Júlia Caetano Martins, PT, MSc, Luci Fuscaldi Teixeira-Salmela, PT, PhD, Lucas Araújo Castro e Souza, PT, MSc, Larissa Tavares Aguiar, PT, Eliza Maria Lara, PT, Juliana Braga Moura, PT and Christina Danielli Coelho de Morais Faria, PT, PhD
From the Department of Physical Therapy, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
OBJECTIVE: To investigate the reliability (test-retest and inter-rater) and criterion-related validity of the modified sphygmomanometer test (MST) for the assessment of upper limb muscle strength in subjects with chronic stroke, and to determine whether the results are affected by the number of trials.
Patients and methods: The strength of 11 upper limb muscle groups of 57 subjects with stroke was bilaterally assessed with portable dynamometers and the MST (measured in mmHg). To investigate whether the number of trials would affect the results, 1-way analysis of variance was applied. For the test-retest/inter-rater reliabilities and criterion-related validity of the MST, intra-class correlation coefficients (ICCs), Pearson’s correlation coefficients, and coefficients of determination were calculated.
RESULTS: Different numbers of trials provided similar values for all assessed muscles (0.01 ≤ F ≤ 0.18; 0.83 ≤ p ≤ 0.99) with adequate test-retest (0.83 ≤ ICC ≤ 0.97; p < 0.0001) and inter-rater reliabilities (0.79 ≤ ICC ≤ 0.97; p < 0.0001) and validity (0.61 ≤ r ≤ 0.95; p < 0.0001). The values obtained with the MST were good predictors of those obtained with portable dynamometers (0.60 ≤ r2 ≤ 0.86), except for pinch strength (0.39 ≤ r2 ≤ 0.54).
CONCLUSION: The MST showed adequate measurement properties for the assessment of the strength of the upper limb muscles of subjects with chronic stroke. After familiarization a single trial provided adequate strength values.
Key words: stroke; muscular strength; upper limbs; evaluation; validity; reliability.
J Rehabil Med 2015; 47: 00–00
Correspondence address: Christina Danielli Coelho de Morais Faria, Department of Physical Therapy, Universidade Federal de Minas Gerais, Avenida Antônio Carlos, 6627, Campus Pampulha, 31270-901 Belo Horizonte, MG, Brazil. E-mail: cdcmf@ufmg.br, chrismoraisf@yahoo.com
Accepted Mar 17, 2015; Epub ahead of print Jun 1, 2015
INTRODUCTION
Adequate functioning of the upper limbs (UL) is required for the performance of most activities of daily living (ADL). UL impairments may affect the performance of meaningful tasks, such as reaching and grasping (1, 2). Despite the non-linear relationships between muscle strength and function throughout the recovery process following a stroke (3), it is well recognized that muscle weakness, especially in the UL, is related to limitations in performance of ADL in subjects with stroke (1, 2). Therefore, UL strength is a relevant outcome to be considered in stroke rehabilitation, for the understanding of the functioning and disability processes (1, 2).
Within clinical contexts, the assessment of strength in subjects with stroke is usually performed using the manual muscle test (4), which provides a subjective measurement of strength, but has low sensitivity (4) and limitations for the identification of important differences in strength, mainly when strength is rated as good or normal (5). The gold standard for the evaluation of isometric strength is the portable dynamometer (6), which provides objective strength values and has good sensitivity (7). However, the clinical applicability of the dynamometer is limited for most professionals, especially in developing countries, where stroke is a burden on the public health system (8).
The modified sphygmomanometer test (MST) is another method that can be applied in clinical settings for the assessment of muscle strength. The MST is applied using a simple adaptation of very common, portable, low-cost equipment; the conventional sphygmomanometer, which is commonly used by health professionals for the assessment of blood pressure (9, 10). The MST has demonstrated adequate measurement properties for the assessment of the strength of various muscle groups and populations (11). A recent study provided information regarding the measurement properties of the MST and the number of trials necessary for the assessment of trunk and lower limb muscle strength in subjects with chronic stroke. Following familiarization, adequate reliability and validity were found for a single trial (12). However, no studies were found that used the MST to assess UL muscle strength in subjects with stroke (11).
Before an instrument or test is applied within a specific context, e.g. population characteristics and muscle groups, its measurement properties should be evaluated (13). Furthermore, it is important to determine the number of trials needed to obtain valid and reliable results (13, 14). Therefore, these issues must be addressed before the MST can be recommended for measurement of UL muscle strength in subjects with stroke.
The aims of the present study were therefore to evaluate the measurement properties (test-retest/inter-rater reliabilities and criterion-related validity) of the MST for the assessment of UL muscle strength in subjects with chronic stroke and to determine the number of trials (first trial, the means of the first 2 trials, and the means of 3 trials) needed to obtain valid and reliable results.
METHODS
The present study was part of a larger research project that aimed to investigate the measurement properties of the MST for the overall assessment of muscle strength in subjects with stroke. Data related to the assessment of the lower limb and trunk muscles have been published previously (12). Some of the participants in the present study also participated in the previous study, and the procedures used were identical (12).
Participants
Subjects with stroke were recruited from the general population and by contacting physical therapists and screening outpatient clinics in university hospitals in the city of Belo Horizonte, Brazil. The inclusion criteria were: subjects diagnosed with stroke at least 6 months previously; over 20 years of age and; ability to perform the data collection procedures. The exclusion criteria were: cognitive impairment (Mini Mental Status Examination), pain and other neurological, rheumatological, and/or orthopaedic impairment that could prevent data collection. It is important to note that subjects were included even if they could not perform all of the muscle group tests. Therefore, the sample size varied for each muscle group analysed, since some subjects were not able to activate some muscles.
According to Balogun et al. (15) and recommended tables (13) for power and sample size calculations for correlation analyses, a sample of 18 subjects would be required for a power of 80%, a correlation coefficient of 0.60 and a significance level of 5%. Based on the assumption related to correlation analyses regarding sample heterogeneity, and in an attempt to obtain variability regarding strength, subjects were recruited into various age groups (20–39, 40–59, and > 60 years), both sexes, and various degrees of motor impairments (severe, moderate, and mild), based on the Fugl-Meyer-UL section scores (2, 16, 17). Therefore, the recruitment included 18 subjects in each age group with different characteristics regarding sex and motor impairments, totalling 54 subjects.
After being informed about the objectives of the study, all participants provided written consent based on previous approval from the university ethics review board. Demographic and clinical data were collected by experienced physiotherapists (PTs). The paretic side was determined by increased tonus of the elbow, wrist, and finger flexor muscles, as determined by the Modified Ashworth Scale scores (18) and/or decreased strength, compared with the non-paretic side.
The following muscle groups were assessed: shoulder flexors, extensors, and abductors; elbow flexors and extensors; wrist flexors and extensors; grip and pinch strength (pulp-to-pulp, palmar, and lateral pinch).
Muscle strength measurement
To investigate the criterion-related validity, the measures obtained with the MST were compared with those obtained with the portable dynamometer, which is considered the gold standard equipment for assessment of isometric strength (6). Three portable dynamometers were used: the Microfet2® digital hand-held dynamometer (Hoggan Health Industries, UT, USA) to measure the strength of the shoulder, elbow, and wrist muscles; the Saehan® hydraulic handgrip dynamometer (Saehan Corp., Korea, Model SH5001) to assess grip strength; and the Saehan® hydraulic pinch dynamometer (Saehan Corp., Model SH5005) to measure pinch strength. All of the equipment was new and was calibrated according to the manufacturers’ instructions.
The aneroid sphygmomanometer DuraShockTM Tycos® (Welch Allyn Inc., NY, USA, Model DS-44), with 2 mmHg increments, was used to perform the MST. The conventional sphygmomanometer was adapted using the bag method, as previously described (9, 12, 19) (Fig. 1). Prior to the evaluation sessions, the sphygmomanometer was inflated to 100 mmHg to remove any wrinkles in its bladder. Sufficient air was, then released to achieve a baseline pressure of 20 mmHg, so that it provided measurement intervals between 20 and 304 mmHg. The valve was closed tightly again to prevent leakage (10). The modified sphygmomanometer was then placed in a position to resist the movement generated by the muscle group to be tested, and the force exerted by the subjects was read from the dial. Before each measurement, the examiner verified that the baseline was exactly 20 mmHg. Therefore, the obtained value with the MST included this baseline value of 20 mmHg, as described previously (9, 10, 12, 15).

Fig. 1. Conventional sphygmomanometer adapted using the bag method and inflated to 20 mmHg.
The stability of the measures obtained with the modified sphygmomanometer was tested prior to assessment with known weights (5–35 kg) (19). The correlations between the known weights and the values obtained (in mmHg) were significant and very high (r = 0.99; p = 0.001), and the coefficients of variation ranged from 2.24% to 4.68%.
Procedures
Two trained PTs (examiners 1 and 2) performed all strength assessments independently and adopted identical test procedures (administration, environment, instructions, and protocols). A third examiner read and recorded all values obtained with the portable dynamometers and MST. No feedback and no further discussions were allowed between the examiners. Both subjects and examiners were blinded regarding all strength values.
Examiner 1 assessed all muscle groups twice over 2 sessions (sessions 1 and 2, 1–4 weeks apart (20), whereas, examiner 2 assessed all muscle groups once at the first session. Inter-rater reliability was investigated taking into account the measurements obtained by the 2 independent examiners (examiners 1 and 2) with the MST during the same session. Test-retest reliability was investigated taking into account the MST measurements obtained by examiner 1 over the 2 sessions (20). Prior to data collection on the second day, the subjects were asked to report any adverse health issues that occurred, as recommended previously (13, 21). The criterion-related validity was investigated, taking into account the MST and portable dynamometer data obtained by examiner 1 during the first day.
Before the strength assessments, the order of the equipment (modified sphygmomanometer and portable dynamometers) was randomized, using simple randomization procedures (sealed envelopes). For both pieces of equipment, the assessment always began with the non-paretic side, followed by the same muscle group on the paretic side. The subjects’ positioning, the stabilization to avoid compensatory movements, and the verbal encouragements provided, were standardized (22).
Subject and segment positioning, equipment positioning, and the location of resistance application, followed protocols that are commonly applied for the assessment of strength with portable dynamometers (22–24) and have been used previously in subjects with stroke (22, 24). All of these procedures were identical for both dynamometer and MST assessments.
The following muscle groups were assessed with the subject in the supine position:
• wrist flexors/extensors: UL next to the body, shoulder extended in neutral position, elbow flexed to 90°, forearm and wrist in neutral positions, fingers flexed, manual stabilization of the forearm, resistance applied to the flexed fingers for wrist flexors and proximal to the metacarpophalangeal joints for the wrist extensors (Fig. 2A);
• elbow flexors/extensors: UL next to the body, shoulder extended in neutral position, elbow flexed to 90°, forearm and wrist in neutral positions, fingers flexed, manual stabilization of the shoulder, resistance just proximal to the wrist on the radial/ulnar surface of forearm (Fig. 2B);
• shoulder flexors/extensors: shoulder flexed to 90° in neutral position of rotation, elbow extended, forearm and wrist in neutral positions, fingers flexed, resistance just proximal to the elbow over the flexor/extensor surface of arm (Fig. 2C);
• shoulder abductors: shoulder abducted to 45° in neutral position of rotation, elbow extended, forearm and wrist in neutral positions, fingers flexed, manual stabilization of the shoulder, resistance just proximal to the elbow on the lateral surface of the arm (Fig. 2D).
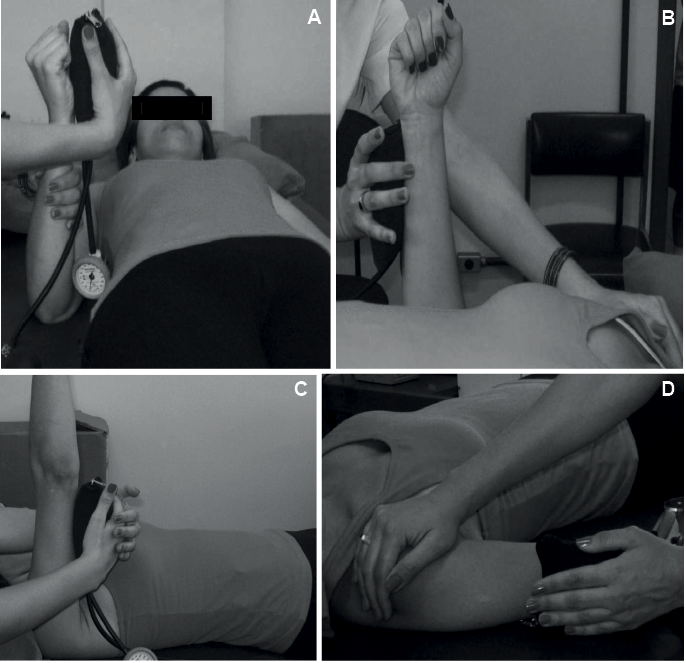
Fig. 2. Assessment of muscle strength with the modified sphygmomanometer test. (A) Wrist flexors; (B) elbow extensors; (C) shoulder extensors; and (D) shoulder abductors.
The following were assessed with the subject in the sitting position (feet and back supported, UL next to the body, shoulder in neutral position, elbow flexed to 90°, forearm in neutral position, wrist extended to 0–30°): grip (Fig. 3A) and pinch strength (Fig. 3B) (pulp-to-pulp, palmar, and lateral pinch) (24).
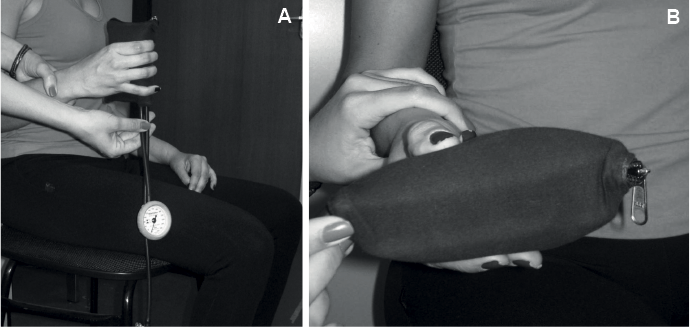
Fig. 3. Assessment of muscle strength with the modified sphygmomanometer test. (A) Grip strength and (B) palmar pinch strength.
Prior to data collection, a demonstration and a familiarization trial (submaximal isometric contraction) were allowed for all procedures. The majority of subjects required only a single trial to become familiar with the procedures, but some subjects required more trials. The participants were then asked to perform a maximal isometric force against the equipment, which was manually stabilized by the examiner, for a period of 5 s, and the peak values were recorded (11). After familiarization, 6 trials of maximal isometric contractions were performed; 3 for each instrument (portable dynamometers and modified sphygmomanometer). Rest intervals (20 s) between trials were allowed in order to avoid fatigue, as in previous studies (2, 22, 25).
Statistical analyses
Descriptive statistics and tests for normality were calculated for all investigated variables. One-way analysis of variance (ANOVA) was used to compare the MST values using different number of trials (first trial, the means of the first 2 trials, and the mean of 3 trials) for all muscle groups, taking into account the values obtained by examiner 1 during session 1.
Intra-class correlation coefficients (ICCs) with 95% confidence intervals (CI) were used to assess the test-retest and inter-rater reliabilities of the MST measures, taking into account the different numbers of trials: first trial (ICC2,1), the means of the first 2 trials (ICC2,2), and the means of 3 trials (ICC2,3). Pearson’s correlation coefficients were calculated to investigate the criterion-related validity between the MST and portable dynamometers measures, taking into account the different numbers of trials. Linear regression analyses were used to identify the model that could better explain the relationships between the measures obtained with both types of equipment and to provide the estimated regression equations that could predict the strength values (in kg) from those obtained with the MST (in mmHg). All analyses took into account the values obtained by examiner 1 during session 1. When ICC values and Pearson’s correlation coefficients reached significance, the strength of the correlations was classified as follows (26): very low = 0–0.25; low = 0.26–0.49; moderate = 0.50–0.69; high = 0.70–0.89; and very high = 0.90–1.00. All analyses were performed with SPSS for Windows, version 17.0 (SPSS Inc., Chicago, IL, USA) (α=5%).
RESULTS
A total of 57 subjects were assessed to determine the validity of the MST (Table I). Test-retest reliability was assessed in 30 of these subjects: 14 women (46.7%) and 16 men (53.3%), mean age 61.20 (SD 14.94) years, mean time since onset of stroke 93.76 (SD 79.85) months, the majority with mild UL motor impairments (66.7%). The investigation of inter-rater reliability also included 30 subjects: 15 women (50%), mean age 58.33 (SD 16.25) years, mean time since onset of stroke 88.33 ± 77.80 months, the majority with mild UL motor impairments (53%).
|
Table I. Subjects’ demographic and clinical characteristics (n = 57) |
|
|
Variables |
Results |
|
Age, years, mean (SD) [range ] |
59.1 (15.0) [25–86] |
|
Time since onset of stroke, months mean (SD) [range] |
95.0 (85.3) [6–371] |
|
Body mass index, kg/m2, mean (SD) |
26.4 (4.8) |
|
Sex, n (%) |
|
|
Men |
29 (50.9) |
|
Women |
28 (49.1) |
|
Paretic side, n (%) |
|
|
Right |
29 (50.9) |
|
Left |
28 (49.1) |
|
Type of stroke, n (%) |
|
|
Ischaemic |
47 (82.5) |
|
Haemorrhagic |
5 (8.8) |
|
Ischaemic and haemorrhagic |
5 (8.8) |
|
Fugl-Meyer Upper Extremity Assessmenta, score (0–66 points), n (%) [range] |
|
|
Mild motor impairments |
32 (56.1) [50–66] |
|
Moderate motor impairments |
12 (21.1) [31–47] |
|
Severe motor impairments |
13 (22.8) [3–28] |
|
aClassification based on Faria-Fortini et al. (2). SD: standard deviation. |
|
The values provided by different numbers of trials were similar (0.01 ≤ F ≤ 0.18; 0.83 ≤ p ≤ 0.99) (Table II). However, for completeness of the investigation, the results for validity and reliability were also investigated taking into account the different numbers of trials.
|
Table II. Descriptive statistics and analysis of variance (ANOVA) results regarding the comparisons between the different numbers of trials for the strength of both upper limb muscles assessed with the modified sphygmomanometer test (mmHg) by examiner 1 during session 1 |
||||
|
Muscle groups (n) |
First trial Mean (SD) |
Means of 2 trials Mean (SD) |
Means of 3 trials Mean (SD) |
ANOVA (F; p-value) |
|
Non-paretic upper limb |
||||
|
Shoulder flexors (56) |
140.8 (52.2) |
139.8 (50.1) |
139.5 (49.3) |
0.01; 0.99 |
|
Shoulder extensors (55) |
184.4 (68.3) |
183.0 (66.9) |
180.7 (65.5) |
0.04; 0.96 |
|
Shoulder abductors (56) |
118.9 (41.3) |
115.5 (38.5) |
114.7 (38.3) |
0.18; 0.83 |
|
Elbow flexors (56) |
185.8 (63.9) |
183.3 (62.1) |
182.3 (61.8) |
0.05; 0.96 |
|
Elbow extensors (56) |
142.7 (43.3) |
143.6 (42.4) |
143.1 (41.7) |
0.01; 0.99 |
|
Wrist flexors (56) |
116.4 (35.8) |
116.0 (35.8) |
114.6 (36.1) |
0.04; 0.96 |
|
Wrist extensors (56) |
103.3 (33.7) |
101.9 (34.3) |
100.5 (34.3) |
0.09; 0.91 |
|
Grip strength (44) |
194.0 (54.0) |
197.9 (54.8) |
196.7 (53.9) |
0.06; 0.94 |
|
Pulp-to-pulp pinch (55) |
113.5 (26.0) |
113.5 (27.0) |
113.8 (27.2) |
0.01; 0.99 |
|
Palmar pinch (56) |
130.9 (32.6) |
132.3 (33.3) |
131.5 (33.3) |
0.03; 0.98 |
|
Lateral pinch (56) |
124.3 (29.1) |
124.4 (28.9) |
124.0 (29.0) |
0.01; 0.99 |
|
Paretic upper limb |
||||
|
Shoulder flexors (42) |
108.2 (40.8) |
109.6 (42.1) |
109.1 (42.3) |
0.01; 0.99 |
|
Shoulder extensors (49) |
147.6 (56.9) |
148.6 (55.8) |
148.4 (54.8) |
0.01; 0.99 |
|
Shoulder abductors (46) |
90.2 (28.5) |
89.8 (28.7) |
89.7 (29.1) |
0.01; 0.99 |
|
Elbow flexors (52) |
131.6 (57.6) |
134.2 (59.4) |
133.9 (58.7) |
0.03; 0.97 |
|
Elbow extensors (49) |
118.0 (36.8) |
118.2 (34.6) |
115.4 (37.4) |
0.09; 0.91 |
|
Wrist flexors (45) |
89.7 (28.4) |
89.2 (28.7) |
88.1 (28.6) |
0.04; 0.96 |
|
Wrist extensors (44) |
78.2 (36.1) |
76.9 (35.4) |
75.5 (33.6) |
0.07; 0.94 |
|
Grip strength (44) |
142.1 (63.6) |
142.0 (62.0) |
141.1 (61.2) |
0.01; 0.99 |
|
Pulp-to-pulp pinch (33) |
98.6 (23.7) |
99.2 (25.2) |
99.1 (25.4) |
0.01; 0.99 |
|
Palmar pinch (30) |
112.7 (28.5) |
112.9 (27.5) |
112.7 (27.7) |
0.01; 0.99 |
|
Lateral pinch (38) |
99.1 (31.1) |
100.8 (29.9) |
100.1 (29.9) |
0.03; 0.97 |
|
SD: standard deviation. |
||||
Reliability
Regarding test-retest reliability, the different number of trials showed high to very high ICC values (0.83 ≤ ICC ≤ 0.97; p < 0.0001) for the muscle groups of the paretic and non-paretic UL (Table III). The 95% CI of the ICC ranged from moderate (lower bounds) to very high (upper bounds) for the majority of the assessed muscles using different numbers of trials.
|
Table III. Test-retest reliability for the assessed muscle groups of both upper limbs with the modified sphygmomanometer test, taking into account the different numbers of trials (data from examiner 1 during both sessions 1 and 2) |
|||||||||
|
Muscle groups (n) |
First trial |
Means of 2 trials |
Means of 3 trials |
||||||
|
ICC2,1 |
95% CI of the ICC2,1 |
ICC2,2 |
95% CI of the ICC2,2 |
ICC2,3 |
95% CI of the ICC2,3 |
||||
|
Non-paretic upper limb |
|||||||||
|
Shoulder flexors (30) |
0.96 |
0.92–0.98 |
0.96 |
0.92–0.98 |
0.96 |
0.91–0.98 |
|||
|
Shoulder extensors (28) |
0.96 |
0.92–0.98 |
0.96 |
0.90–0.98 |
0.92 |
0.83–0.97 |
|||
|
Shoulder abductors (30) |
0.96 |
0.91–0.98 |
0.95 |
0.90–0.98 |
0.95 |
0.90–0.98 |
|||
|
Elbow flexors (29) |
0.95 |
0.90–0.98 |
0.97 |
0.93–0.98 |
0.97 |
0.94–0.99 |
|||
|
Elbow extensors (30) |
0.96 |
0.91–0.98 |
0.95 |
0.90–0.98 |
0.95 |
0.90–0.98 |
|||
|
Wrist flexors (30) |
0.91 |
0.81–0.96 |
0.88 |
0.76–0.95 |
0.87 |
0.73–0.94 |
|||
|
Wrist extensors (30) |
0.94 |
0.88–0.97 |
0.95 |
0.90–0.98 |
0.95 |
0.89–0.98 |
|||
|
Grip strength (22) |
0.92 |
0.81–0.97 |
0.90 |
0.75–0.96 |
0.91 |
0.79–0.96 |
|||
|
Pulp-to-pulp pinch (30) |
0.88 |
0.76–0.94 |
0.90 |
0.78–0.95 |
0.91 |
0.81–0.96 |
|||
|
Palmar pinch (30) |
0.95 |
0.89–0.98 |
0.96 |
0.91–0.98 |
0.96 |
0.92–0.98 |
|||
|
Lateral pinch (30) |
0.83 |
0.65–0.92 |
0.89 |
0.78–0.95 |
0.92 |
0.83–0.96 |
|||
|
Paretic upper limb |
|||||||||
|
Shoulder flexors (23) |
0.92 |
0.81–0.97 |
0.93 |
0.84–0.97 |
0.94 |
0.86–0.97 |
|||
|
Shoulder extensors (26) |
0.93 |
0.85–0.97 |
0.94 |
0.87–0.97 |
0.95 |
0.89–0.98 |
|||
|
Shoulder abductors (25) |
0.93 |
0.83–0.97 |
0.93 |
0.85–0.97 |
0.94 |
0.85–0.97 |
|||
|
Elbow flexors (29) |
0.92 |
0.83–0.96 |
0.94 |
0.87–0.97 |
0.95 |
0.90–0.98 |
|||
|
Elbow extensors (27) |
0.92 |
0.83–0.96 |
0.95 |
0.89–0.98 |
0.94 |
0.88–0.97 |
|||
|
Wrist flexors (25) |
0.86 |
0.67–0.94 |
0.89 |
0.76–0.95 |
0.91 |
0.80–0.96 |
|||
|
Wrist extensors (24) |
0.93 |
0.83–0.97 |
0.96 |
0.91–0.98 |
0.96 |
0.91–0.98 |
|||
|
Grip strength (26) |
0.91 |
0.81–0.96 |
0.93 |
0.84–0.97 |
0.94 |
0.86–0.97 |
|||
|
Pulp-to-pulp pinch (21) |
0.88 |
0.68–0.95 |
0.91 |
0.77–0.96 |
0.92 |
0.81–0.97 |
|||
|
Palmar pinch (22) |
0.90 |
0.75–0.96 |
0.92 |
0.81–0.97 |
0.90 |
0.77–0.96 |
|||
|
Lateral pinch (21) |
0.83 |
0.57–0.93 |
0.90 |
0.76–0.96 |
0.89 |
0.73–0.96 |
|||
|
p < 0.0001 for all ICC values. ICC: intra-class correlation; CI: confidence interval. |
|||||||||
For the inter-rater reliability, the different number of trials showed high to very high ICC values for all muscle groups (0.79 ≤ ICC ≤ 0.97; p < 0.0001). The 95% CI of the ICC were more variable and ranged from very low (lower bounds) to very high (upper bounds) for the majority of the assessed muscles using different numbers of trials (Table IV).
|
Table IV. Inter-rater reliability for the assessed muscle groups of both upper limbs with the modified sphygmomanometer test, taking into account the different numbers of trials (data from examiners 1 and 2 at the same session) |
|||||||||
|
Muscle groups (n) |
First trial |
Means of 2 trials |
Means of 3 trials |
||||||
|
ICC2,1 |
95% CI of the ICC2,1 |
ICC2,2 |
95% CI of the ICC2,2 |
ICC2,3 |
95% CI of the ICC2,3 |
||||
|
Non-paretic upper limb |
|||||||||
|
Shoulder flexors (30) |
0.93 |
0.86–0.97 |
0.96 |
0.91–0.98 |
0.96 |
0.91–0.98 |
|||
|
Shoulder extensors (28) |
0.92 |
0.82–0.96 |
0.93 |
0.85–0.97 |
0.89 |
0.76–0.95 |
|||
|
Shoulder abductors (30) |
0.91 |
0.56–0.97 |
0.91 |
0.47–0.97 |
0.90 |
0.56–0.97 |
|||
|
Elbow flexors (29) |
0.89 |
0.77–0.95 |
0.90 |
0.78–0.95 |
0.89 |
0.76–0.95 |
|||
|
Elbow extensors (30) |
0.91 |
0.80–0.96 |
0.93 |
0.85–0.97 |
0.94 |
0.87–0.97 |
|||
|
Wrist flexors (30) |
0.80 |
0.47–0.92 |
0.79 |
0.50–0.91 |
0.81 |
0.54–0.92 |
|||
|
Wrist extensors (30) |
0.86 |
0.71–0.93 |
0.89 |
0.72–0.95 |
0.89 |
0.71–0.96 |
|||
|
Grip strength (21) |
0.94 |
0.86–0.98 |
0.93 |
0.83–0.97 |
0.92 |
0.79–0.97 |
|||
|
Pulp-to-pulp pinch (29) |
0.92 |
0.82–0.96 |
0.94 |
0.83–0.98 |
0.94 |
0.77–0.98 |
|||
|
Palmar pinch (29) |
0.95 |
0.89–0.98 |
0.97 |
0.93–0.99 |
0.97 |
0.93–0.99 |
|||
|
Lateral pinch (29) |
0.88 |
0.62–0.95 |
0.89 |
0.29–0.97 |
0.89 |
0.18–0.97 |
|||
|
Paretic upper limb |
|||||||||
|
Shoulder flexors (22) |
0.91 |
0.78–0.96 |
0.92 |
0.82–0.97 |
0.93 |
0.82–0.97 |
|||
|
Shoulder extensors (24) |
0.93 |
0.83–0.97 |
0.95 |
0.87–0.98 |
0.96 |
0.90–0.98 |
|||
|
Shoulder abductors (24) |
0.88 |
0.73–0.95 |
0.89 |
0.75–0.95 |
0.90 |
0.77–0.96 |
|||
|
Elbow flexors (27) |
0.84 |
0.66–0.93 |
0.88 |
0.74–0.95 |
0.89 |
0.76–0.95 |
|||
|
Elbow extensors (26) |
0.85 |
0.66–0.93 |
0.88 |
0.74–0.95 |
0.88 |
0.73–0.95 |
|||
|
Wrist flexors (22) |
0.85 |
0.64–0.94 |
0.82 |
0.56–0.92 |
0.81 |
0.56–0.92 |
|||
|
Wrist extensors (22) |
0.81 |
0.55–0.92 |
0.84 |
0.62–0.93 |
0.85 |
0.63–0.94 |
|||
|
Grip strength (23) |
0.92 |
0.80–0.96 |
0.94 |
0.85–0.97 |
0.94 |
0.86–0.98 |
|||
|
Pulp-to-pulp pinch (18) |
0.89 |
0.69–0.96 |
0.91 |
0.74–0.97 |
0.91 |
0.76–0.97 |
|||
|
Palmar pinch (19) |
0.95 |
0.87–0.98 |
0.95 |
0.86–0.98 |
0.95 |
0.88–0.98 |
|||
|
Lateral pinch (21) |
0.90 |
0.74–0.96 |
0.93 |
0.82–0.97 |
0.93 |
0.80–0.98 |
|||
|
p < 0.0001 for all ICC values. ICC: intra-class correlation; CI: confidence interval. |
|||||||||
Validity
For all muscle groups, significant correlations were found between the portable dynamometers and the MST measures when different numbers of trials were considered. The correlations were classified as moderate to very high for all muscle groups and the numbers of trials of both the non-paretic (0.61 ≤ r ≤ 0.93; p < 0.0001) and paretic UL (0.65 ≤ r ≤ 0.95; p < 0.0001).
Except for the pulp-to-pulp and palmar pinch strength of both UL and lateral pinch strength of the paretic UL (0.39 ≤ r2 ≤ 0.54), the coefficients of determination were 0.60 ≤ r2 ≤ 0.86 for the muscles of both sides, indicating that at least 60% of the strength values obtained with the dynamometer were explained by the measures obtained with the MST (Table V). The equations provided in Table V could be used to predict the strength values (in kg) from those obtained with the MST (in mmHg).
|
Table V. Descriptive statistics, Pearson’s correlation coefficients, and regression analysis results for the first trial of strength of both upper limb muscles (data from examiner 1 during session 1) |
|||||
|
Muscle groups (n) |
Portable dynamometers (kg) Mean (SD) |
MST (mmHg) Mean (SD) |
Correlation (r) |
Regression (r2) |
Regression (equations) |
|
Non-paretic upper limb |
|||||
|
Shoulder flexors (56) |
11.5 (5.1) |
140.8 (52.2) |
0.86* |
0.74* |
y = –0.311 + 0.084 MSTm |
|
Shoulder extensors (55) |
15.0 (7.05) |
184.4 (68.3) |
0.87* |
0.75* |
y = –1.513 + 0.089 MSTm |
|
Shoulder abductors (56) |
9.0 (4.2) |
118.9 (41.3) |
0.92* |
0.85* |
y = –2.151 + 0.093 MSTm |
|
Elbow flexors (56) |
14.0 (5.7) |
185.8 (63.9) |
0.85* |
0.72* |
y = –0.133 + 0.076 MSTm |
|
Elbow extensors (56) |
10.6 (4.5) |
142.7 (43.3) |
0.89* |
0.79* |
y = –2.626 + 0.092 MSTm |
|
Wrist flexors (56) |
7.5 (3.4) |
116.4 (35.8) |
0.82* |
0.68* |
y = –1.574 + 0.078 MSTm |
|
Wrist extensors (56) |
6.8 (3.3) |
103.3 (33.7) |
0.85* |
0.73* |
y = –1.846 + 0.084 MSTm |
|
Grip strength (44) |
24.8 (7.6) |
194.0 (54.0) |
0.80* |
0.65* |
y = 2.991 + 0.113 MSTm |
|
Pulp-to-pulp pinch (55) |
5.4 (2.0) |
113.5 (26.0) |
0.62* |
0.39* |
y = –0.092 + 0.048 MSTm |
|
Palmar pinch (56) |
5.5 (2.0) |
130.9 (32.6) |
0.62* |
0.39* |
y = 0.502 + 0.038 MSTm |
|
Lateral pinch (56) |
6.8 (2.0) |
124.3 (29.1) |
0.78* |
0.61* |
y = 0.223 + 0.053 MSTm |
|
Paretic upper limb |
|||||
|
Shoulder flexors (42) |
8.7 (4.6) |
108.2 (40.8) |
0.86* |
0.74* |
y = –1.680 + 0.096 MSTm |
|
Shoulder extensors (49) |
11.6 (5.6) |
147.6 (56.9) |
0.93* |
0.86* |
y = –1.790 + 0.091 MSTm |
|
Shoulder abductors (46) |
6.3 (3.0) |
90.2 (28.5) |
0.89* |
0.80* |
y = –2.290 + 0.095 MSTm |
|
Elbow flexors (52) |
9.8 (5.1) |
131.6 (57.6) |
0.85* |
0.72* |
y = –0.039 + 0.074 MSTm |
|
Elbow extensors (49) |
8.0 (3.7) |
118.0 (36.9) |
0.89* |
0.80* |
y = –2.534 + 0.089 MSTm |
|
Wrist flexors (45) |
5.2 (2.6) |
89.7 (28.4) |
0.77* |
0.60* |
y = –1.116 + 0.070 MSTm |
|
Wrist extensors (44) |
4.4 (3.0) |
78.2 (36.1) |
0.82* |
0.67* |
y = –1.062 + 0.069 MSTm |
|
Grip strength (44) |
17.6 (9.3) |
142.1 (63.6) |
0.84* |
0.71* |
y = –0.224 + 0.123 MSTm |
|
Pulp-to-pulp pinch (33) |
3.6 (1.8) |
98.6 (23.7) |
0.69* |
0.48* |
y = –1.692 + 0.054 MSTm |
|
Palmar pinch (30) |
4.1 (1.7) |
112.7 (28.5) |
0.65* |
0.42* |
y = –0.426 + 0.040 MSTm |
|
Lateral pinch (38) |
4.9 (2.0) |
99.1 (31.1) |
0.74* |
0.54* |
y = 0.130 + 0.048 MSTm |
|
*p < 0.0001; SD: standard deviation; MST: modified sphygmomanometer test; r: Pearson’s correlation coefficient; r2: Coefficient of determination; y: dependent or criterion variable (portable dynamometer measure); MSTm (modified sphygmomanometer test measure): independent or predictor variable. |
|||||
DISCUSSION
In this study, the MST provided adequate values of test-retest/inter-rater reliabilities and validity for all assessed muscles of both UL in subjects with chronic stroke for all investigated numbers of trials.
Portney & Watkins (13) found that most researchers recommend making more than 1 measurement of a behaviour or characteristic, whenever possible. However, the question as to which value best represents the individual’s true scores remains unanswered. The majority of studies that used the MST to assess UL strength reported the mean of 3 trials (11). A recent study (12) investigated the effects of different numbers of trials on the MST values for the assessment of trunk and lower limb muscles in subjects with chronic stroke, and found that the first and the means of 2 and 3 trials showed similar results for all assessed muscles. Coldham et al. (27) reported that a single trial of maximal grip strength with a portable dynamometer in healthy subjects was appropriate, less painful, and as reliable as the mean of 3 trials or the best measure.
In the present study, no significant differences were found between the MST measures when different numbers of trials were considered for the assessment of UL muscles. The use of a single trial for the MST assessment of strength improves its applicability and feasibility, since a significant time-saving can be made in addition to avoiding muscular and general fatigue.
Reliability
Considering that ICC is the most recommended method for reliability analyses, since it reflects both the associations and the agreement levels between 2 or more measures (13), the present results were compared only with those of earlier studies that provided ICC values to report the reliability of MST measures. Perossa et al. (28) assessed the test-retest reliability of the MST for assessment of the strength of the shoulder flexors, extensors, abductors, internal, and external rotators in healthy individuals and found ICCs ranging from 0.86 to 0.97. Isherwood et al. (29) assessed the MST strength measures of the elbow flexors in healthy women and found ICCs ranging from 0.84 to 0.89 for the test-retest and from 0.87 to 0.95 for the inter-rater reliabilities. Kaegi et al. (10) assessed the test-retest reliability of the MST measures of the elbow extensors of elderly inpatients and found ICC values of 0.87. Helewa et al. (30) assessed the inter-rater reliability of the MST measures of the shoulder abductors in patients with rheumatoid arthritis and reported ICC values of 0.93. Suresh et al. (31) assessed the grip and pinch (pulp-to-pulp and lateral pinch) strength in leprosy and found poor inter-rater reliability coefficient only for the lateral pinch strength (ICC = 0.48). The ICC values for both test-retest and inter-rater reliabilities found in the present study were similar to those of previous studies and, for the lateral pinch strength in particular, they were even higher (0.88 ≤ ICC ≤ 0.93). These results illustrate that there were no systematic differences between the MST values obtained by examiner 1 over 2 sessions and between 2 raters. Furthermore, the number of trials did not affect the MST results regarding test-retest and inter-rater reliabilities for the assessment of UL muscle strength. Similar results were also reported for MST assessment of the lower limb and trunk muscles of subjects with chronic stroke (12).
Validity
Previous studies investigating the criterion-related validity of the MST for the assessment of UL strength (11) compared the measures provided by the modified sphygmomanometer with those obtained with the portable dynamometer, only for the elbow flexors of healthy individuals (32) and grip strength of elderly and healthy adults (15, 33–35). These studies found significant and high correlations between the measures provided by the 2 devices (0.75 ≤ r ≤ 0.96), as observed in the present study for the same muscle groups. Furthermore, in the present study, the validity of the MST was also investigated for 9 other muscle groups and the results were also significant, with high to very high correlations, except for the pulp-to-pulp and palmar pinch strength, which had moderate to high correlations (0.61 ≤ r ≤ 0.76). The number of trials did not affect the results regarding the criterion-related validity. Similar results were reported with the MST for the assessment of the lower limb and trunk muscles of subjects with chronic stroke (12).
Three studies that investigated the criterion-related validity of the MST also used regression analyses to identify the best model to explain the relationships between the MST and the criterion measures (15, 32, 35). Bohannon & Lusardi (32) used the mean of 2 trials of the strength of the elbow flexors with healthy subjects and reported curvilinear relationships with coefficients of determination of r2 = 0.82 between the measures provided by both instruments. In the present study, for the same muscle group, using the first trial, linear relationships between the MST and the dynamometer measures were found, with coefficient of determinations of r2 = 0.72. Balogun et al. (15) assessed grip strength using the highest value of 2 trials with healthy adults and described a linear relationship (r2 = 0.71). Hamilton et al. (35) assessed the same muscle group using the mean of 3 trials with healthy women and also described a linear relationship (r2 = 0.56). In the present study, the linear regression model revealed coefficients of determination of r2 = 0.65 and 0.71 for grip strength using the first trial.
Although the MST uses a device designed to assess blood pressure, when it was properly adapted and the technique described in this study was applied, at least 60% of the variation in the dynamometer measurements could be explained by the MST measurements for the majority of the muscle groups. The established prediction equations of the present study could be used to estimate the strength values (in kg) by using the MST measures (in mmHg) for subjects with stroke. Thus, the results for UL muscle strength obtained with the MST could be compared with those of previous studies using the dynamometer (1, 2, 36). For example, there are some cut-off values regarding grip strength measures assessed with portable dynamometers used to classify the degree of clinically relevant muscular weakness: < 26 kg in men and < 16 kg in women are classified as “weak”; between 26–31.9 kg in men and 16–19.9 kg in women are classified as “intermediate”; and ≥ 32 kg in men and ≥ 20 kg in women are classified as “normal strength” (36). Clinicians who assess grip strength with the MST, which provides force values in mmHg, can apply the regression equations provided by the present study to determine the concurrent force values in kg and could therefore apply this categorization to classify clinically relevant weakness.
Within both clinical and research contexts, the residual deficit of the paretic upper limb is usually determined. One of the proposed approaches to quantify this deficit is the ratio between the strength data of the paretic and non-paretic sides (37). It is interesting to note that, when taking these ratio values, the units of measurement (mmHg or kg) do not matter. Therefore, for a specific muscle group, the ratio values provided by the dynamometer (kg) are similar to those provided by the MST (mmHg). This can easily be observed by calculating the strength ratio between the paretic and non-paretic sides, using the mean values of the dynamometer and the MST measures shown in Table V. Therefore, if clinicians are interested in ratio values, it is not necessary to apply the regression equations provided by the present study. It is important to note that a limitation of using the ratio values is that the strength of the non-paretic side is also impaired in subjects with stroke (38, 39) and, therefore, the non-paretic side should not be used as a reference (39).
In the present study, the pulp-to-pulp and palmar pinch strength of both UL and lateral pinch strength of the paretic UL showed significant and lower coefficients of determination. This could be explained by the difficulty that the subjects had in assuming the standard positioning, since they required assistance to maintain their finger positioning on the equipment. In addition, the pinch dynamometer had a thin surface, where the fingers were positioned. On the other hand, the MST has a large surface. Thus, the length-tension of the muscles was different between the devices.
Another important issue to be discussed is related to the greater variability observed for all the strength measures of the non-paretic UL compared with the paretic one. The force generated in a joint that is crossed by several muscles can be the result of the combination of a large number of forces. Furthermore, a specific level of muscle activation, which is necessary to generate force, can be the result of various combinations of sub-sets of motor units, which can be recruited in different frequencies (40). These natural motor variabilities, which result in movements that are “both variable and optimal”, demonstrate specific changes that can be associated with neurological disorders (40), such as stroke.
The results of the present study, together with other results published recently (12), indicate that the MST may be applied within clinical settings for the assessment of the strength of the most commonly affected muscles of the trunk, upper and lower limbs of subjects with chronic stroke. Considering the adequate levels of reliability and validity, future studies should investigate the responsiveness of the MST and include analyses of the minimal clinically important differences. It is important to determine how much change in strength measured with the MST is necessary to characterize a meaningful and worthwhile change, taking into account the subjects’ perspectives (41).
Study limitations
A limitation of this study was that few subjects with high levels of disability were evaluated. In addition, to avoid bias related to internal validity investigation, a third examiner was responsible for reading and recording all strength measures, which is not a procedure commonly observed within clinical contexts. Moreover, the predicted equations for the pulp-to-pulp and palmar pinch strength of both UL and the lateral pinch strength of the paretic UL should be used with caution, since the coefficients of determination were low for these muscle groups and there may be other predictor factors that were not considered in this study. Finally, considering that subjects with stroke have more difficulty performing dynamic contractions, it would be interesting to verify whether the MST could be applied to evaluate strength in dynamic contractions. However, in the present study, only isometric strength was assessed. The majority of studies that used portable dynamometry with subjects with stroke also evaluated isometric muscular contractions (22). In addition, a recently published systematic review (11) reported that all of the studies related to the MST measures also evaluated isometric strength. Therefore, future studies should investigate whether the MST can be used for the assessment of strength during dynamic contractions.
Conclusion and clinical applicability
The MST can be used to assess the strength of the UL muscles in subjects with chronic stroke, since it shows adequate reliability and validity. In addition, after familiarization, a single trial is sufficient to generate valid and reliable measures. As the device is portable and available worldwide, the MST could easily be used in a range of clinical contexts. Adaptation of the conventional aneroid sphygmomanometer is simple, requiring only the addition of a cotton bag costing approximately US$15. Furthermore, it is not necessary to use the sphygmomanometer exclusively for the assessment of strength, since the adaptation is not permanent. Thus, the MST enables health professionals to perform objective assessments of UL strength in subjects with stroke, with good-quality results, at a low cost, and with good feasibility.
ACKNOWLEDGMENTS
Financial support for this research was provided by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and PRPq/UFMG (Pró-reitoria de Pesquisa da Universidade Federal de Minas Gerais). The authors are grateful to Dr John Henry Salmela for copyediting the manuscript.
Conflicts of interest. None of the authors have a relationship with any entities that have a financial interest in this topic.
REFERENCES
