Maria Bäck, RPT, PhD1, Lennart Jivegård, MD, PhD2, Anna Johansson, RPT1, Joakim Nordanstig, MD, PhD2, Therese Svanberg, MSc3, Ulla Wikberg Adania, MA4 and Petteri Sjögren, DDS, PhD3
From the 1Department of Occupational Therapy and Physiotherapy, Sahlgrenska University Hospital, Gothenburg, 2Department of Vascular Surgery, Sahlgrenska University Hospital/Sahlgrenska, 3HTA-Centrum, Region Västra Götaland and 4Medical Library, Sahlgrenska University Hospital/Mölndal, Gothenburg, Sweden
OBJECTIVE: To evaluate the effects of home-based supervised exercise vs hospital-based supervised exercise, and the effects of home-based supervised exercise vs unsupervised “go home and walk advice” on daily life and corridor-walking capacity, health-related quality of life and patient-reported functional walking capacity in patients with intermittent claudication.
DATA SOURCES: Systematic literature searches were conducted in PubMed, EMBASE, ProQuest, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED), the Cochrane Library, and a number of Health Technology Assessment (HTA)-databases in October 2014.
STUDY SELECTION: Randomized controlled trials and non-randomized controlled trials (> 100 patients) were considered for inclusion.
DATA EXTRACTION: Data extraction and risk of bias assessment was performed independently and discussed in meetings.
DATA SYNTHESIS: Seven randomized controlled trials and 2 non-randomized controlled studies fulfilled the inclusion criteria. The included studies had some, or major, limitations.
CONCLUSION: Based on a low quality of evidence, home-based supervised exercise may lead to less improvement in maximum and pain-free walking distance, and in more improvement in daily life walking capacity, compared with hospital-based supervised exercise. Home-based supervised exercise may improve maximum and pain-free walking distance compared with “go home and walk advice” and result in little or no difference in health-related quality of life and functional walking capacity compared with hospital-based supervised exercise or “go home and walk advice”. Further research is needed to establish the optimal exercise modality for these patients.
Key words: intermittent claudication; exercise, health-related quality of life; patient-reported outcomes.
J Rehabil Med 2015; 47: 801–808
Correspondence address: Maria Bäck, Department of Occupational Therapy and Physiotherapy, Vita Stråket 13, Sahlgrenska University Hospital/Sahlgrenska, SE-413 45 Gothenburg, Sweden. E-mail: maria.m.back@vgregion.se
Accepted Jun 22, 2015; Epub ahead of print Sep 11, 2015
INTRODUCTION
Intermittent claudication (IC) is the most common symptomatic presentation in peripheral arterial disease. Approximately 20–40 million individuals worldwide experience typical IC symptoms (1). Attributable mainly to increasing life expectancy, the prevalence of IC has increased substantially during the last decade (1). IC entails a reduction in ambulatory function, ranging from very mild symptoms to severe impairment of walking capacity, which may restrict even basic activities of daily living (2). Although the risk for worsening ischaemia and amputation is low and constitutes a very rare outcome in IC, health-related quality of life (HRQoL) is often markedly reduced in patients with IC compared with age- and sex-matched controls (3–6). As a manifestation of atherosclerosis, IC also confers an increased risk for cardiovascular and cerebrovascular events as well as premature death (7). International guidelines indicate that treatment of IC should consist of risk factor management, medical treatment and exercise. This approach is recommended before considering vascular surgery (8, 9).
Hospital-based supervised exercise (SET) provided by physiotherapists is often advised because of improved treadmill walking performance at short-term follow-up compared with an unsupervised “go home and walk advice” (GHWA) (10). However, current practice is different, as SET programmes are largely unavailable in most countries (11). In addition, a recent systematic review showed uncertain and probably poor adherence to SET programmes (12). Furthermore, the clinical efficacy of SET programmes has been studied mainly in terms of treadmill walking capacity, and has not definitely been demonstrated with regard to patient-reported functional walking capacity (13), HRQoL and long-term follow-up (10). Walking on a treadmill may be an artificial measure of walking capacity and it is unclear to which extent such results reflect functional performance in patients’ daily life. One study has shown that decreased daily life walking capacity impaired HRQoL in patients with IC (14). Daily life walking capacity could therefore be an important outcome and could be measured by, e.g. global-positioning system (GPS) monitoring of normal outdoor walking. Recent studies indicate that performance on corridor-based walk tests, such as the 6-min walk test, are more closely linked to daily life walking capacity than treadmill tests (14–16), and accordingly such measures constitute important end-points when studying the effects of different exercise therapies in patients with IC.
Home-based supervised exercise (HET) has been suggested as a promising and more practical alternative to SET programmes, potentially addressing some of the shortcomings of SET. Recent studies have indicated that HET programmes have higher adherence than SET programmes and are equally efficacious in improving treadmill walking capacity as SET programmes (17–19). The HET interventions described in the literature are heterogeneous and consist of different types of supervision, most typically step activity monitoring, log-book feedback and weekly telephone calls (20).
Most recent systematic reviews regarding the effects of exercise in patients with IC have focused mainly on SET programmes and treadmill walking capacity (10, 11, 21), thus the evidence-base for HET programmes, as compared with both SET programmes and unsupervised GHWA, are not entirely clear for patients with IC, especially when also considering corridor-walking, daily life walking capacity and patient-reported outcomes.
The aim of this systematic review was therefore to evaluate walking capacity, including corridor-walking capacity, daily life walking capacity, HRQoL, and patient-reported functional walking capacity following HET programmes in comparison with either SET programmes or unsupervised GHWA for patients with IC.
METHODS
The present systematic review is based on a previous Health Technology Assessment (HTA) report from the regional HTA-centre (HTA-centrum), Region Västra Götaland, Sweden (22). The protocol, including the PICO (Participants, Intervention, Comparisons, Outcomes) and the search strategies, was set at the regional HTA-centre, but was not published. In the HTA report, but not in the current systematic review, previously published systematic reviews were considered for inclusion. This systematic review was reported according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (23).
Data sources
Systematic literature searches were originally conducted by 2 of the authors (TS, UWA) on 13 December 2013 and updated for this review in 14 October 2014. Databases searched included PubMed, EMBASE, ProQuest, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and the Cochrane Library. A complete list of databases searched and search strategies used is given in Appendix SI1.
Study selection
Inclusion criteria and, accordingly, the PICO (Participants, Intervention, Comparisons, Outcomes), were as follows: publications studying “P”: adults with IC with current symptoms during at least 6 months (not recently operated), and comparing the intervention “I”: “HET” with “C”: “SET” or “C”: “GHWA”, using any of the outcome measures “O”: “maximum walking distance (or time)”, “pain-free walking distance (or time)”, “daily life walking capacity”, “corridor-walking distance”, “HRQoL”, “symptom change according to the Walking Impairment Questionnaire (WIQ)”, or “risks/complications associated with the studied type of exercise”.
Selection criteria for articles were: study design: randomized controlled trials (RCT), non-randomized controlled studies with more than 100 patients; languages: English, Swedish, Norwegian, and Danish. Two authors (TS, UWA) selected studies and independently assessed the obtained abstracts and a first selection of full-text articles for inclusion. Any disagreements were resolved in consensus. Reference lists of relevant articles were scrutinized for additional references. A flow chart of the selection process is shown in Fig. 1. The selected full-text articles were sent to all authors, who read the articles independently, and decided in a consensus meeting which articles should be included.
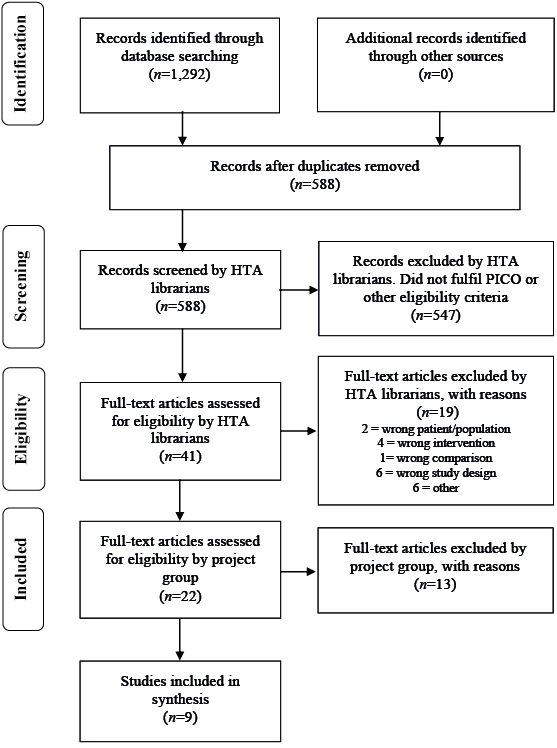
Fig. 1. Flow chart of selection process.
Risk of bias assessment and synthesis
Two authors from the regional HTA-centre Region Västra Götaland (LJ, PS) trained the other authors in risk of bias assessment and rating of the evidence. All included studies were critically appraised. The appraisal of RCTs and non-randomized controlled studies was based on checklists from The Swedish Council on Health Technology Assessment (SBU) (24). Data extraction from the included studies was verified by at least 2 authors for each outcome. In a separate meeting the quality of evidence was rated, for all the studied outcomes separately, across the studies, using the GRADE approach (25). Because of the heterogeneity among the included RCTs in outcome measures, interventions and length of follow-up, no meta-analysis was conducted. Findings were synthesized in tables and summarized narratively.
RESULTS
Study selection and characteristics
The literature search identified a total of 588 articles (after removal of duplicates). Two of the authors (TS, UWA) then excluded 547 articles after reading their abstracts. Another 19 articles were excluded by the librarians after reading the articles in full text. The remaining 22 articles were sent to the other authors, and 9 of them were finally included in the report. One article was included from the update search in October 2014. There were 7 RCTs and 2 non-randomized controlled studies. The studies were mainly from the USA, with a follow-up period of 3–6 months. The mean age of the included patients was 65–70 years and a larger proportion was men. Characteristics of the included studies are presented in Table I, and the excluded articles in Table II.
|
Table I. Included primary publications – design and patient characteristics |
|||||||
|
Author, year Country |
Study design |
Follow-up period (months) |
Study groups intervention (I) control (C) |
Patients (n) |
Mean age (years) |
Men/women |
Outcome variables |
|
Collins et al., 2011 (32) USA |
RCT |
6 |
I = HET C = GHWA |
145 |
67 |
100/45 |
Maximum walking distance Pain-free walking distance HRQoL WIQ |
|
Gardner et al., 2011 (17) USA |
RCT |
3 |
I = HET C1 = GHWA C2 = SET |
119 |
65 |
57/62 |
Maximum walking distance Pain-free walking distance HRQoL WIQ |
|
Gardner et al., 2014 (19) USA |
RCT |
3 |
I = HET C = SET |
120 |
65–67 (group means) |
29/31 |
Maximum walking distance Pain-free walking distance HRQoLa WIQa 6-min walk test |
|
Patterson et al., 1997 (26) USA |
RCT |
6 |
I = HET C = SET |
60b |
69 |
29/26 |
Maximum walking distance Pain-free walking distance HRQoL |
|
Regensteiner et al., 1997 (27) USA |
RCT |
3 |
I = HET C = SET |
20 |
64 |
Not reported |
Maximum walking distance Pain-free walking distance HRQoL WIQ |
|
Sandercock et al., 2007 (28) UK |
RCT |
3 |
I = HET C = SET |
50b |
62–67 (group means) |
32/12 |
Maximum walking distance |
|
Savage et al., 2001 (29) USA |
RCT |
6 |
I = HET C = SET |
21 |
66 |
15/6 |
Maximum walking distance Pain-free walking distance HRQoL |
|
Fakhry et al., 2011 (30) Netherlands |
Non-randomized controlled study |
12 |
I = HET C = SET |
217 |
67–68 (group means) |
135/82 |
Maximum walking distance Pain-free walking distance HRQoL |
|
Manfredini et al., 2008 (31) Italy |
Non-randomized controlled study |
6 |
I = HET C = GHWA |
143 |
68 |
117/26 |
Maximum walking distance Pain-free walking distance |
|
aBetween-group differences for HRQoL and WIQ were not reported. bFive patients were excluded at onset, after randomization (i.e. 55 were included in baseline data). cData for 44 patients presented in baseline characteristics. RCT: randomized controlled study; GHWA: “go home and walk advice”; HET: home-based supervised exercise; HRQoL: health-related quality of life; SET: hospital-based supervised exercise; WIQ: Walking Impairment Questionnaire. |
|||||||
|
Table II. Excluded studies |
|
|
Study |
Reason for exclusion |
|
Al-jundi et al., 2013 (20) |
Review article |
|
Bermingham et al., 2013 (43) |
Wrong outcome |
|
Fokkenrood et al., 2013 (10) |
Wrong intervention |
|
Gardner et al., 2014 (44) |
Wrong intervention and comparison |
|
Gardner & Poehlman, 1995 (45) |
No comparison |
|
Gommans et al., 2014 (21) |
Review article |
|
Langbein et al., 2002 (46) |
Wrong intervention |
|
Makris et al., 2012 (11) |
Review article |
|
McDermott et al., 2013 (18) |
Wrong intervention |
|
Nicolai et al., 2010 (47) |
Wrong intervention |
|
Nielsen et al., 1977 (48) |
Wrong intervention |
|
Pinto et al., 1997 (49) |
Duplicate publication (Patterson, 1997) |
|
Wind & Koelemay, 2007 (50) |
Wrong intervention |
Risk of bias assessment
The included studies had some, or major, study limitations (risk of bias), mainly regarding blinding of outcome assessor, directness and/or precision. Regarding the RCTs there were moderate or major study limitations regarding, for example, blinding of outcome assessor, and there were also problems with directness and/or precision. The non-randomized controlled studies had moderate or major study limitations, e.g. blinding of outcome assessor, directness and precision. The overall study limitations for the individual studies are shown in Table III.
|
Table III. Study limitations |
|||||||||
|
Article/ Study design |
Random sequence generation |
Allocation concealment |
Blinding of participants and personnel |
Blinding of outcome assessment |
Incomplete outcome data |
Selective reporting |
Other bias |
Directness |
Precision |
|
Collins et al., 2011 (32) RCT |
+ |
? |
– |
– |
+ |
+ |
+ |
+ |
? |
|
Gardner et al., 2011 (17) RCT |
+ |
+ |
? |
+ |
+ |
+ |
+ |
+ |
– |
|
Gardner et al., 2014 (19) RCT |
+ |
+ |
? |
+ |
– |
+ |
+ |
? |
? |
|
Patterson et al., 1997 (26) RCT |
+ |
+ |
? |
– |
+ |
+ |
+ |
+ |
– |
|
Regensteiner et al., 1997 (27) RCT |
? |
? |
? |
– |
+ |
+ |
+ |
– |
? |
|
Sandercock et al., 2007 (28) RCT |
+ |
+ |
? |
– |
+ |
+ |
+ |
+ |
– |
|
Savage et al., 2001 (29) RCT |
? |
? |
? |
– |
? |
? |
? |
– |
– |
|
Fakhry et al., 2011 (30) Non-randomized controlled study |
Na |
Na |
? |
? |
? |
? |
? |
– |
? |
|
Manfredini et al., 2008 (31) Non-randomized controlled study |
Na |
Na |
– |
– |
? |
? |
? |
? |
? |
|
+: low-risk/no problems; ?: unclear risk/some problems; -: high-risk/major problems; Na: not applicable; RCT: randomized controlled trial. |
|||||||||
Summary of findings and quality of evidence
Home-based supervised exercise vs “go home and walk advice”. Two RCTs and 1 non-randomized controlled study reported maximum walking distance (or time), most commonly evaluated with a graded treadmill test. Two studies reported this outcome in distance and 1 in time. One RCT and the non-randomized controlled study showed increased maximum walking distance/time for HET vs GHWA at 3 months (+124 s vs –10 s, p < 0.05) and at 6 months (+83 m vs + 44 m, p < 0.0001). The second RCT showed no significant differences between groups.
Two RCTs and 1 non-randomized controlled study reported pain-free walking distance (or time), most commonly evaluated with a graded treadmill test. Two studies reported this outcome in distance and 1 in time. One RCT and the non-randomized controlled study showed increased pain-free walking distance for HET vs GHWA at 3 months (+134 vs –16 s, p < 0.05) and at 6 months (+51 vs + 27 m, p < 0.0001). The second RCT showed no significant differences between groups.
Two RCTs reported HRQoL with the Short-Form 36 (SF-36). One study demonstrated significantly increased HRQoL for HET in the mental health domain, but in no other domain, at 6 months, compared with GHWA (+3.2 vs –2.4, p < 0.01). The other RCT showed no significant differences between-groups.
Two RCTs rated patient reported functional walking capacity with the WIQ. One study showed significantly improved results for HET in the walking speed domain, but in no other domain, of the WIQ at 6 months, compared with GHWA (+5.7 vs –1.9 WIQ scores, p = 0.034). The other RCT showed no significant differences between groups.
Daily life walking capacity was not reported in any study.
In summary, HET may improve the short-term maximum and pain-free walking distance in patients with IC compared with GHWA (low quality of evidence (GRADE + +) (Tables SI and SII1)). Moreover, HET may result in little or no difference in HRQoL and little or no improvement in functional walking capacity (WIQ) in patients with IC compared with GHWA(low quality of evidence (GRADE + +) (Tables SIII and SIV1)).
Home-based supervised exercise vs hospital-based supervised exercise. Six RCTs and 1 non-randomized controlled study reported maximum walking distance or time. Maximum walking distance was evaluated at 3 or 6 months with a graded treadmill test, and graded treadmill walking was used as exercise modality in the SET, but not in the HET groups. Two studies measured this outcome in distance and 4 studies in time. Three RCTs and 1 non-randomized controlled study demonstrated less improvement in maximum walking distance/time for HET compared with SET. Three RCTs showed no significant differences between groups.
Five RCT studies and one non-randomized controlled study reported pain-free walking distance (or time). Pain-free walking distance was evaluated at 3 or 6 months with a graded treadmill test, and graded treadmill walking was used as exercise modality in the SET groups. Two studies reported this outcome in distance and 4 studies in time. Two RCTs showed no significant differences between groups. Three RCTs and the non-randomized controlled study showed less improvement in pain-free graded treadmill walking distance for HET compared with SET.
Five RCTs and one non-randomized controlled study reported HRQoL with SF-36 or SF-20.
The non-randomized controlled study showed significantly improved adjusted mean difference for SET vs HET in the general health domain at 6 months (8.39, p < 0.03), but not in any other domain. The RCTs demonstrated no significant differences between groups regarding HRQoL.
Three RCTs rated patient reported functional walking ability with the WIQ, without significant differences between the study groups.
Daily life walking capacity, as measured with the 6-min walk test, was reported in one RCT, with significantly more improvement in walking distance for HET compared with SET (+45 vs +15 m, p < 0.05).
In summary, HET may result in less short-term improvement in graded treadmill maximum and pain-free walking distance in patients with IC compared with SET (low quality of evidence (GRADE + +) (Tables SV and SVI1)). Moreover, HET may result in little or no difference in HRQoL and self-reported functional walking capacity compared with SET (low quality of evidence (GRADE + +). (Tables SVII and SVIII1)).
HET may result in more improvement in 6-min walk test distance in patients with IC compared with SET (low quality of evidence (GRADE + +) (Table SIX1)).
Risks/complications associated with exercise were not reported in any of the studies.
The assessments regarding the quality of evidence (GRADE), for each outcome across the studies, are listed in the summary of findings (Table IV).
|
Table IV. Summary of findings |
|||||||||
|
Outcome variable Number of studies |
Design |
Study limitations |
Consistency |
Directness |
Precision |
Publication bias |
Magnitude of effect |
Absolute effect |
Quality of evidence GRADE |
|
Home-based supervised exercise (I) vs ”go home and walk advice” (C) |
|||||||||
|
Maximal walking distance 3 |
2 RCT 1 non-randomized controlled study |
Some limitations (?)1 |
Some inconsistency (?)2 |
Some uncertainty (?)3 |
Serious imprecision (–1)4 |
Unlikely |
Not relevant |
I: Δ 25–83* m C: Δ 39–44* m I: Δ 124* s C: Δ –10* s |
+ + |
|
Pain-free walking distance 3 |
2 RCT 1 non-randomized controlled study |
Some limitations (?)1 |
Some inconsistency (?)2 |
Some uncertainty (?)3 |
Serious imprecision (–1)5 |
Unlikely |
Not relevant |
I: Δ 51–67* m C: Δ 27–52* m I: Δ 134* s C: Δ –16* s |
+ + |
|
Health-related quality of life 2 |
2 RCT |
Some limitations (?)1 |
No serious inconsistency |
Serious indirectness (–1)3 |
Serious imprecision (–1)6 |
Unlikely |
Not relevant |
SF 36 physical function* I: Δ 8 C: Δ –1 SF 36 mental health* I: Δ 3 C: Δ –2 |
+ + |
|
Walking impairment questionnaire 2 |
2 RCT |
Some limitations (?)1 |
No serious inconsistency |
Serious indirectness (–1)3 |
Serious imprecision (–1)6 |
Unlikely |
Not relevant |
Speed score* I: Δ 10%, or Δ 6 C: Δ 4%, or Δ –2 |
+ + |
|
Home-based supervised exercise (I) vs hospital-based supervised exercise (C) |
|||||||||
|
Maximal walking distance 7 |
6 RCT 1 non-randomized controlled study |
Serious limitations (–1)7 |
Some inconsistency (?)2 |
Some uncertainty (?)8 |
Uncertain precision (?)9 |
Unlikely |
Not relevant |
I: Δ 18–124 s* C: Δ 170–378 s* |
+ + |
|
Pain-free walking distance 6 |
5 RCT 1 non-randomized controlled study |
Serious limitations (–1)7 |
Some inconsistency (?)2 |
Some uncertainty (?)8 |
Uncertain precision (?)9 |
Unlikely |
Not relevant |
I: Δ 36–134 s* C: Δ 165–180* s |
+ + |
|
Health-related quality of life 6 |
5 RCT 1 non-randomized controlled study |
Serious limitations (–1)7 |
No serious inconsistency |
Some uncertainty (?)8 |
Uncertain precision (?)10 |
Unlikely |
Not relevant |
No significant differences |
+ + |
|
Walking impairment questionnaire 3 |
3 RCT |
Serious limitations (–1)11 |
No serious inconsistency |
Some uncertainty (?)12 |
Uncertain precision (?)10 |
Unlikely |
Not relevant |
Speed score* I: Δ 6–11% C: Δ 9–15% |
+ + |
|
6-min walk test 1 |
1 RCT |
Some limitations (?)7 |
One study |
Serious indirectness (–1)8 |
Uncertain precision (?)9 |
Unlikely |
Not relevant |
I: Δ 45 (SD 53) m C: Δ 15 (SD 52) m |
+ + |
|
High quality of evidence = + + + +, Moderate quality of evidence = + + +, Low quality of evidence = + +, Very low quality of evidence = +. *Pooled analysis not suitable due to different outcome measures and heterogeneous interventions. Ranges of mean changes from individual studies presented in table, when applicable. 1Problems with baseline data and randomization. 2Different effect estimates across the studies. 3Short follow-up period, specificity of the intervention? 4Possible unfavourable effects, outcome measure the same as exercised in the intervention group, no power calculation for non-inferiority design for secondary outcomes. 5Outcome measure the same as exercised in the intervention group, no power calculation for non-inferiority design for secondary outcomes. 6Possibly unfavourable effects, no power calculation for non-inferiority design for secondary outcomes. 7Large proportion of patients lost to follow-up, unclear randomization, no intention-to-treat analysis, comparable groups? 8Different interventions, short follow-up. 9Outcome measure the same as exercised in the intervention group, possibly unfavourable effects. 10Possibly unfavourable effects. 11Unclear randomization, comparable groups? 12Short follow-up period. RCT: randomized controlled trial. |
|||||||||
DISCUSSION
In this systematic review, the effectiveness of HET programmes was compared with SET programmes or unsupervised GHWA in terms of walking performance, including corridor-walking capacity, as well as patient-reported and disease-specific functional outcomes and HRQoL in patients with IC.
Seven RCTs and 2 non-randomized controlled studies were identified. Regarding walking capacity, there was low quality of evidence (GRADE + +) that HET, as compared with GHWA, may improve maximum and pain-free walking distance, whereas there was little or no difference in HRQoL, and patient-reported functional walking ability. There was low quality of evidence (GRADE + +) that HET may improve maximum and pain-free graded treadmill walking distance slightly less than SET and result in little or no difference in HRQoL, and functional walking ability. By contrast, HET may result in a greater improvement in daily life walking capacity compared with SET (low quality of evidence (GRADE + +). Only 1 of the included RCTs reported this outcome.
Most of the included studies had moderate or major study limitations, mainly regarding blinding of outcome assessor, and had problems with directness and/or precision. Given both the heterogeneity in outcome measures and exercise interventions, a meta-analysis was not undertaken. The design of HET programmes, in particular, varied significantly, from the design of exercise interventions to the use of feedback and behaviour change techniques from caregivers (26–32).
In most of the published studies, the outcome measure of graded treadmill walking was the same as the exercise intervention in the SET programmes (19, 26–30). This introduces an unknown, and possibly major, risk of bias. In our opinion, the quality of evidence for SET programmes is seriously hampered by this, as it is known that a gradual improvement in treadmill results over time is observed with repeated testing, attributed to a “learning” effect (15). Hence, also patients without active treatment will generally improve treadmill performance during the course of a study, introducing a bias that may affect study results (33). A recent RCT comparing SET with HET adds further information regarding this issue (19). In that study patients treated with SET, including graded treadmill walking, performed better than HET patients on graded treadmill assessment, while patients treated with HET, including home-based exercise at a self-selected pace, performed better than SET patients on a corridor-based walk test (19).
The overall management of patients with IC has 2 main objectives (34). Firstly, to reduce the risk for major vascular complications (cardio- and cerebrovascular disease) attributable to atherosclerotic disease and, secondly, to improve daily life walking capacity and HRQoL. In theory, both of these goals could be reached by exercise. Extensive scientific evidence has established the benefits of exercise in improving walking capacity in patients with IC, thus exercise is recommended to all these patients as a part of standard care (35). In a previous systematic review, SET programmes have been shown to further improve short-term walking distance compared with GHWA (10), and to enhance the effect of revascularization when used as an adjunctive treatment (36). However, long-term adherence with SET programmes is generally low in patients with IC (37, 38) and, even though it has been concluded that SET is an effective treatment (10), such programmes are currently not available to the majority of patients with IC (11). Moreover, the long-term efficacy of SET programmes has not been firmly established (10, 12), and the effects on daily life walking capacity and corridor-based walking distance are largely unknown. This limitation was also found in the current systematic review, as both long-term follow-up results on the graded treadmill and reports on daily life walking capacity were scarce in the included studies (10). To overcome some of these shortcomings, interest has turned to HET programmes with a presumed potential to increase long-term adherence to exercise by promoting the integration of exercise into daily life, and thereby inducing changes towards a more active lifestyle (18, 20).
Exercise has also been suggested to reduce the long-term risk for major vascular complications, although little is known about the magnitude and details of such potential effects in IC. Improved serum lipid profiles have been demonstrated in patients with IC participating in exercise (39, 40) and a recent study reported improved surrogate variables for endothelial function and vascular repair mechanisms imposed by a SET programme (41). More data about the effect of different exercise therapies on the risk for major vascular complications in IC are warranted, and constitute another important topic for further research.
Many studies included in this review failed to include outcome measures from the patient perspective, in addition to measures of walking capacity. The need for supplementary studies using patient-reported measures is also highlighted in the review by Fokkenrood et al. (10). We believe that it is crucial to integrate patient-reported end-points in the clinical practice of patients with IC, because such measurements offer important information that could be used in different treatment strategies (42).
The current systematic review is limited by relatively few included studies, and also by the heterogeneity among the included studies regarding different outcome measures, different exercise interventions and lengths of follow-up. On the other hand, this review is strengthened by its recent and comprehensive literature search strategy and inclusion of new outcome measures that have not been summarized previously.
In conclusion, this systematic review has shown low quality of evidence that HET was superior to GHWA regarding graded treadmill maximum and pain-free walking distance, but not different regarding HRQoL and patient-reported walking ability. Moreover, there was low quality of evidence that HET may result in less short-term improvement in graded treadmill maximum and pain-free walking distance and little and or no difference in functional walking ability and HRQoL compared with SET. On the other hand, HET may result in more improvement in daily life walking performance compared with SET. Our study adds to the current literature in patients with IC, in that it provides information about the effectiveness of the various treatment options, which is important to guide rehabilitation to this patient group.
Our systematic review indicates scarce high-quality data on the efficacy of HET to mitigate leg symptoms in patients with IC. With respect to the findings of the current systematic review, we believe that HET programmes can be used as an alternative to SET programmes in patients with IC. Nevertheless, we recommend consideration of using HET programmes before simple GHWA. However, further well-designed randomized controlled trials are needed to establish the optimal exercise components for patients with IC, focusing on long-term comprehensive clinical outcomes and cost-effectiveness. Moreover, interventions to increase adherence with prescribed exercise programmes are essential and must be evaluated and considered from the perspective of clinical transferability.

1http://www.medicaljournals.se/jrm/content/?doi=10.2340 /16501977-2012
REFERENCES
1http://www.medicaljournals.se/jrm/content/?doi=10.2340/16501977-2012
