses available data and generates analyses with reliable results, even in case of some missing data (37). It is possible that we overestimated the between-group difference when using 15-mm VAS 7 days for power calculations, since the baseline values were approximately 45 mm for the whole group (MIC 45 mm=13.5 mm). If so, our study might be slightly underpowered concerning the number of participants.
A conclusion from the pilot study (12) was to expect a delayed treatment response for the HLL intervention, and therefore these participants were encouraged to attend all sessions (mean 11.0 sessions). The LMC intervention was more individualized and ended when the participants considered themselves recovered (mean 6.1 sessions). It is possible that treatment effect can be achieved with fewer treatment sessions for LMC exercises than HLL exercise. Our study had similar inclusion and exclusion criteria as previous studies on LBP (38, 39), with the difference that only participants with nociceptive mechanical pain were included (3, 22). Our participants are representative regarding pain intensity and disability compared with Brooks et al. (39), who also included participants diagnosed with nociceptive LBP (3), while previous studies (38) that did not use this inclusion criteria show slightly higher baseline values.
Conclusion
No difference was observed between the HLL and LMC interventions regarding pain intensity, disability, or health-related quality of life at the 2-, 12- or 24-month follow-ups. Both interventions included retraining of movement patterns and pain education, thus challenging beliefs about pain interference in everyday life. These components might explain the positive results over time.
ACKNOWLEDGEMENTS
This study was founded by grants from Visare Norr, Sweden and Norrbottens County Council, Sweden.
The authors declare no conflicts of interest.
REFERENCES
